Methods Used and Application of the Mouse Grimace Scale in Biomedical Research 10 Years on: A Scoping Review
Abstract
Simple Summary
Abstract
1. Introduction
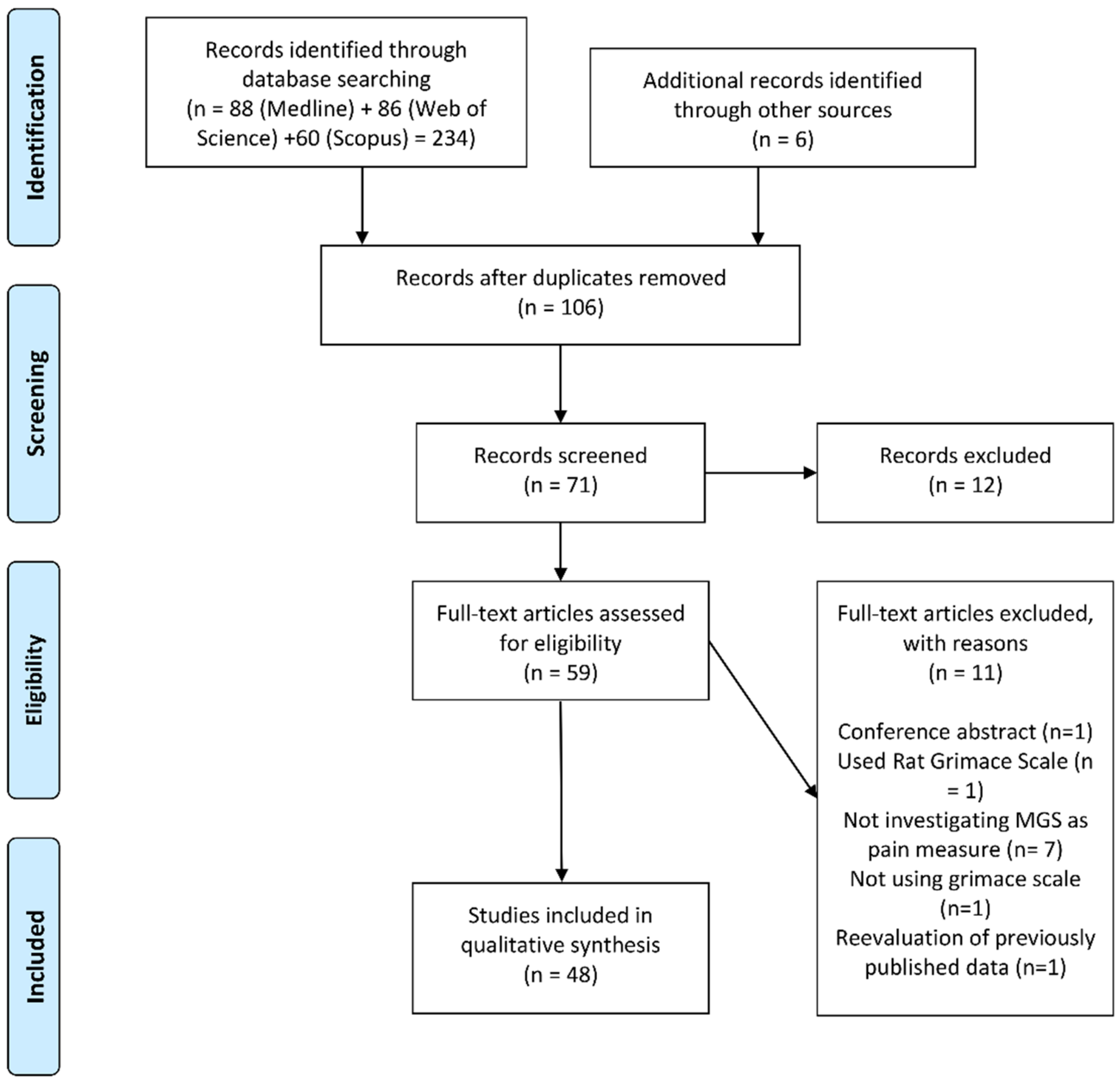
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
3. Results
3.1. Study Characteristics
3.2. Animal Model Characteristics
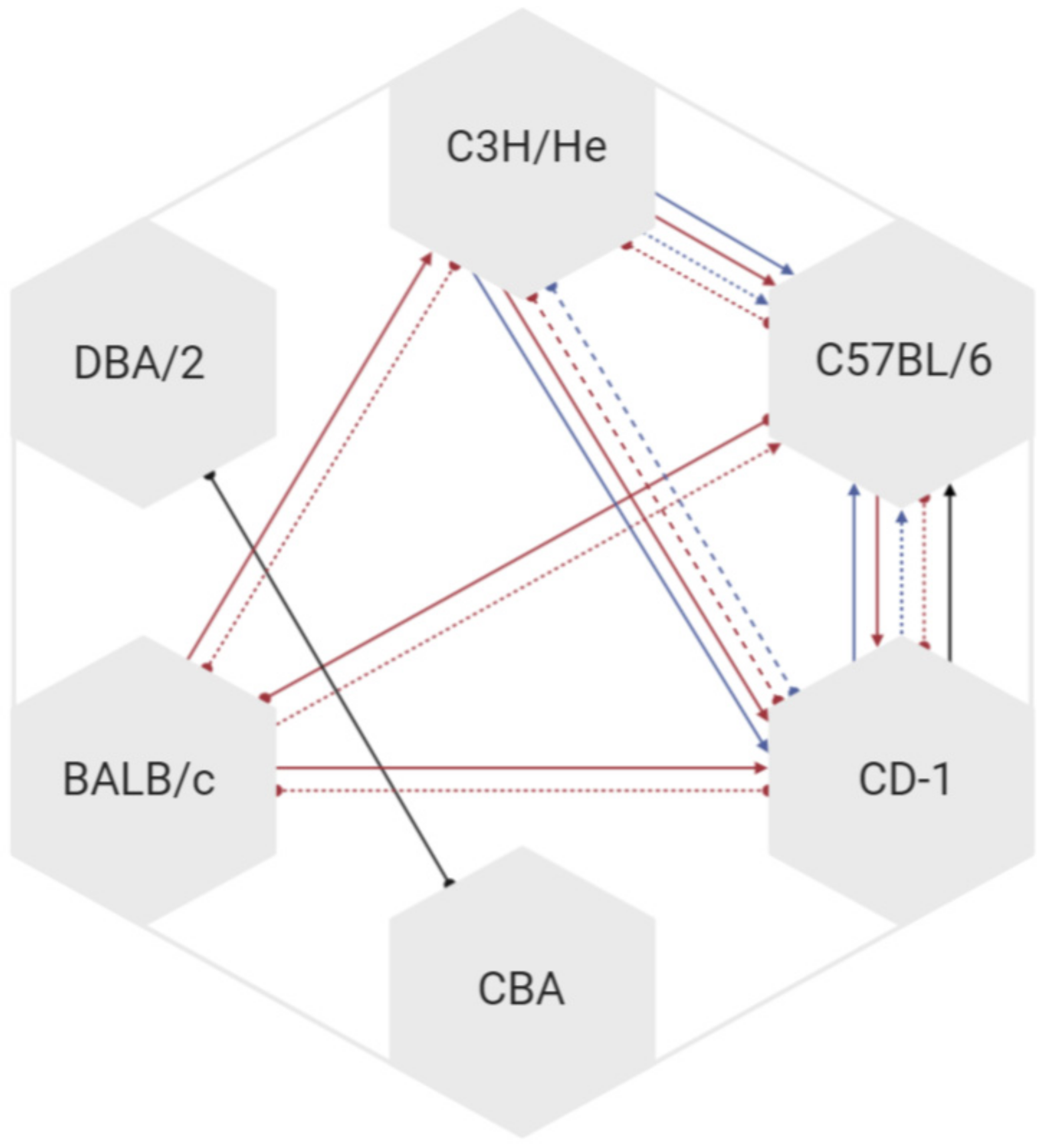
3.3. Mouse Characteristics
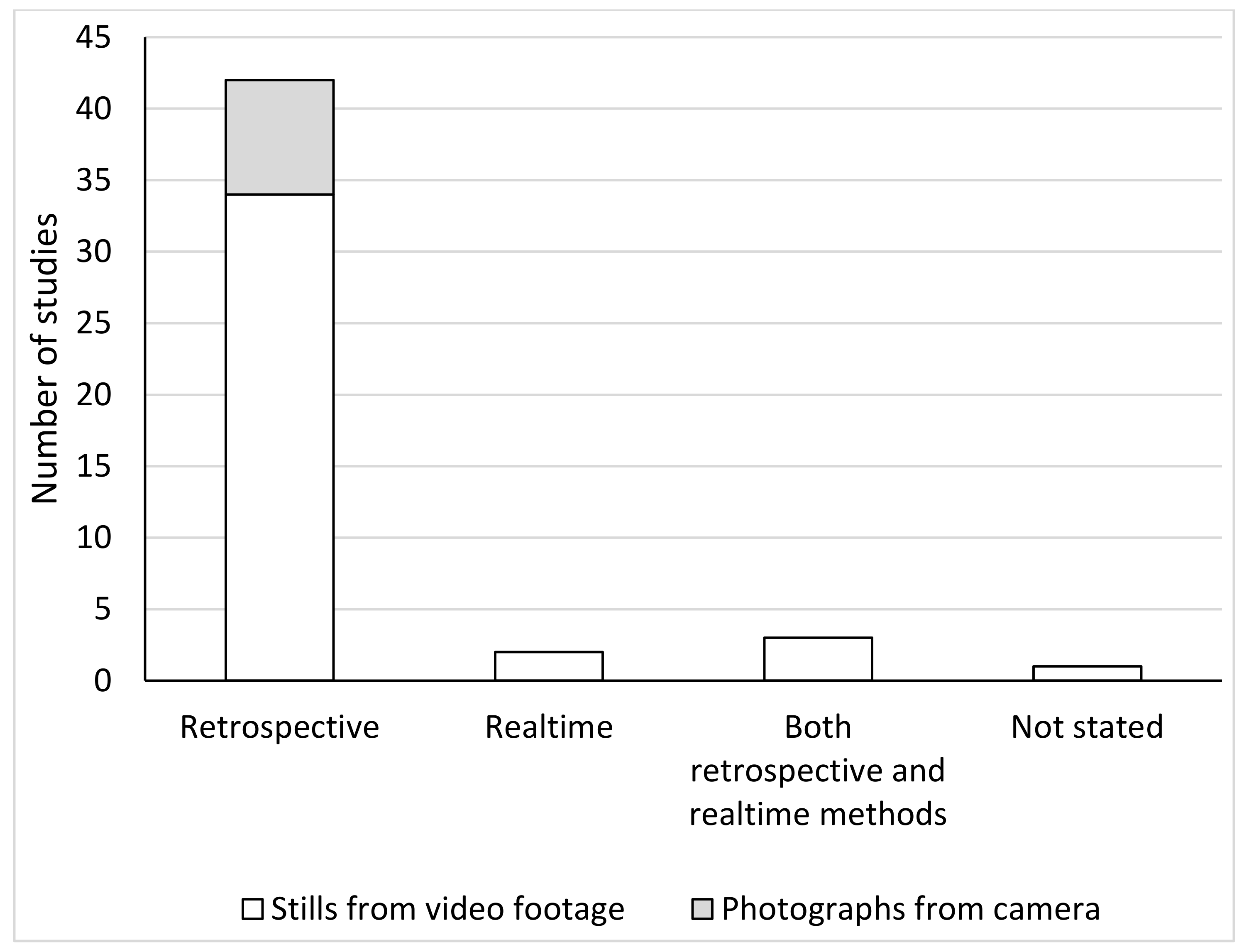
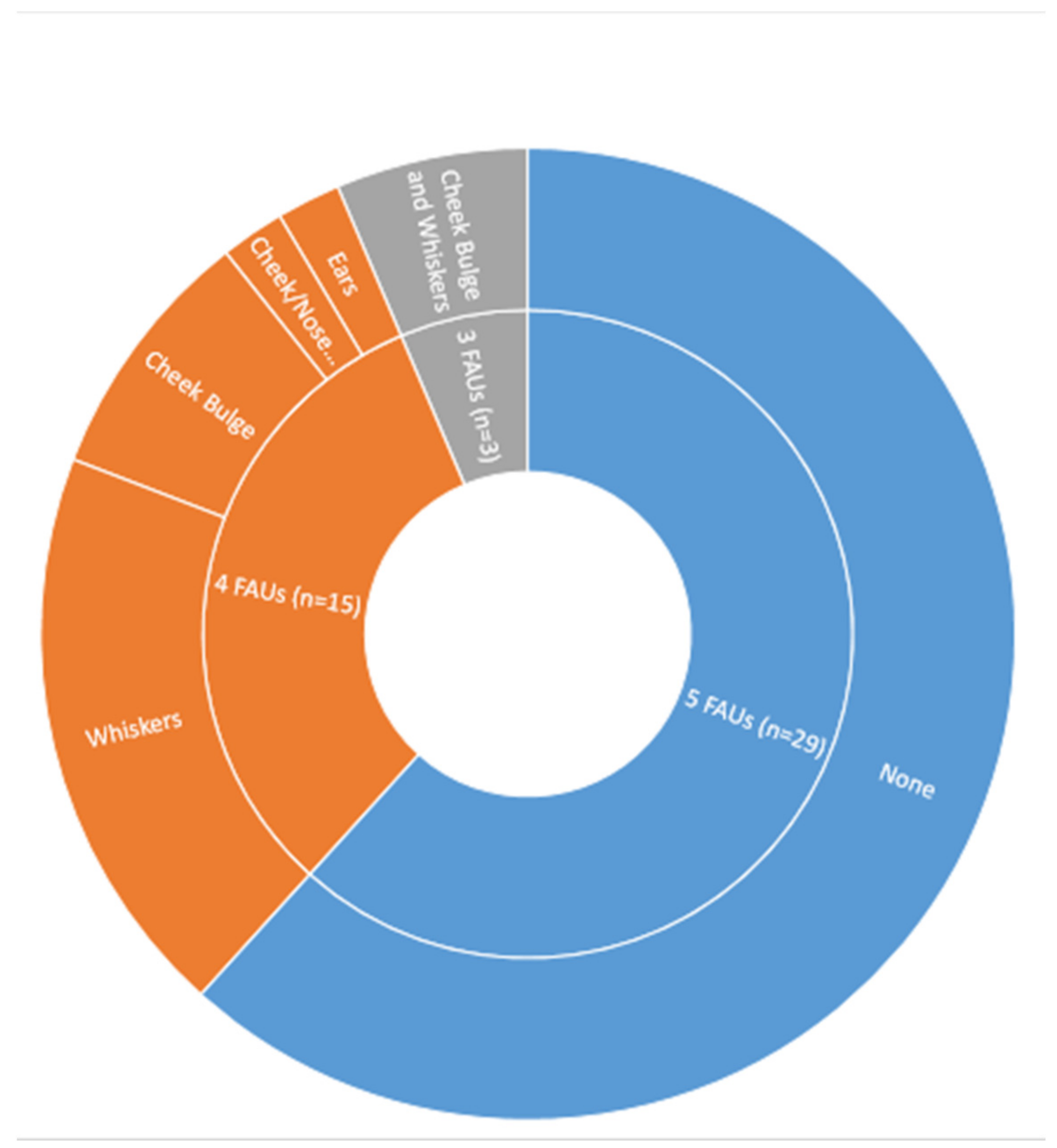
3.4. MGS Measurement Methods
3.5. Corroborating Methods of Affective State Assessment Used
3.6. Impact of External Factors on MGS
3.6.1. Circadian Rhythm
3.6.2. Variability Arising from Observers
4. Discussion
4.1. Methods Used
4.2. Validity of the MGS across a Range of Pain Types
4.3. Reliability
4.4. The Impact of Biological Variation and the External Environment on the MGS
4.5. Conclusions and Recommendations for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Search Strategy |
|---|---|
| Medline | (Mouse [tiab]) OR (Mice [tiab])) OR (Murine [tiab])) OR ((Murin*) [tiab])) OR (Mus [tiab])) OR (Musculus [tiab])) OR (Transgenic Animal [tiab])) OR (Mice [mh])) AND (Grimace Scale)) OR (Grimace Score[tiab])) OR (Facial grimace[tiab])) |
| Scopus | TITLE-ABS-KEY (“Mouse” OR “Mice” OR “Murine” OR “Murin* “ OR “Mus “ OR “Musculus” OR “Transgenic Animal”) AND TITLE-ABS-KEY (“Grimace Scale” OR “Grimace Score” OR “Facial grimace”) |
| Web of Science | TS = (Mouse OR Mice OR Murine OR Murin*OR Mus OR Musculus OR Transgenic Animal OR Mice) AND TS = (Grimace Scale OR Grimace Score OR Facial grimace) |
References
- European Commission. Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union in 2015–2017. 2019. Available online: https://op.europa.eu/en/publication-detail/-/publication/04a890d4-47ff-11ea-b81b-01aa75ed71a1 (accessed on 5 December 2020).
- Finlayson, K.; Lampe, J.F.; Hintze, S.; Würbel, H.; Melotti, L. Facial indicators of positive emotions in rats. PLoS ONE 2016, 11, e0166446. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Marsh, L.E. The role of behavioural assessment in determining ‘positive’ affective states in animals. CAB Rev. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Phys. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Panksepp, J. Affective consciousness: Core emotional feelings in animals and humans. Conscious. Cogn. 2005, 14, 30–80. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Hernández, E.; Martínez-Burnes, J.; Whittaker, A.L. The utility of grimace scales for practical pain assessment in laboratory animals. Animals 2020, 10, 1838. [Google Scholar] [CrossRef]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y. The need for fundamental reforms in the pain research field to develop innovative drugs. Expert Opin. Drug Dis. 2017, 12, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, G.T.; Pomonis, J.D.; Kennedy, J.D. An industry perspective on the role and utility of animal models of pain in drug discovery. Neurosci. Lett. 2013, 557, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- González-Cano, R.; Montilla-García, Á.; Ruiz-Cantero, M.; Bravo-Caparrós, I.; Tejada, M.; Nieto, F.; Cobos, E. The search for translational pain outcomes to refine analgesic development: Where did we come from and where are we going? Neurosci. Biobehav. Rev. 2020, 113, 238–261. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Austin, J. Pain and laboratory animals: Publication practices for better data reproducibility and better animal welfare. PLoS ONE 2016, 11, e0155001. [Google Scholar] [CrossRef]
- Peterson, N.C.; Nunamaker, E.A.; Turner, P.V. To treat or not to treat: The effects of pain on experimental parameters. Comp. Med. 2017, 67, 469–482. [Google Scholar] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Whittaker, A.L.; Howarth, G.S. Use of spontaneous behaviour measures to assess pain in laboratory rats and mice: How are we progressing? Appl. Anim. Behav. Sci. 2014, 151, 1–12. [Google Scholar] [CrossRef]
- Mogil, J.S.; Pang, D.S.; Dutra, G.G.S.; Chambers, C.T. The development and use of facial grimace scales for pain measurement in animals. Neurosci. Biobehav. Rev. 2020, 116, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Miwa, M.; Yoshida, M.; Miura, R.; Tanei, S.; Tsuji, M.; Takeda, H. Spontaneous pain-associated facial expression and efficacy of clinically used drugs in the reserpine-induced rat model of fibromyalgia. Eur. J. Pharmacol. 2019, 864, 172716. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, K.; Tomizawa-Shinohara, H.; Yasuno, H.; Yogo, K.; Matsumoto, Y. Anti-il-6 receptor antibody inhibits spontaneous pain at the pre-onset of experimental autoimmune encephalomyelitis in mice. Front. Neurol. 2019, 10, 341. [Google Scholar] [CrossRef]
- LeResche, L. Facial expression in pain: A study of candid photographs. J. Nonverbal Behav. 1982, 7, 46–56. [Google Scholar] [CrossRef]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Meth. 2010, 7, 447–449. [Google Scholar] [CrossRef]
- Descovich, K.; Wathan, J.; Leach, M.C.; Buchanan-Smith, H.M.; Flecknell, P.; Farningham, D.; Vick, S.-J. Facial expression: An under-utilised tool for the assessment of welfare in mammals. ALTEX 2017. [Google Scholar] [CrossRef]
- McLennan, K.M.; Miller, A.L.; Dalla Costa, E.; Stucke, D.; Corke, M.J.; Broom, D.M.; Leach, M.C. Conceptual and methodological issues relating to pain assessment in mammals: The development and utilisation of pain facial expression scales. Appl. Anim. Behav. Sci. 2019, 217, 1–15. [Google Scholar] [CrossRef]
- Homberg, J.R.; Wöhr, M.; Alenina, N. Comeback of the rat in biomedical research. ACS Chem. Neurosci. 2017, 8, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, H.L.; Levac, D.; O’Brien, K.K.; Straus, S.; Tricco, A.C.; Perrier, L.; Kastner, M.; Moher, D. Scoping reviews: Time for clarity in definition, methods, and reporting. J. Clin. Epidemiol. 2014, 67, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. JBI Evid. Implement. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Chartier, L.C.; Hebart, M.L.; Howarth, G.S.; Whittaker, A.L.; Mashtoub, S. Affective state determination in a mouse model of colitis-associated colorectal cancer. PLoS ONE 2020, 15, e0228413. [Google Scholar] [CrossRef]
- Dwivedi, D.J.; Grin, P.M.; Khan, M.; Prat, A.; Zhou, J.; Fox-Robichaud, A.E.; Seidah, N.G.; Liaw, P.C. Differential expression of pcsk9 modulates infection, inflammation, and coagulation in a murine model of sepsis. Shock 2016, 46, 672–680. [Google Scholar] [CrossRef]
- Hassan, A.M.; Jain, P.; Mayerhofer, R.; Fröhlich, E.E.; Farzi, A.; Reichmann, F.; Herzog, H.; Holzer, P. Visceral hyperalgesia caused by peptide yy deletion and y2 receptor antagonism. Sci. Rep. 2017, 7, 40968. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Akintola, T.; Raver, C.; Studlack, P.; Uddin, O.; Masri, R.; Keller, A. The grimace scale reliably assesses chronic pain in a rodent model of trigeminal neuropathic pain. Neurobiol. Pain 2017, 2, 13–17. [Google Scholar] [CrossRef]
- Bu, X.; Liu, Y.; Lu, Q.; Jin, Z. Effects of “danzhi decoction” on chronic pelvic pain, hemodynamics, and proinflammatory factors in the murine model of sequelae of pelvic inflammatory disease. Evid. Based Complement. Alternat. Med. 2015, 2015, 547251. [Google Scholar] [CrossRef]
- Burgos-Vega, C.C.; Quigley, L.D.; Trevisan Dos Santos, G.; Yan, F.; Asiedu, M.; Jacobs, B.; Motina, M.; Safdar, N.; Yousuf, H.; Avona, A.; et al. Non-invasive dural stimulation in mice: A novel preclinical model of migraine. Cephalalgia 2019, 39, 123–134. [Google Scholar] [CrossRef]
- Cho, C.; Michailidis, V.; Lecker, I.; Collymore, C.; Hanwell, D.; Loka, M.; Danesh, M.; Pham, C.; Urban, P.; Bonin, R.P.; et al. Evaluating analgesic efficacy and administration route following craniotomy in mice using the grimace scale. Sci. Rep. 2019, 9, 359. [Google Scholar] [CrossRef]
- De Almeida, A.S.; Rigo, F.K.; De Prá, S.D.; Milioli, A.M.; Dalenogare, D.P.; Pereira, G.C.; Ritter, C.D.S.; Peres, D.S.; Antoniazzi, C.T.D.; Stein, C.; et al. Characterization of cancer-induced nociception in a murine model of breast carcinoma. Cell. Mol. Neurobiol. 2019, 39, 605–617. [Google Scholar] [CrossRef]
- De Almeida, A.S.; Rigo, F.K.; De Prá, S.D.; Milioli, A.M.; Pereira, G.C.; Lückemeyer, D.D.; Antoniazzi, C.T.; Kudsi, S.Q.; Araújo, D.; Oliveira, S.M.; et al. Role of transient receptor potential ankyrin 1 (trpa1) on nociception caused by a murine model of breast carcinoma. Pharmacol. Res. 2020, 152, 104576. [Google Scholar] [CrossRef]
- Duffy, S.S.; Perera, C.J.; Makker, P.G.; Lees, J.G.; Carrive, P.; Moalem-Taylor, G. Peripheral and central neuroinflammatory changes and pain behaviors in an animal model of multiple sclerosis. Front. Immunol. 2016, 7, 369. [Google Scholar] [CrossRef] [PubMed]
- Faller, K.M.; McAndrew, D.J.; Schneider, J.E.; Lygate, C.A. Refinement of analgesia following thoracotomy and experimental myocardial infarction using the mouse grimace scale. Exp. Physiol. 2015, 100, 164–172. [Google Scholar] [CrossRef]
- Gallo, M.S.; Karas, A.Z.; Pritchett-Corning, K.; Garner Guy Mulder, J.P.; Gaskill, B.N. Tell-tale tint: Does the time to incorporate into nest test evaluate postsurgical pain or welfare in mice? J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zou, S.; Mohammad, Z.; Wang, S.; Yang, J.; Li, H.; Dubner, R.; Wei, F.; Chung, M.K.; Ro, J.Y.; et al. Voluntary biting behavior as a functional measure of orofacial pain in mice. Physiol. Behav. 2019, 204, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Hassler, S.N.; Ahmad, F.B.; Burgos-Vega, C.C.; Boitano, S.; Vagner, J.; Price, T.J.; Dussor, G. Protease activated receptor 2 (par2) activation causes migraine-like pain behaviors in mice. Cephalalgia 2019, 39, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Bolton, F.; Arias, A.S.; Harrison, R.A.; Gutiérrez, J.M. Analgesic effect of morphine and tramadol in standard toxicity assays in mice injected with venom of the snake bothrops asper. Toxicon 2018, 154, 35–41. [Google Scholar] [CrossRef]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thöne-Reineke, C. Severity classification of repeated isoflurane anesthesia in c57bl/6jrj mice-assessing the degree of distress. PLoS ONE 2017, 12, e0179588. [Google Scholar] [CrossRef]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thöne-Reineke, C. Impact of repeated anesthesia with ketamine and xylazine on the well-being of c57bl/6jrj mice. PLoS ONE 2018, 13, e0203559. [Google Scholar] [CrossRef] [PubMed]
- Hohlbaum, K.; Corte, G.M.; Humpenöder, M.; Merle, R.; Thöne-Reineke, C. Reliability of the mouse grimace scale in c57bl/6jrj mice. Animals 2020, 10, 1648. [Google Scholar] [CrossRef]
- Hsi, Z.Y.; Stewart, L.A.; Lloyd, K.C.K.; Grimsrud, K.N. Hypoglycemia after bariatric surgery in mice and optimal dosage and efficacy of glucose supplementation. Comp. Med. 2020, 70, 111–118. [Google Scholar] [CrossRef]
- Jirkof, P.; Abdelrahman, A.; Bleich, A.; Durst, M.; Keubler, L.; Potschka, H.; Struve, B.; Talbot, S.R.; Vollmar, B.; Zechner, D.; et al. A safe bet? Inter-laboratory variability in behaviour-based severity assessment. Lab. Anim. 2020, 54, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Jurik, A.; Ressle, A.; Schmid, R.M.; Wotjak, C.T.; Thoeringer, C.K. Supraspinal trpv1 modulates the emotional expression of abdominal pain. Pain 2014, 155, 2153–2160. [Google Scholar] [CrossRef]
- Kim, J.Y.; Tillu, D.V.; Quinn, T.L.; Mejia, G.L.; Shy, A.; Asiedu, M.N.; Murad, E.; Schumann, A.P.; Totsch, S.K.; Sorge, R.E.; et al. Spinal dopaminergic projections control the transition to pathological pain plasticity via a d1/d5-mediated mechanism. J. Neurosci. 2015, 35, 6307–6317. [Google Scholar] [CrossRef]
- Leach, M.C.; Klaus, K.; Miller, A.L.; Scotto di Perrotolo, M.; Sotocinal, S.G.; Flecknell, P.A. The assessment of post-vasectomy pain in mice using behaviour and the mouse grimace scale. PLoS ONE 2012, 7, e35656. [Google Scholar] [CrossRef]
- Mai, S.H.C.; Sharma, N.; Kwong, A.C.; Dwivedi, D.J.; Khan, M.; Grin, P.M.; Fox-Robichaud, A.E.; Liaw, P.C. Body temperature and mouse scoring systems as surrogate markers of death in cecal ligation and puncture sepsis. Intensive Care Med. Exp. 2018, 6, 20. [Google Scholar] [CrossRef]
- Matsumiya, L.C.; Sorge, R.E.; Sotocinal, S.G.; Tabaka, J.M.; Wieskopf, J.S.; Zaloum, A.; King, O.D.; Mogil, J.S. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 42–49. [Google Scholar] [PubMed]
- Meyer, N.; Kröger, M.; Thümmler, J.; Tietze, L.; Palme, R.; Touma, C. Impact of three commonly used blood sampling techniques on the welfare of laboratory mice: Taking the animal’s perspective. PLoS ONE 2020, 15, e0238895. [Google Scholar] [CrossRef]
- Miller, A.; Kitson, G.; Skalkoyannis, B.; Leach, M. The effect of isoflurane anaesthesia and buprenorphine on the mouse grimace scale and behaviour in cba and dba/2 mice. Appl. Anim. Behav. Sci. 2015, 172, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Leach, M.C. The mouse grimace scale: A clinically useful tool? PLoS ONE 2015, 10, e0136000. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Leach, M.C. Using the mouse grimace scale to assess pain associated with routine ear notching and the effect of analgesia in laboratory mice. Lab. Anim. 2015, 49, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Kitson, G.L.; Skalkoyannis, B.; Flecknell, P.A.; Leach, M.C. Using the mouse grimace scale and behaviour to assess pain in cba mice following vasectomy. Appl. Anim. Behav. Sci. 2016, 181, 160–165. [Google Scholar] [CrossRef]
- Miller, A.L.; Leach, M.C. The effect of handling method on the mouse grimace scale in two strains of laboratory mice. Lab. Anim. 2016, 50, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Howarth, G.S.; Chartier, L.C.; Trinder, D.; Lawrance, I.C.; Huang, L.S.; Mashtoub, S. Orally administered emu oil attenuates disease in a mouse model of crohn’s-like colitis. Exp. Biol. Med. 2020, 245, 1697–1707. [Google Scholar] [CrossRef]
- Mittal, A.; Gupta, M.; Lamarre, Y.; Jahagirdar, B.; Gupta, K. Quantification of pain in sickle mice using facial expressions and body measurements. Blood Cells Mol. Dis. 2016, 57, 58–66. [Google Scholar] [CrossRef]
- Rea, B.J.; Wattiez, A.S.; Waite, J.S.; Castonguay, W.C.; Schmidt, C.M.; Fairbanks, A.M.; Robertson, B.R.; Brown, C.J.; Mason, B.N.; Moldovan-Loomis, M.C.; et al. Peripherally administered calcitonin gene-related peptide induces spontaneous pain in mice: Implications for migraine. Pain 2018, 159, 2306–2317. [Google Scholar] [CrossRef]
- Rosen, S.F.; Ham, B.; Drouin, S.; Boachie, N.; Chabot-Dore, A.-J.; Austin, J.-S.; Diatchenko, L.; Mogil, J.S. T-cell mediation of pregnancy analgesia affecting chronic pain in mice. J. Neurosci. 2017, 37, 9819–9827. [Google Scholar] [CrossRef]
- Rossi, H.L.; See, L.P.; Foster, W.; Pitake, S.; Gibbs, J.; Schmidt, B.; Mitchell, C.H.; Abdus-Saboor, I. Evoked and spontaneous pain assessment during tooth pulp injury. Sci. Rep. 2020, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Roughan, J.V.; Bertrand, H.G.; Isles, H.M. Meloxicam prevents cox-2-mediated post-surgical inflammation but not pain following laparotomy in mice. Eur. J. Pain 2016, 20, 231–240. [Google Scholar] [CrossRef]
- Roughan, J.V.; Sevenoaks, T. Welfare and scientific considerations of tattooing and ear tagging for mouse identification. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 142–153. [Google Scholar] [CrossRef]
- Sorge, R.E.; Martin, L.J.; Isbester, K.A.; Sotocinal, S.G.; Rosen, S.; Tuttle, A.H.; Wieskopf, J.S.; Acland, E.L.; Dokova, A.; Kadoura, B.; et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Meth. 2014, 11, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Tillu, D.V.; Hassler, S.N.; Burgos-Vega, C.C.; Quinn, T.L.; Sorge, R.E.; Dussor, G.; Boitano, S.; Vagner, J.; Price, T.J. Protease-activated receptor 2 activation is sufficient to induce the transition to a chronic pain state. Pain 2015, 156, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, A.H.; Molinaro, M.J.; Jethwa, J.F.; Sotocinal, S.G.; Prieto, J.C.; Styner, M.A.; Mogil, J.S.; Zylka, M.J. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol. Pain 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lim, J.; Joseph, J.; Wang, S.; Wei, F.; Ro, J.Y.; Chung, M.K. Spontaneous and bite-evoked muscle pain are mediated by a common nociceptive pathway with differential contribution by trpv1. J. Pain 2017, 18, 1333–1345. [Google Scholar] [CrossRef]
- Wang, S.; Brigoli, B.; Lim, J.; Karley, A.; Chung, M.K. Roles of trpv1 and trpa1 in spontaneous pain from inflamed masseter muscle. Neuroscience 2018, 384, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kim, M.; Ali, Z.; Ong, K.; Pae, E.K.; Chung, M.K. Trpv1 and trpv1-expressing nociceptors mediate orofacial pain behaviors in a mouse model of orthodontic tooth movement. Front. Physiol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Zhu, X.; Renn, C.L.; Dorsey, S.G.; Faden, A.I. Cell cycle inhibition limits development and maintenance of neuropathic pain following spinal cord injury. Pain 2016, 157, 488–503. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Long, H.; Zhu, J.; Jian, F.; Ye, N.; Lai, W. Effect of static magnetic field on pain level and expression of p2x3 receptors in the trigeminal ganglion in mice following experimental tooth movement. Bioelectromagnetics 2017, 38, 22–30. [Google Scholar] [CrossRef]
- Melnikova, I. Pain market. Nat. Rev. Drug Dis. 2010, 9, 589–590. [Google Scholar] [CrossRef]
- Hau, J.; Schapiro, S. Handbook of Laboratory Animal Science; CRC Press: Boca Raton, FL, USA, 2010; Volume 1. [Google Scholar]
- Leung, V.; Zhang, E.; Pang, D.S. Real-time application of the rat grimace scale as a welfare refinement in laboratory rats. Sci. Rep. 2016, 6, 31667. [Google Scholar] [CrossRef]
- Leung, V.S.Y.; Benoit-Biancamano, M.-O.; Pang, D.S.J. Performance of behavioral assays: The rat grimace scale, burrowing activity and a composite behavior score to identify visceral pain in an acute and chronic colitis model. Pain Rep. 2019, 4, e718. [Google Scholar] [CrossRef] [PubMed]
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Meth. 2014, 234, 139–146. [Google Scholar] [CrossRef]
- Jirkof, P.; Leucht, K.; Cesarovic, N.; Caj, M.; Nicholls, F.; Rogler, G.; Arras, M.; Hausmann, M. Burrowing is a sensitive behavioural assay for monitoring general wellbeing during dextran sulfate sodium colitis in laboratory mice. Lab. Anim. 2013, 47, 274–283. [Google Scholar] [CrossRef]
- Cunningham, C.; Campion, S.; Teeling, J.; Felton, L.; Perry, V.H. The sickness behaviour and cns inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded rna (poly i:C). Brain Behav. Immun. 2007, 21, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Gaskill, B.N.; Pritchett-Corning, K.R. Nest building as an indicator of illness in laboratory mice. Appl. Anim. Behav. Sci. 2016, 180, 140–146. [Google Scholar] [CrossRef]
- Whittaker, A.L.; Lymn, K.A.; Nicholson, A.; Howarth, G.S. The assessment of general well-being using spontaneous burrowing behaviour in a short-term model of chemotherapy-induced mucositis in the rat. Lab. Anim. 2015, 49, 30–39. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Barker, T.H. The impact of common recovery blood sampling methods, in mice (mus musculus), on well-being and sample quality: A systematic review. Animals 2020, 10, 989. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.L.; West, R.S. Taming anxiety in laboratory mice. Nat. Meth. 2010, 7, 825–826. [Google Scholar] [CrossRef]
- Good, M.; Stiller, C.; Zauszniewski, J.A.; Anderson, G.C.; Stanton-Hicks, M.; Grass, J.A. Sensation and distress of pain scales: Reliability, validity, and sensitivity. J. Nurs. Meas. 2001, 9, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.W.; Adams, M.C.B. Sex, gender, and pain: An overview of a complex field. Anesth. Analg. 2008, 107, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, W.F.; Smith, L.; Scorr, L. Nociception and antinociception during the first week of life in mice: Sex differences and test dependence. J. Pain 2004, 5, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Gaumond, I.; Arsenault, P.; Marchand, S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002, 958, 139–145. [Google Scholar] [CrossRef]
- Dina, O.A.; Aley, K.; Isenberg, W.; Messing, R.O.; Levine, J.D. Sex hormones regulate the contribution of pkcε and pka signalling in inflammatory pain in the rat. Eur. J. Neurosci. 2001, 13, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.C.; Smith, E.S.; Picker, M.J. Sex-related differences in mechanical nociception and antinociception produced by μ-and κ-opioid receptor agonists in rats. Eur. J. Pharmacol. 2002, 452, 163–173. [Google Scholar] [CrossRef]
- Mogil, J.S.; Chesler, E.; Wilson, S.; Juraska, J.M.; Sternberg, W. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci. Biobehav. Rev. 2000, 24, 375–389. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. Policy: Nih to balance sex in cell and animal studies. Nat. News 2014, 509, 282. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Hickman, D.L. The impact of social and behavioral factors on reproducibility in terrestrial vertebrate models. ILAR J. 2020, 60, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Konecka, A.M.; Sroczynska, I. Circadian rhythm of pain in male mice. Gen. Pharmacol. 1998, 31, 809–810. [Google Scholar] [CrossRef]
- Ripperger, J.A.; Jud, C.; Albrecht, U. The daily rhythm of mice. FEBS Lett. 2011, 585, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, R.C.; Burgis, V.; Edwards, J.D. Hyperalgesia induced by naloxone follows diurnal rhythm in responsivity to painful stimuli. Science 1977, 198, 756–758. [Google Scholar] [CrossRef]
- Hamra, J.G.; Kamerling, S.; Wolfsheimer, K.; Bagwell, C. Diurnal variation in plasma ir-beta-endorphin levels and experimental pain thresholds in the horse. Life Sci. 1993, 53, 121–129. [Google Scholar] [CrossRef]
- Oliverio, A.; Castellano, C.; Puglisi-Allegra, S. Opiate analgesia: Evidence for circadian rhythms in mice. Brain Res. 1982, 249, 265–270. [Google Scholar] [CrossRef]
- Naber, D.; Cohen, R.M.; Pickar, D.; Kalin, N.H.; Davis, G.; Pert, C.B.; Bunney, W.E., Jr. Episodic secretion of opioid activity in human plasma and monkey csf: Evidence for a diurnal rhythm. Life Sci. 1981, 28, 931–935. [Google Scholar] [CrossRef]
- Yang, M.; Weber, M.; Crawley, J. Light phase testing of social behaviors: Not a problem. Front. Neurosci. 2008, 2, 29. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Martin, P.R.; Bateson, P.P.G. Measuring Behaviour: An Introductory Guide; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Langford, D.J.; Crager, S.E.; Shehzad, Z.; Smith, S.B.; Sotocinal, S.G.; Levenstadt, J.S.; Chanda, M.L.; Levitin, D.J.; Mogil, J.S. Social modulation of pain as evidence for empathy in mice. Science 2006, 312, 1967–1970. [Google Scholar] [CrossRef]





| Reference | Study Design § | Strain/Stock | Age/Weight | Sex | Type of Intervention | Intervention (Model Created or Procedure Investigated) | Pain Classification Assigned | Intervention Effect on MGS Score * |
|---|---|---|---|---|---|---|---|---|
| Akintola et al., 2017 [30] | RCT | C57BL/6 | 10–12 weeks | M | Animal Model | Chronic constriction injury model for pain | Neuropathic | ↑ |
| Bu et al., 2015 [31] | RCT | BALB/c | 6–8 weeks | F | Animal Model | Chronic pelvic pain | Visceral | ↑ |
| Burgos-Vega et al., 2019 [32] | RCT | ICR | 6–8 weeks | M,F | Animal Model | Migraine | Neuropathic | ↑ |
| Chartier et al., 2020 [26] | RCT | C57BL/ 6JArc | 8 weeks | F | Animal Model | Colitis-associated colorectal cancer | Visceral | Nil |
| Cho et al., 2019 [33] | RCT | CD-1, C57BL/6N | 7–9 weeks | M,F | Animal Model | Craniotomy with different analgesics | Neuropathic | ↑ (reduced by analgesics) |
| de Almeida et al., 2019 [34] | Pre-test, post-test | BALB/c | 20–30 g | F | Animal Model | Cancer-induced nociception | Mixed | ↑ |
| de Almeida et al., 2020 [35] | RCT | BALB/c | F | Animal Model | Cancer-induced nociception | Mixed | ↑ | |
| Duffy et al., 2016 [36] | RCT | C57BL/6J | 10–12 weeks | F | Animal Model | Experimental autoimmune encephalomyelitis (EAE) | Neuropathic | ↑ |
| Dwivedi et al., 2016 [27] | RCT | Transgenic (BL/6 background) PCSK9 KO mice and PCSK9 overexpression | 10–12 weeks | M,F | Animal Model | Caecal Ligation and Puncture model of sepsis | Visceral | ↑ |
| Faller et al., 2015 [37] | RCT | C57BL/6J, transgenic overexpressing creatine transporter in the heart (BL/6 background) | 12–16 weeks | F | Animal Model | Myocardial infarction created through thoracotomy | Visceral | ↑ (reduced by analgesics) |
| Gallo et al., 2020 [38] | RCT (factorial) | Crl:CD1(ICR) | 8–9 weeks | M | Husbandry/Procedural | Carotid artery catheterisation | Acute | ↑ |
| Guo et al., 2019 [39] | RCT | C57BL/6 | 9 weeks | M,F | Animal Model | Orofacial pain | Acute | |
| Hassan et al., 2017 [28] | RCT | C57BL/6N, PYY knockout | 10 weeks | M | Animal Model | Colonic nociception | Visceral | ↑ |
| Hassler et al., 2019 [40] | RCT | ICR, C57BL/6J, and PAR2 (BL/6 background) | 20–30 g | M | Animal Model | Migraine | Neuropathic | ↑ |
| Herrera et al., 2018 [41] | RCT | CD-1 | 18–20 g | nr | Animal Model | Bothops Asper venom | Visceral | ↑ |
| Hohlbaum et al., 2017 [42] | RCT | C57BL/6JRj | 11–13 weeks | M, F | Husbandry/Procedural | Isoflurane anaesthesia | None/momentary | ↑ (female mice only) |
| Hohlbaum et al., 2018 [43] | RCT | C57BL/6JRj | 11–13 weeks | M, F | Husbandry/Procedural | Ketamine/xylazine anaesthesia | None/momentary | ↑ |
| Hohlbaum et al., 2020 [44] ⁋ | Quasi-experimental | C57BL/6JRj | 11–13 weeks | M, F | Biological | Ketamine /xylazine anaesthesia | N/A | Study investigated inter-observer reliability in scoring |
| Hsi et al., 2020 [45] | RCT | C57BL/6N | 7–9 weeks | F | Animal Model | Animals with hypoglycaemia following Roux-en-Y Gastric Bypass surgery | None/momentary (outcome of interest is the hypoglycaemia) | Nil ǂ |
| Jirkof et al., 2020 [46] | RCT | C57BL/6J | F | Husbandry/Procedural | Tramadol treatment effect on MGS between laboratories | None/momentary | Nil | |
| Jurik et al., 2014 [47] | RCT | TRPV1 knock-out (BL/6 background) | 8–16 weeks | M | Animal Model | Abdominal constriction test and acute pancreatitis as models of pain. Effects of knockout versus wildtype genotype | Visceral | No effect of genotype on MGS scores |
| Kim et al., 2015 [48] | RCT | ICR | M,F | Animal Model | Hyperalgesic priming via IL-6 and Carrageenan injection | Acute | ↑ | |
| Langford et al., 2010 [20] | RCT | CD-1 (ICR:Crl) | 6–18 weeks | M,F | Animal Model | 14 models of pain | Acute | ↑ |
| Leach et al., 2012 [49] | RCT | CD-1 | M | Husbandry/Procedural | Vasectomy surgery | Acute | ↑ (reduced by analgesics) | |
| Mai et al., 2018 [50] | RCT | C57BL/6J | 8–12 weeks | M | Animal Model | Caecal ligation and puncture model of sepsis | Visceral | ↑ |
| Matsumiya et al., 2012 [51] | RCT | CD1 (ICR:Crl) | 6–8 weeks | M,F | Husbandry/Procedural | Ventral ovariectomy and response to analgesics | Acute | ↑(reduced by analgesics) |
| Meyer et al., 2020 [52] | RCT | C57BL/6J | 10–12 weeks | M | Husbandry/Procedural | Common recovery blood sampling routes (facial vein, retrobulbar, tail vein with anaesthetic and handling control) | None/momentary | ↑—anaesthetic, facial vein bleeding, or retrobulbar compared to handling |
| Miller et al., 2015 [53] | Pre-test, post-test | CBA, DBA/2 | M | Husbandry/Procedural | Isoflurane anaesthesia and buprenorphine analgesic | None/momentary | Nil (↑ by isoflurane in DBA/2 strain) | |
| Miller and Leach, 2015a [54] | RCT | C57BL/6, C3H/He, CD-1 BALB/c | 8 weeks | M,F | Biological | Impact of sex, strain, time of day or habituation | None/momentary | Nil-order ↑- males compared to females (but strain dependant and not always consistent) Strain effects present Time of day effects with sex and strain differences |
| Miller and Leach, 2015b [55] | RCT | C57BL/6 | 8 weeks | M | Husbandry/Procedural | Ear notching and analgesic effects | None/momentary | Nil |
| Miller et al., 2016 [56] | Pre-test, post-test | CBA | 25.6–28.7 g | M | Husbandry/Procedural | Vasectomy surgery | Acute | ↑ |
| Miller and Leach, 2016 [57] | RCT | CBA, DBA/2 | M | Husbandry/Procedural | Handling method: tail versus tube | None/momentary | Nil | |
| Mitchell et al., 2020 [58] | RCT | ArcCrl:CD | 12 weeks | F | Animal Model | TNBS-induced Crohn’s-like colitis | Visceral | ↑ |
| Mittal et al., 2016 [59] | RCT | Transgenic HbSS-BERK (with relevant controls) | M,F | Animal Model | Sickle cell disease and effects of cold | Acute | ↑—in females, cold also had impact | |
| Rea et al., 2018 [60] | RCT | C57BL/6J, CD1 | 10–14 weeks | M,F | Animal Model | Pain as result of migraine | Neuropathic | ↑ |
| Rosen et al., 2017 [61] | RCT | CD-1 (Crl:ICR), Nude (Crl:CD1- Foxn1nu), C57BL/6J, C57BL/6-Rag1 tm1Mom/J, mutant mice lacking expression of the Oprd1 (-opioid receptor) gene | 7–12 weeks | M,F | Animal Model | Pregnancy analgesia after inflammatory insult induced by administration of complete Freund’s adjuvant (CFA) | Visceral (pregnancy state). | ↑—in late-pregnant mice compared to nulliparous females |
| Rossi et al., 2020 [62] | RCT | Mixed CD-1 and C57BL/6J background | 17–21 weeks | M,F | Animal Model | Tooth pulp injury | Acute | ↑ |
| Roughan et al., 2016 [63] | RCT | BALB/c | 25–30 g | M | Husbandry/Procedural | Handling method: tail versus cupping at time of surgery | None/momentary | Nil (although surgery itself increased MGS) |
| Roughan and Sevenoaks, 2019 [64] | RCT | BALB/cAnNCrl | 10–13 weeks | M,F | Husbandry/Procedural | Ear tattooing and tagging, with tail handling method or tunnel | None/momentary | ↑ tail versus tunnel ↑ males versus females ↑ ear tagging versus tattoo |
| Sorge et al., 2014 [65] | RCT | CD-1 (ICR:Crl), C57BL/6J | 6–12 weeks | M,F | Husbandry/Procedural | Effect of gender/gender-specific and other animal pheromones on response to nociceptive assays | None/momentary (intervention of interest is the pheromones) | ↓ with male observer or male’s T-shirt compared to no observer |
| Serizawa et al., 2019 [18] | RCT | C57BL/6J | 7 weeks | F | Animal Model | Experimental autoimmune encephalomyelitis (EAE) | Neuropathic | ↑ |
| Tillu et al., 2015 [66] | RCT | ICR, C57BL/6 | 20–25 g | M | Animal Model | Hyperalgesic priming | Acute | ↑ |
| Tuttle et al., 2018 [67] | RCT | CD-1 (ICR:Crl) | 6–12 weeks | M,F | Husbandry/Procedural | Ventral ovariectomy and response to analgesics, xymogen assay (validation of automated scoring) | Acute | ↑ (reduced by analgesic) |
| Wang et al., 2017 [68] | RCT | C57BL/6, TRPV1 KO | 8–12 weeks | M | Animal Model | Masseter inflammation | Acute | ↑ |
| Wang et al., 2018 [69] | RCT | C57BL/6, TRPV1 KO (C57BL/6 background), TRPA1 KO (mixed B6; 129 background) | 8–12 weeks | M | Animal Model | Masseter inflammation | Acute | ↑ |
| Wang et al., 2019 [70] | RCT | C57BL/6 | 12 weeks | M | Animal Model | Orthodontic tooth movement | Acute | ↑ |
| Wu et al., 2016 [71] | RCT | C57BL/6 | 8–19 weeks | M | Animal Model | Spinal cord injury | Neuropathic | ↑ |
| Zhu et al., 2017 [72] | RCT | BALB/c | 25–30 g | M | Animal Model | Orthodontic tooth movement | Acute | ↑ |
| Reference | Intervention | Strain | Direction of Effect (F vs. M) |
|---|---|---|---|
| [32] | Migraine | ICR | Not directly compared |
| [33] | Craniotomy with different analgesics | CD-1 C57BL/6N | = = |
| [27] | Caecal Ligation and Puncture model of sepsis | Transgenic (BL/6 background) PCSK9 KO mice and PCSK9 overexpression | Not reported |
| [39] | Orofacial pain | C57BL/6 | Not reported |
| [42] | Isoflurane anaesthesia | C57BL/6JRj | = |
| [43] | Repeated ketamine anaesthesia | C57BL/6JRj | = |
| [48] | Hyperalgesic priming via IL-6 and Carrageenan injection | ICR | Not reported |
| [20] | 14 models of pain | CD-1 (ICR:Crl) | = |
| [51] | Ventral ovariectomy and response to analgesics | CD1 (ICR:Crl) | = |
| [54] | Biological | Live Scoring | |
| C57BL/6 | = | ||
| C3H/He | F < M | ||
| CD-1 | F < M (at 1 time point) | ||
| Retrospective Scoring | |||
| C57BL/6 | F > M | ||
| C3H/He | = | ||
| CD-1 | = | ||
| [59] | Sickle cell disease and effects of cold | Transgenic HbSS-BERK (with relevant controls) | F > M |
| [60] | CGRP- induced migraine | C57BL/6J CD1 | = |
| [61] | CD-1 (Crl:ICR), Nude (Crl:CD1- Foxn1 nu), C57BL/6J, C57BL/6-Rag1 tm1Mom, mutant mice lacking expression of the Oprd1 (-opioid receptor) gene | Pregnancy analgesia | Not directly compared for grimace outcome |
| [62] | Tooth pulp injury | Mixed CD1 and C57BL6/J background | = |
| [64] | Ear tattooing and tagging, with tail handling method or tunnel | BALB/cAnNCrl | F < M |
| [65] | Effect of gender/gender-specific and other animal pheromones on response to nociceptive assays | CD-1 (ICR:Crl) C57BL/6J | F > M (baseline values) Females displayed greater ‘male observer’ effect, e.g., increased reduction in grimace scores. |
| [67] | Ventral ovariectomy and response to analgesics, xymogen assay (validation of automated scoring) | CD-1 (ICR:Crl) | = |
| Reference. | Reporting Detail | Intervention | Compared with Measures in Light (Y/N) | Direction of Effect for Comparison between Light and Dark |
|---|---|---|---|---|
| [51] | Conducted circadian study comparing light and dark recordings | Ventral ovariectomy and response to analgesics | Y | Compared mice which had surgery in the morning versus the evening with measurement timepoints of baseline, and every 6 h past surgery for 48 h There was no circadian effect on baseline MGS scores. However, mice operated on in the morning displayed larger MGS increases 12 h after surgery compared to 24 h, whilst mice operated on in the evening showed smaller increases at these time points. This suggests that mice experience higher levels of postoperative pain at night (dark phase) |
| [54] | No dark cycle recording but did compare MGS across the light phase | Biological | N | Live scoring There was no difference in MGS score between three time points (9 am, 12.30 pm, 4 pm) for C57BL/6, CD-1 or C3H/He mice. BALB/c mice showed a greater score at Noon compared to AM. Retrospective Scoring There was no significant difference in MGS scores between the three time points for CD-1, C3H/He or BALB/c mice. C57BL/6 mice showed a greater MGS scores at both Noon and PM time points compared to the AM time point |
| [60] | Performed a restrained grimace technique in dark (manipulated dark condition- not part of cycle) | CGRP-induced migraine | Y (compared bright light) | Grimace scores were higher in the dark than in bright light for the CD1 mice. Light transition led to decreased orbital tightening and nose bulge. C57BL/6J mice showed no significant difference between the CGRP-induced grimace in light and dark. Responses to CGRP were generally similar in direction as those recorded in the light. |
| Reference | Number of Observers | Consistency Metrics |
|---|---|---|
| Inter-Observer Variability | ||
| [37] | 2 | There was an excellent correlation between the two observers for MGS measurement (r = 0.98) assessed using Type II regression analysis. However, Bland–Altman analysis showed that the slope differed from unity with a bias towards higher MGS scores in one observer. |
| [44] | 4 (2 Novice, 2 Expert Scorers) | Good agreement between all observers was observed (ICC = 0.851) when all three time points were examined. However, interrater reliability differed across timepoints. The best agreement was achieved for orbital tightening, and the poorest agreement for nose and cheek bulge, and this depended on the observers’ experience levels. In general, experienced observers produced scores of higher consistency when compared to inexperienced. |
| [20] | 7 | Inter-rater reliability was high as assessed by intra-class correlation coefficient (ICC average = 0.90). When high-definition video cameras were used, over 97% of pain versus no-pain images were categorised correctly. |
| [59] | 6 | ICC and Cronbach’s alpha values were low (ICC average < 0.7, α < 0.8). This resulted from large intra-coder variability for three of the coders. Therefore, only the results of the coders with low variability were used in data presentation (updated metrics not reported). |
| [60] | 2 | Correlation coefficients ranged between 0.89 and 0.92. |
| [63] | 4 | There was high inter-observer consistency, with ICC values ranging from 0.75–0.84. |
| [64] | 6 Novice and 6 Expert Scorers | The α values for experts and novices were high (0.88 to 0.94; 0.78 to 0.87 respectively). Agreement between novices and experts was generally good (ICC ranging from 0.7 to 0.84 across the timepoints). |
| [65] | 2 | Moderate to high inter-rater correlation (r = 0.64, p < 0.001). Group data from one rater compared to the other were almost identical. |
| [67] | 2 | High inter-rater consistency with Cronbach’s alpha of 0.89. |
| Inter-Laboratory Variability | ||
| [46] | 3 | Median MGS scores were significantly different at a number of timepoints between the 3 laboratories. They were however qualitatively similar i.e., direction of effect. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whittaker, A.L.; Liu, Y.; Barker, T.H. Methods Used and Application of the Mouse Grimace Scale in Biomedical Research 10 Years on: A Scoping Review. Animals 2021, 11, 673. https://doi.org/10.3390/ani11030673
Whittaker AL, Liu Y, Barker TH. Methods Used and Application of the Mouse Grimace Scale in Biomedical Research 10 Years on: A Scoping Review. Animals. 2021; 11(3):673. https://doi.org/10.3390/ani11030673
Chicago/Turabian StyleWhittaker, Alexandra L., Yifan Liu, and Timothy H. Barker. 2021. "Methods Used and Application of the Mouse Grimace Scale in Biomedical Research 10 Years on: A Scoping Review" Animals 11, no. 3: 673. https://doi.org/10.3390/ani11030673
APA StyleWhittaker, A. L., Liu, Y., & Barker, T. H. (2021). Methods Used and Application of the Mouse Grimace Scale in Biomedical Research 10 Years on: A Scoping Review. Animals, 11(3), 673. https://doi.org/10.3390/ani11030673







