Sire Effects on Carcass of Beef-Cross-Dairy Cattle: A Case Study in New Zealand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Slaughter
2.3. Measurements
2.3.1. Weights
2.3.2. In-Chiller Assessments for Carcass Traits
2.4. Statistical Analysis
2.4.1. Data Cleaning
2.4.2. Contemporary Groups
2.4.3. Statistical Models
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bown, M.D.; Muir, P.D.; Thomson, B.C. Dairy and beef breed effects on beef yield, beef quality and profitability: A review. N. Z. J. Agric. Res. 2016, 59, 174–184. [Google Scholar] [CrossRef]
- New Zealand Meat Classification Authority. New Zealand Meat: Guide to Beef Carcass Classification; New Zealand Meat Classification Authority: Wellington, New Zealand, 2004. [Google Scholar]
- Morris, S.T. Presidential Address 2012: The New Zealand beef cattle industry. In Proceedings of the New Zealand Society of Animal Production, Hamilton, New Zealand, 2–4 July 2013; pp. 1–4. [Google Scholar]
- Purchas, R. Factors affecting carcass composition and beef quality. In Profitable Beef Production in New Zealand. New Zealand Beef Council Report; Smeaton, D., Ed.; MPI: Wellington, New Zealand, 2003; pp. 124–152. [Google Scholar]
- Warner, R.D.; Greenwood, P.L.; Pethick, D.W.; Ferguson, D.M. Genetic and environmental effects on meat quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef]
- Pethick, D.; Harper, G.; Oddy, V. Growth, development and nutritional manipulation of marbling in cattle: A review. Aust. J. Exp. Agric. 2004, 44, 705–715. [Google Scholar] [CrossRef]
- Warriss, P.D. The handling of cattle pre-slaughter and its effects on carcass and meat quality. Appl. Anim. Behav. Sci. 1990, 28, 171–186. [Google Scholar] [CrossRef]
- Young, O.A.; West, J.; Hart, A.L.; van Otterdijk, F.F.H. A method for early determination of meat ultimate pH. Meat Sci. 2004, 66, 493–498. [Google Scholar] [CrossRef]
- Coleman, L.W.; Hickson, R.E.; Schreurs, N.M.; Martin, N.P.; Kenyon, P.R.; Lopez-Villalobos, N.; Morris, S.T. Carcass characteristics and meat quality of Hereford sired steers born to beef-cross-dairy and Angus breeding cows. Meat Sci. 2016, 121, 403–408. [Google Scholar] [CrossRef]
- Schreurs, N.M.; Hickson, R.E.; Coleman, L.W.; Kenyon, P.R.; Martín, N.P.; Morris, S.T. BRIEF COMMUNICATION: Quality of meat from steers born to beef-cross-dairy cows and sired by Hereford bulls. In Proceedings of the New Zealand Society of Animal Production, Napier, New Zealand, 30 June–1 July 2014; pp. 229–232. [Google Scholar]
- Purchas, R.W.; Morris, S.T. A comparison of carcass characteristics and meat quality for Angus, Hereford x Friesian, and Jersey x Friesian steers. In Proceedings of the New Zealand Society of Animal Production, Wanaka, New Zealand, 20–22 June 2007; pp. 18–22. [Google Scholar]
- Purchas, R.W.; Burnham, D.L.; Morris, S.T. Effects of growth potential and growth path on tenderness of beef longissimus muscle from bulls and steers. J. Anim. Sci. 2002, 80, 3211–3221. [Google Scholar] [CrossRef] [PubMed]
- Purchas, R.W. An assessment of the role of pH differences in determining the relative tenderness of meat from bulls and steers. Meat Sci. 1990, 27, 129–140. [Google Scholar] [CrossRef]
- Purchas, R.W.; Aungsupakorn, R. Further investigations into the relationship between ultimate pH and tenderness for beef samples from bulls and steers. Meat Sci. 1993, 34, 163–178. [Google Scholar] [CrossRef]
- Ertbjerg, P.; Puolanne, E. Muscle structure, sarcomere length and influences on meat quality: A review. Meat Sci. 2017, 132, 139–152. [Google Scholar] [CrossRef]
- Van Selm, B.; de Boer, I.J.M.; Ledgard, S.F.; van Middelaar, C.E. Reducing greenhouse gas emissions of New Zealand beef through better integration of dairy and beef production. Agric. Syst. 2021, 186, 102936. [Google Scholar] [CrossRef]
- Davison, R. Export Cattle Slaughter by Beef and Dairy Origin; Executive Director for Beef+Lamb New Zealand Economic Service & Insights: Wellington, New Zealand, 2020. [Google Scholar]
- Beef+Lamb New Zealand Economic Service. Compendium of New Zealand Farm Facts 2019. In Farm Facts, 43th ed.; Beef+Lamb New Zealand: Wellington, New Zealand, 2019. [Google Scholar]
- Morris, S.T. Living Legend Address 2019: A precis of beef-cattle research over the last 45 years. In Proceedings of the New Zealand Society of Animal Production, Palmerston North, New Zealand, 2–4 July 2019; pp. 188–197. [Google Scholar]
- Geenty, K.; Morris, S. (Eds.) Beef+Lamb New Zealand. Genetic Improvement. In Guide to New Zealand Cattle Farming; Beef+Lamb New Zealand: Wellington, New Zealand, 2017; pp. 62–81. [Google Scholar]
- Cunningham, B.E.; Magee, W.T.; Ritchie, H.D. Effects of using sires selected for yearling weight and crossbreeding with beef and dairy breeds: Birth and weaning traits. J. Anim. Sci. 1987, 64, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.T.; Parker, W.J.; Purchas, R.W.; McCutcheon, S.N. Dairy crossbreeding alternatives to improve New Zealand beef production. In Proceedings of the New Zealand Grassland Association, Gore, New Zealand, 2–5 November 1992; pp. 19–22. [Google Scholar]
- Dal Zotto, R.; Penasa, M.; De Marchi, M.; Cassandro, M.; López-Villalobos, N.; Bittante, G. Use of crossbreeding with beef bulls in dairy herds: Effect on age, body weight, price, and market value of calves sold at livestock auctions. J. Anim. Sci. 2009, 87, 3053–3059. [Google Scholar] [CrossRef]
- Berry, D.P.; Amer, P.R.; Evans, R.D.; Byrne, T.; Cromie, A.R.; Hely, F. A breeding index to rank beef bulls for use on dairy females to maximize profit. J. Dairy Sci. 2019, 102, 10056–10072. [Google Scholar] [CrossRef] [PubMed]
- BREEDPLAN. A Basic Guide to BREEDPLAN EBVs; International Beef Recording Scheme: Armidale, Australia, 2015; p. 26. [Google Scholar]
- Beef+Lamb New Zealand Economic Service. Compendium of New Zealand Farm Facts 2018. In Farm Facts, 41th ed.; Beef+Lamb New Zealand: Wellington, New Zealand, 2018; p. 32. [Google Scholar]
- Agricultural Business Research Institute. BREEDPLAN: International Beef Recording Scheme. Available online: https://breedplan.une.edu.au/ (accessed on 1 April 2020).
- Coleman, L.W. The Use of High Genetic Merit Angus and Hereford Bulls in a New Zealand Dairy Herd. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2020. [Google Scholar]
- Martín, N.; Schreurs, N.; Morris, S.; López-Villalobos, N.; McDade, J.; Hickson, R. Sire effects on post-weaning growth of beef-cross-dairy cattle: A case study in New Zealand. Animals 2020, 10, 2313. [Google Scholar] [CrossRef]
- Animal and Animal Products Directorate, R.A.B. Ministry for Primary Industries. Operational Code: Slaughter and Dressing; Ministry for Primary Industries: Wellington, New Zealand, 2017. [Google Scholar]
- Clark, G. Cattle Visual Assessment Prior to Slaughter; Livestock Buyer for Greenlea Premier Meats Ltd.: Hamilton, New Zealand, 2018. [Google Scholar]
- AUS-MEAT Limited. Handbook of Australian Beef Processing; AUS-MEAT Limited: Queensland, Australia, 2018. [Google Scholar]
- Ministry for Primary Industries. Livestock Slaughter Statistics. Available online: http://www.mpi.govt.nz/news-and-resources/open-data-and-forecasting/agriculture (accessed on 5 December 2019).
- Geenty, K.; Morris, S. (Eds.) Beef+Lamb New Zealand. Post weaning systems. In Guide to New Zealand Cattle Farming; Beef+Lamb New Zealand: Wellington, New Zealand, 2017; pp. 23–36. [Google Scholar]
- Thonney, M.L. Genetics of Growth and Body Composition. In The Genetics of Cattle, 2nd ed.; Garrick, D.J., Ruvinsky, A., Eds.; CAB International: Wallingford, UK, 2015; pp. 523–543. [Google Scholar] [CrossRef]
- Nour, A.Y.M.; Thonney, M.L.; Stouffer, J.R.; White, W.R.C., Jr. Changes in carcass weight and characteristics with increasing weight of large and small cattle. J. Anim. Sci. 1983, 57, 1154–1165. [Google Scholar] [CrossRef]
- Chambaz, A.; Morel, I.; Scheeder, M.R.L.; Kreuzer, M.; Dufey, P.A. Characteristics of steers of six beef breeds fattened from eight months of age and slaughtered at a target level of intramuscular fat: I. Growth performance and carcass quality. Arch. Anim. Breed. 2001, 44, 395–412. [Google Scholar] [CrossRef]
- Beef+Lamb New Zealand Genetics. Sire Report: Cohort 2; Beef+Lamb New Zealand Genetics: Dunedin, New Zealand, 2019; p. 16. [Google Scholar]
- McPhee, M.; Walmsley, B.; Oddy, H.; Andrews, T.; Gudex, B. Enhancing BeefSpecs Systems for Improving Market Compliance of Pasturefed Beef in Southern Australia; B.SBP.0111; Meat and Livestock Australia: Sydney, Australia, 2016; p. 109. [Google Scholar]
- Angus New Zealand Inc. Indexes. Available online: http://angusnz.com/ (accessed on 1 September 2020).
- New Zealand AngusPure. AngusPure Source & Trace. Available online: https://www.anguspure.co.nz/anguspure-source-trace-0 (accessed on 14 April 2020).
- Reverter, A.; Johnston, D.J.; Ferguson, D.M.; Perry, D.; Goddard, M.E.; Burrow, H.M.; Oddy, V.H.; Thompson, J.M.; Bindon, B.M. Genetic and phenotypic characterisation of animal, carcass, and meat quality traits from temperate and tropically adapted beef breeds. 4. Correlations among animal, carcass, and meat quality traits. Aust. J. Agric. Res. 2003, 54, 149–158. [Google Scholar] [CrossRef]
- Realini, C.; E Williams, R.; Pringle, D.; Bertrand, J. Gluteus medius and rump fat depth as additional live animal ultrasound measurements for predicting retail product and trimmable fat in beef carcasses. J. Anim. Sci. 2001, 79, 1378–1385. [Google Scholar] [CrossRef]
- Boles, J.A.; Kohlbeck, K.S.; Meyers, M.C.; Perz, K.A.; Davis, K.C.; Thomson, J.M. The use of blood lactate concentration as an indicator of temperament and its impact on growth rate and tenderness of steaks from Simmental x Angus steers. Meat Sci. 2015, 103, 68–74. [Google Scholar] [CrossRef]
- NSW Department of Primary Industries. Frame Scoring of Beef Cattle. Available online: http://www.dpi.nsw.gov.au/animals-and-livestock/beef-cattle/appraisal/publications/frame-scoring (accessed on 20 October 2020).
- King, D.A.; Shackelford, S.D.; Kuehn, L.A.; Kemp, C.M.; Rodriguez, A.B.; Thallman, R.M.; Wheeler, T.L. Contribution of genetic influences to animal-to-animal variation in myoglobin content and beef lean color stability. J. Anim. Sci. 2010, 88, 1160–1167. [Google Scholar] [CrossRef]
- Johnston, D.J.; Reverter, A.; Ferguson, D.M.; Thompson, J.M.; Burrow, H.M. Genetic and phenotypic characterisation of animal, carcass, and meat quality traits from temperate and tropically adapted beef breeds. 3. Meat quality traits. Aust. J. Agric. Res. 2003, 54, 135–147. [Google Scholar] [CrossRef]
- Dixon, H.; Thomson, B.; Graafhuis, A. Producing quality beef: Practical experience, current practice and future direction. In Proceedings of the New Zealand Grassland Association, Waitangi, New Zealand, 4–7 March 1996; pp. 183–188. [Google Scholar]
- McDade, J.L. The Effects of On-Farm Mixing of Bulls on Beef Quality Characteristics. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2010. [Google Scholar]
- Njisane, Y.Z.; Muchenje, V. Farm to abattoir conditions, animal factors and their subsequent effects on cattle behavioural responses and beef quality—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 755–764. [Google Scholar] [CrossRef]
- Reverter, A.; Johnston, D.J.; Perry, D.; Goddard, M.E.; Burrow, H.M. Genetic and phenotypic characterisation of animal, carcass, and meat quality traits from temperate and tropically adapted beef breeds. 2. Abattoir carcass traits. Aust. J. Agric. Res. 2003, 54, 119–134. [Google Scholar] [CrossRef]
- Morgan, J.; Pickering, F.; Everitt, G. Some factors affecting yellow fat colour in cattle. In Proceedings of the New Zealand Society of Animal Production, Hamilton, New Zealand, 11–13 February 1969; pp. 164–175. [Google Scholar]
- Dunne, P.G.; Monahan, F.J.; O’Mara, F.P.; Moloney, A.P. Colour of bovine subcutaneous adipose tissue: A review of contributory factors, associations with carcass and meat quality and its potential utility in authentication of dietary history. Meat Sci. 2009, 81, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants: From forages to dairy products. Animal Feed Science and Technology 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Buchanan, D.S.; Lenstra, J.A. Breeds of Cattle. In The Genetics of Cattle, 2nd ed.; Garrick, D.J., Ruvinsky, A., Eds.; CAB International: Wallingford, UK, 2015; pp. 33–66. ISBN 978-1-78064-221-5. [Google Scholar]
- Barton, R.A.; Pleasants, A.B. Fat colour and meat colour in different breeds of steers in five consecutive years raised on pasture and slaughtered at 30 months of age. In Proceedings of the New Zealand Society of Animal Production, Hamilton, New Zealand, 17–19 February 1993; pp. 389–391. [Google Scholar]
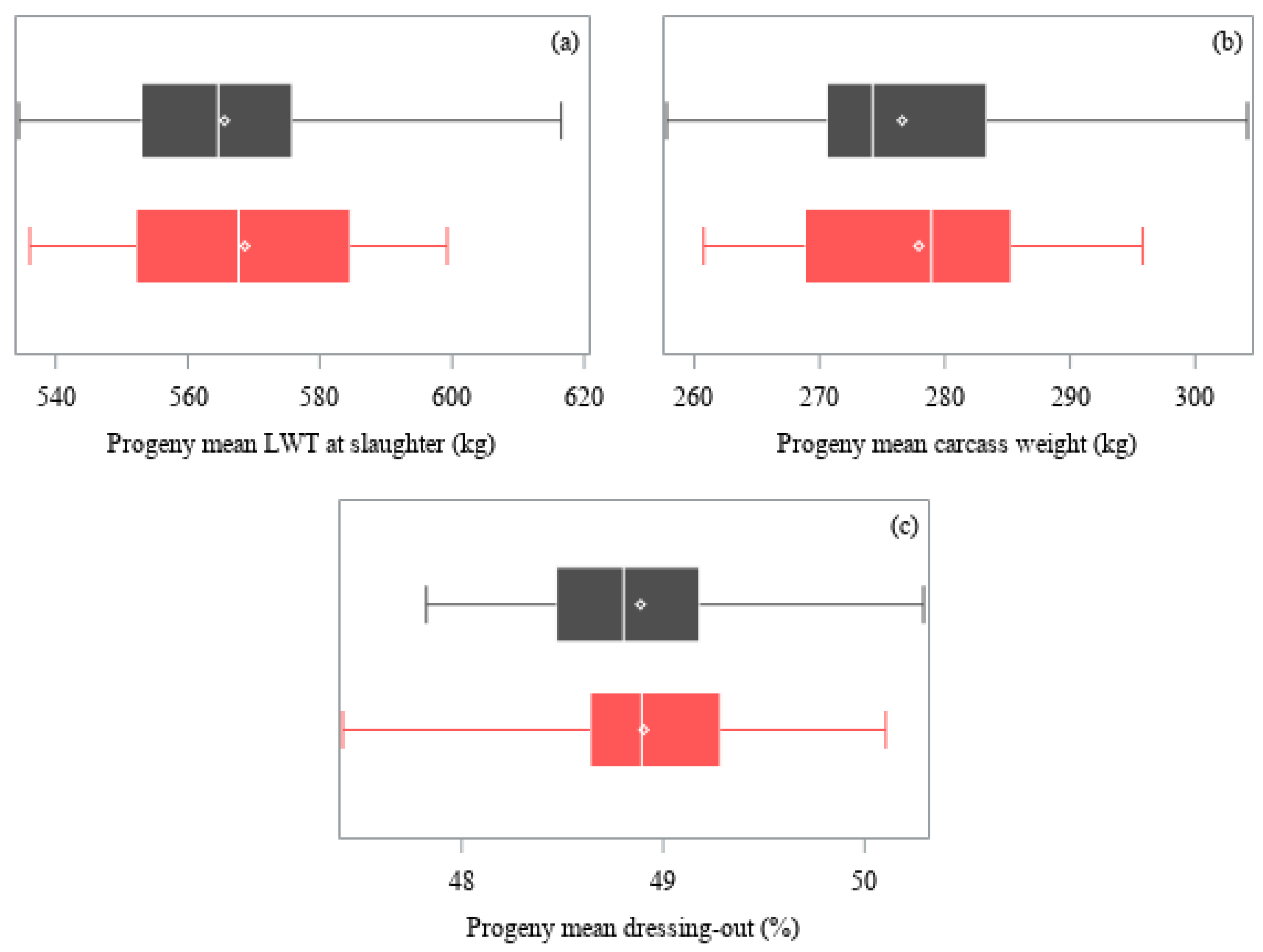

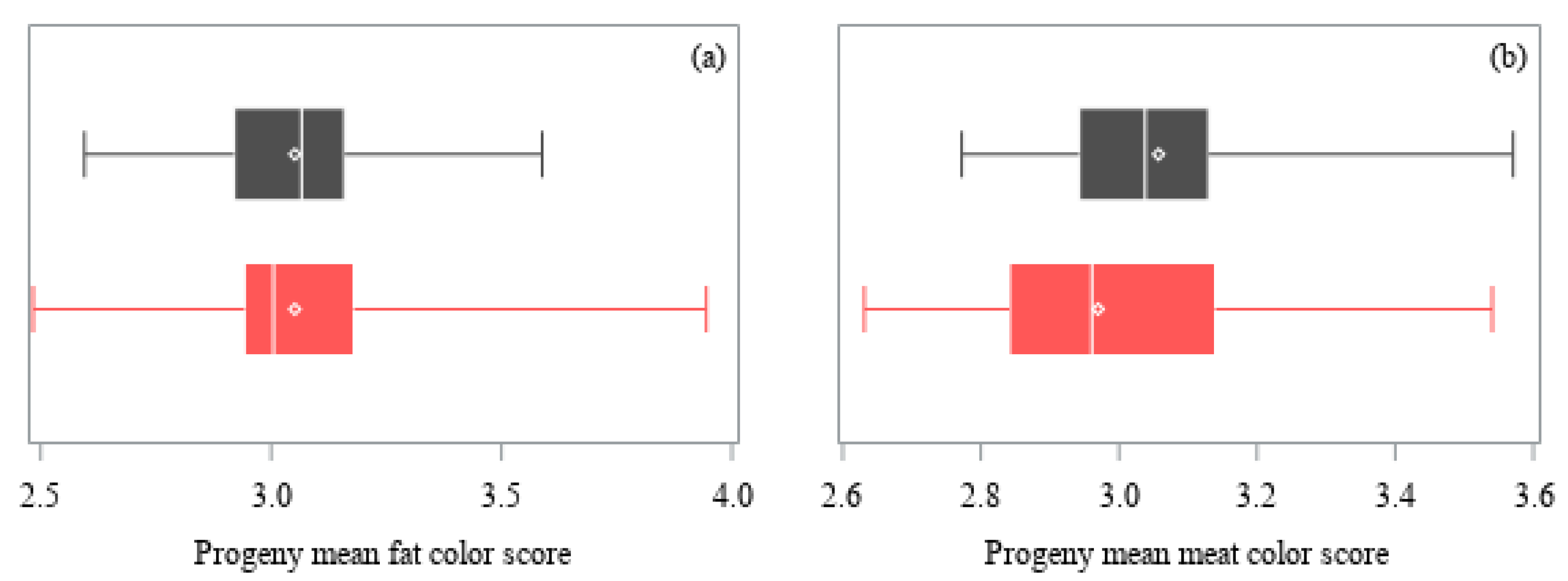
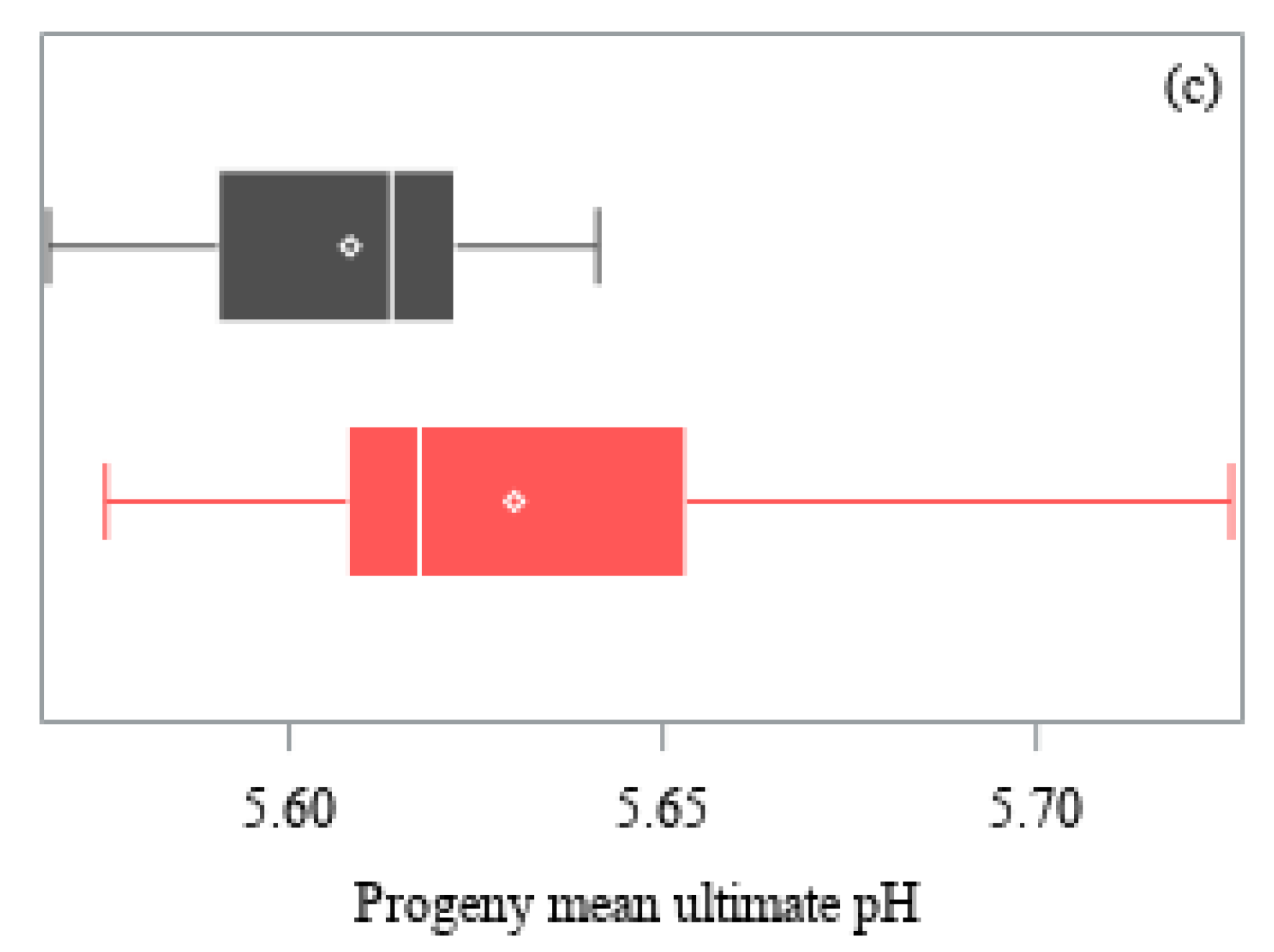
| Trait | Angus | Hereford | ||||
|---|---|---|---|---|---|---|
| n | EBV | EBV Range | n | EBV | EBV Range | |
| Carcass weight (kg) | 37 | 49 ± 16 | (18 to 80) | 40 | 53 ± 14 | (25 to 84) |
| Eye muscle area (cm2) | 37 | 4.6 ± 2.5 | (−2.2 to 9.7) | 40 | 2.9 ± 1.9 | (0.3 to 8.0) |
| Rib fat (mm) | 37 | 0.9 ± 1.8 | (−2.0 to 6.1) | 40 | 0.8 ± 0.9 | (−1.8 to 2.7) |
| Intramuscular fat (%) | 37 | 1.3 ± 1.4 | (−2.1 to 4.4) | 40 | 0.4 ± 0.7 | (−1.0 to 2.0) |
| Trait | n | Mean ± SD | CV% | Sire Effect p-Value |
|---|---|---|---|---|
| Age (days) | 1015 | 854.6 ± 21.8 | 3% | <0.001 |
| Live weight (kg) 1 | 1015 | 567.2 ± 17.9 | 3% | <0.001 |
| Carcass weight (kg) 1 | 1000 | 277.3 ± 9.3 | 3% | <0.001 |
| Dressing-out (%) 1 | 1000 | 48.9 ± 0.6 | 1% | <0.001 |
| Carcass length (cm) 2 | 999 | 240.3 ± 2.3 | 1% | <0.001 |
| Eye muscle area (cm2) 2 | 995 | 73.6 ± 3.5 | 5% | <0.001 |
| Rib fat depth (mm) 2 | 994 | 7.4 ± 1.4 | 19% | <0.001 |
| Marble score 2 | 902 | 0.91 ± 0.32 | 35% | <0.001 |
| Fat color score | 914 | 3.05 ± 0.25 | 8% | 0.058 |
| Meat color score | 914 | 3.01 ± 0.19 | 6% | 0.212 |
| Ultimate pH 3 | 486 | 5.62 ± 0.03 | 1% | 0.961 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, N.; Schreurs, N.; Morris, S.; López-Villalobos, N.; McDade, J.; Hickson, R. Sire Effects on Carcass of Beef-Cross-Dairy Cattle: A Case Study in New Zealand. Animals 2021, 11, 636. https://doi.org/10.3390/ani11030636
Martín N, Schreurs N, Morris S, López-Villalobos N, McDade J, Hickson R. Sire Effects on Carcass of Beef-Cross-Dairy Cattle: A Case Study in New Zealand. Animals. 2021; 11(3):636. https://doi.org/10.3390/ani11030636
Chicago/Turabian StyleMartín, Natalia, Nicola Schreurs, Stephen Morris, Nicolás López-Villalobos, Julie McDade, and Rebecca Hickson. 2021. "Sire Effects on Carcass of Beef-Cross-Dairy Cattle: A Case Study in New Zealand" Animals 11, no. 3: 636. https://doi.org/10.3390/ani11030636
APA StyleMartín, N., Schreurs, N., Morris, S., López-Villalobos, N., McDade, J., & Hickson, R. (2021). Sire Effects on Carcass of Beef-Cross-Dairy Cattle: A Case Study in New Zealand. Animals, 11(3), 636. https://doi.org/10.3390/ani11030636







