Expeller-Pressed Canola (Brassica napus) Meal Modulates the Structure and Function of the Cecal Microbiota, and Alters the Metabolome of the Pancreas, Liver, and Breast Muscle of Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design and Diet Treatments
2.3. Broilers
2.4. Cecal Content Collection for Chick Inoculation
2.5. Enteric Microbiota Establishment
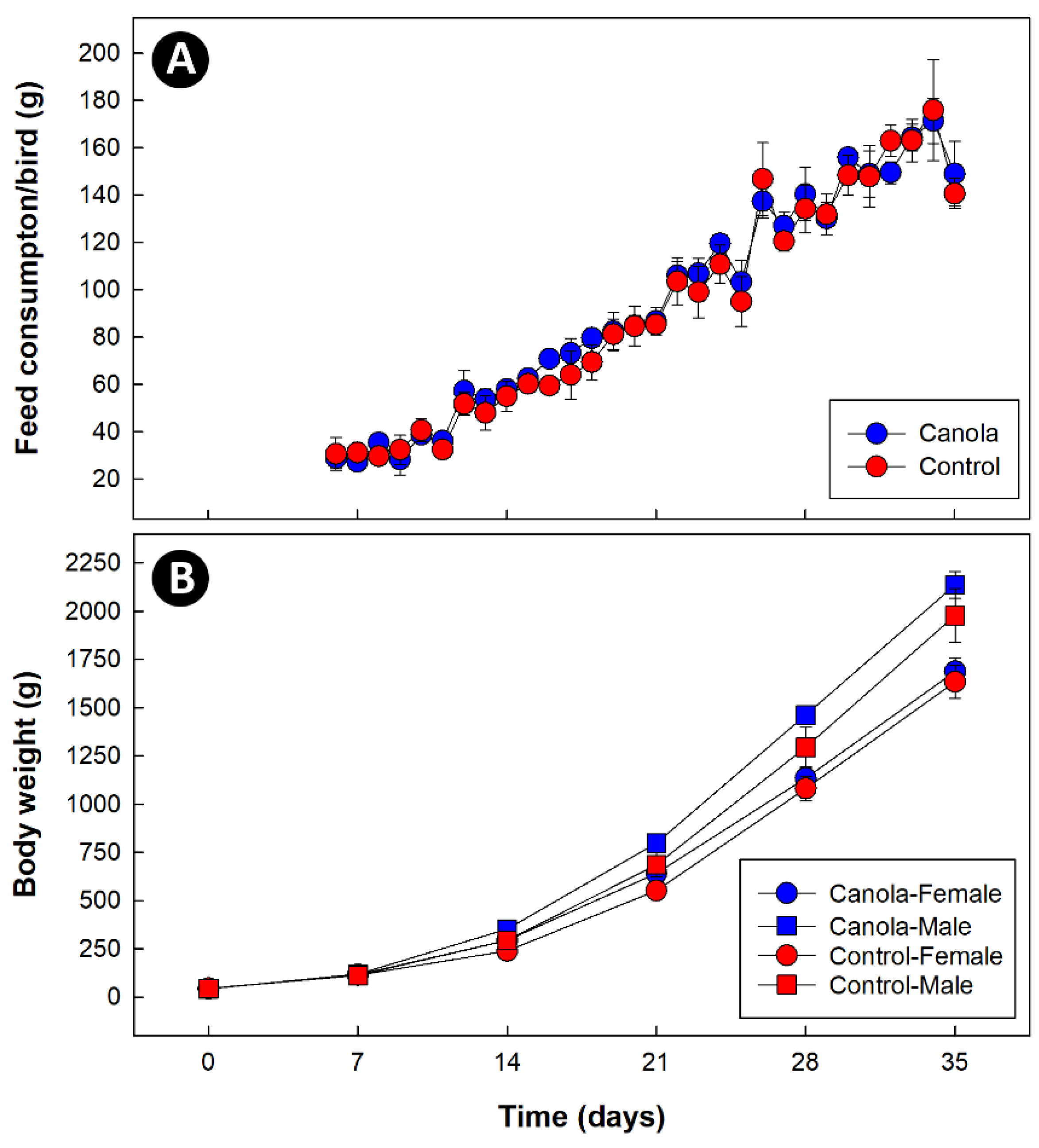
2.6. Feed Consumption and Weight Gain
2.7. Necropsies
2.8. Short-Chain Fatty Acid Analysis
2.9. Characterization of Bacterial Communities
2.10. Metabolomics
2.11. Statistical Analyses
3. Results
3.1. Supplementation of Diets with Canola Meal Did Not Affect Feed Consumption or Broiler Weight Gain
3.2. The Canola Meal Diet Did Not Affect the Richness, Evenness, or Diversity of Cecal Bacterial Communities
3.3. The Canola Meal Diet Affected the Structure of the Cecal Bacterial Community
3.4. The Canola Meal Diet Contained More Dietary Fiber, and Resulted in Increased Levels of Cecal Fermentation
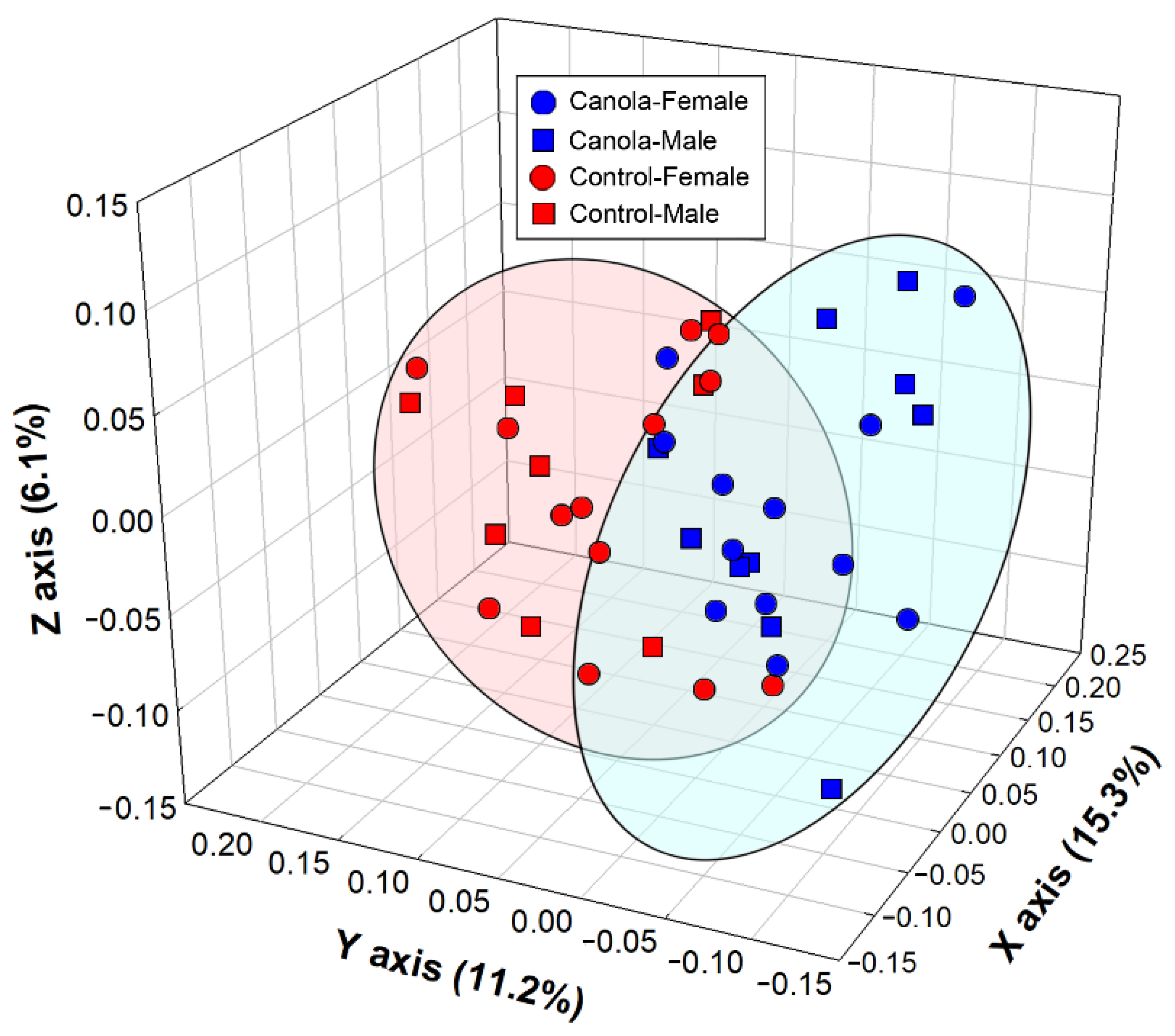
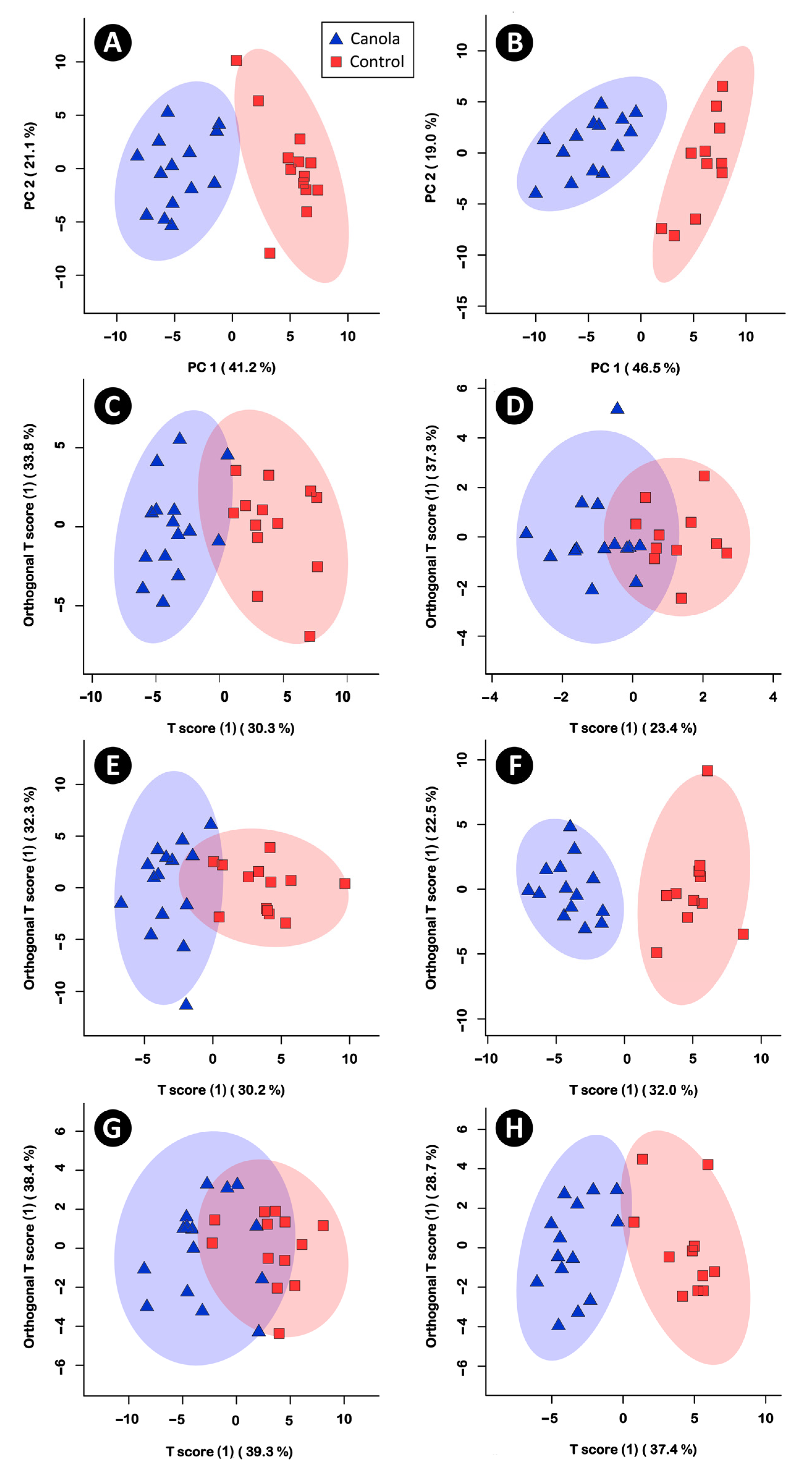
3.5. Metabolite Profiles in the Cecal Digesta, Pancreas, Liver, and Breast Muscle Were Altered in Broilers Fed the Canola Meal Diet
4. Discussion
4.1. Inoculation of Birds with Cecal Digesta
4.2. Canola Meal Characteristics and Impacts on Bird Growth and Nutrition
4.3. Impacts of Canola Meal on the Cecal Microbiota
4.4. Impacts of Canola Meal on the Cecal Metabolome
4.5. Impacts of Canola Meal on the Metabolome of the Pancreas
4.6. Impacts of Canola Meal on the Metabolome of the Liver
4.7. Impacts of Canola Meal on the Metabolome of Breast Muscle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Government of Canada. Canola: A Canadian Success Story. Statistics Canada, 2020. Available online: https://www150.statcan.gc.ca/n1/pub/96-325-x/2007000/article/10778-eng.htm (accessed on 17 February 2021).
- Canola Council of Canada. Canola Meal Feeding Guide. Canola Council of Canada, 2015. Available online: https://www.canolacouncil.org/download/215/pages/5344/2015_canola_meal_feed_industry_guide (accessed on 17 February 2021).
- Pratt, S. New Canola Could Open Up Feed Market. Western Producer. 2016. Available online: https://www.producer.com/news/new-canola-could-open-up-feed-market/ (accessed on 17 February 2021).
- Khajali, F.; Slominski, B.A. Factors that affect the nutritive value of canola meal for poultry. Poult. Sci. 2012, 91, 2564–2575. [Google Scholar] [CrossRef]
- Adewole, D.I.; Rogiewicz, A.; Dyck, B.; Slominski, B.A. Effects of canola meal source on the standardized ileal digestible amino acids and apparent metabolizable energy contents for broiler chickens. Poult. Sci. 2017, 96, 4298–4306. [Google Scholar] [CrossRef]
- Adewole, D.I.; Rogiewicz, A.; Dyck, B.; Slominski, B.A. Chemical and nutritive characteristics of canola meal from Canadian processing facilities. Anim. Feed Sci. Technol. 2016, 222, 17–30. [Google Scholar] [CrossRef]
- Recoules, E.; Lessire, M.; Labas, V.; Duclos, M.J.; Combes-Soia, L.; Lardic, L.; Peyronnet, C.; Quinsac, A.; Narcy, A.; Rehault-Godbert, S. Digestion dynamics in broilers fed rapeseed meal. Sci. Rep. 2019, 9, 3052. [Google Scholar] [CrossRef]
- Long, C.; Wang, J.; Zhang, H.J.; Wu, S.G.; Qi, G.H. Effects of dietary rapeseed meal supplementation on cecal microbiota in laying hens with different flavin-containing monooxygenase 3 genotypes. Poult. Sci. 2017, 96, 1748–1758. [Google Scholar] [CrossRef]
- Toghyani, M.; Girish, C.K.; Wu, S.B.; Iji, P.A.; Swick, R.A. Effect of elevated dietary amino acid levels in high canola meal diets on productive traits and cecal microbiota population of broiler chickens in a pair-feeding study. Poult. Sci. 2017, 96, 1268–1279. [Google Scholar] [CrossRef]
- Konieczka, P.; Czerwiński, J.; Jankowiak, J.; Ząbek, K.; Smulikowska, S. Effects of partial replacement of soybean meal with rapeseed meal, narrow-leaved lupin, DDGS, and probiotic supplementation, on performance and gut microbiota activity and diversity in broilers. Ann. Anim. Sci. 2019, 19, 1115–1131. [Google Scholar] [CrossRef]
- Vertiprakhov, V.G.; Grozina, A.A.; Fisinin, V.I.; Egorov, I.A. The correlation between the activities of digestive enzymes in the pancreas and blood serum in chicken. Open J. Anim. Sci. 2018, 08, 215–222. [Google Scholar] [CrossRef]
- Lv, Z.P.; Peng, Y.Z.; Zhang, B.B.; Fan, H.; Liu, D.; Guo, Y.M. Glucose and lipid metabolism disorders in the chickens with dexamethasone-induced oxidative stress. J. Anim. Physiol. Anim. Nutr. 2018, 102, e706–e717. [Google Scholar] [CrossRef]
- Zaytsoff, S.J.M.; Brown, C.L.J.; Montina, T.; Metz, G.A.S.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Corticosterone-mediated physiological stress modulates hepatic lipid metabolism, metabolite profiles, and systemic responses in chickens. Sci. Rep. 2019, 9, 19225. [Google Scholar] [CrossRef]
- Oryschak, M.; Korver, D.; Zuidhof, M.; Meng, X.; Beltranena, E. Comparative feeding value of extruded and nonextruded wheat and corn distillers dried grains with solubles for broilers. Poult. Sci. 2010, 89, 2183–2196. [Google Scholar] [CrossRef]
- Jiminez, J.A.; Uwiera, T.C.; Abbott, D.W.; Uwiera, R.R.; Inglis, G.D. Impacts of resistant starch and wheat bran consumption on enteric inflammation in relation to colonic bacterial community structures and short-chain fatty acid concentrations in mice. Gut Pathog. 2016, 8, 67. [Google Scholar] [CrossRef]
- Jiminez, J.A.; Uwiera, T.C.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with Citrobacter rodentium. mSphere 2017, 2, e00243-17. [Google Scholar] [CrossRef]
- Playne, M.J. Determination of ethanol volatile fatty-acids lactic-acid and succinic-acid in fermentation liquids by gas chromatography. J. Sci. Food Agric. 1985, 36, 638–644. [Google Scholar] [CrossRef]
- Cottyn, B.G.; Boucque, C.V. Rapid method for the gas-chromatographic determination of volatile fatty acids in rumen fluid. J. Agric. Food Chem. 1968, 16, 105–107. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Tiziani, S.; Emwas, A.H.; Lodi, A.; Ludwig, C.; Bunce, C.M.; Viant, M.R.; Gunther, U.L. Optimized metabolite extraction from blood serum for 1H nuclear magnetic resonance spectroscopy. Anal. Biochem. 2008, 377, 16–23. [Google Scholar] [CrossRef]
- Paxman, E.J.; Boora, N.S.; Kiss, D.; Laplante, D.P.; King, S.; Montina, T.; Metz, G.A.S. Prenatal maternal stress from a natural disaster alters urinary metabolomic profiles in project ice storm participants. Sci. Rep. 2018, 8, 12932. [Google Scholar] [CrossRef]
- Kiss, D.; Ambeskovic, M.; Montina, T.; Metz, G.A. Stress transgenerationally programs metabolic pathways linked to altered mental health. Cell Mol. Life Sci. 2016, 73, 4547–4557. [Google Scholar] [CrossRef]
- Anderson, P.E.; Mahle, D.A.; Doom, T.E.; Reo, N.V.; DelRaso, N.J.; Raymer, M.L. Dynamic adaptive binning: An improved quantification technique for NMR spectroscopic data. Metabolomics 2011, 7, 179–190. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER. Guide to Software and Statistical Methods. ScienceOpen. 2008. Available online: https://www.scienceopen.com/document?vid=19bb860e-6a20-42d4-a081-a1f62bc8dfa1 (accessed on 17 February 2021).
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Goodpaster, A.M.; Romick-Rosendale, L.E.; Kennedy, M.A. Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Anal. Biochem. 2010, 401, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.; Zhao, X.Q.; Knudsen, K.E.; Eggum, B.O. The influence of dietary fibre source and level on the development of the gastrointestinal tract, digestibility and energy metabolism in broiler chickens. Br. J. Nutr. 1996, 75, 379–395. [Google Scholar] [CrossRef]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Schokker, D.; Jansman, A.J.; Veninga, G.; de Bruin, N.; Vastenhouw, S.A.; de Bree, F.M.; Bossers, A.; Rebel, J.M.; Smits, M.A. Perturbation of microbiota in one-day old broiler chickens with antibiotic for 24 hours negatively affects intestinal immune development. BMC Genom. 2017, 18, 241. [Google Scholar] [CrossRef]
- Baba, E.; Nagaishi, S.; Fukata, T.; Arakawa, A. The role of intestinal microflora on the prevention of Salmonella colonization in gnotobiotic chickens. Poult. Sci. 1991, 70, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, E.E.; Stanley, D.; Hughes, R.J.; Moore, R.J. The time-course of broiler intestinal microbiota development after administration of cecal contents to incubating eggs. PeerJ 2017, 5, e3587. [Google Scholar] [CrossRef]
- Low, K.E.; Xing, X.; Moote, P.E.; Inglis, G.D.; Venketachalam, S.; Hahn, M.G.; King, M.L.; Jones, C.Y.; Jones, D.R.; Willats, W.G.T.; et al. Combinatorial glycomic analyses to direct CAZyme discovery for the tailored degradation of canola meal non-starch dietary polysaccharides. Microorganisms 2020, 8, 1888. [Google Scholar] [CrossRef] [PubMed]
- Van der Eijk, J.A.J.; Rodenburg, T.B.; de Vries, H.; Kjaer, J.B.; Smidt, H.; Naguib, M.; Kemp, B.; Lammers, A. Early-life microbiota transplantation affects behavioural responses, serotonin and immune characteristics in chicken lines divergently selected on feather pecking. Sci. Rep. 2020, 10, 2750. [Google Scholar] [CrossRef]
- Mailer, R. Canola Meal: Limitations and Opportunities. Australian Oilseed Feederation. 2004. Available online: http://www.australianoilseeds.com/__data/assets/pdf_file/0011/1271/AOF_Canola_Meal_Report-Limitations__and__Opportunities_2004.pdf (accessed on 17 February 2021).
- Wickramasuriya, S.S.; Yi, Y.J.; Yoo, J.; Kang, N.K.; Heo, J.M. A review of canola meal as an alternative feed ingredient for ducks. J. Anim. Sci. Technol. 2015, 57, 29. [Google Scholar] [CrossRef]
- Newkirk, R.W.; Classen, H.L.; Edney, M.J. Effects of prepress-solvent extraction on the nutritional value of canola meal for broiler chickens. Anim. Feed Sci. Technol. 2003, 104, 111–119. [Google Scholar] [CrossRef]
- Clark, W.D.; Classen, H.L.; Newkirk, R.W. Assessment of tail-end dehulled canola meal for use in broiler diets. Can. J. Anim. Sci. 2001, 81, 379–386. [Google Scholar] [CrossRef]
- Inglis, G.D.; Ramezani, N.; Taboada, E.N.; Boras, V.F.; Uwiera, R.R.E. Analysis of Campylobacter jejuni subtype distribution in the chicken broiler production continuum: Longitudinal examination of birds from three farms over a 1.5-year period in Southwestern Alberta, Canada to identify primary contamination points. Appl. Environ. Microbiol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.Z.; Khan, S.H.; Yousaf, M. Effect of feeding various levels of canola meal on the performance of broiler chicks. J. Anim. Plant Sci. 2006, 16, 78. [Google Scholar]
- Newkirk, R.W.; Classen, H.L. The effects of toasting canola meal on body weight, feed conversion efficiency, and mortality in broiler chickens. Poult. Sci. 2002, 81, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.R.; Devegowda, G.; Khosravinia, H. Effects of enzyme addition to broiler diets containing varying levels of double zero rapeseed meal. Asian-Aust. J. Anim. Sci. 2006, 19, 1354–1360. [Google Scholar] [CrossRef]
- Oryschak, M.; Beltranena, E. Solvent-extracted vs. expeller-pressed B. napus and B. juncea fed to layers: Effects on feed intake, egg production and physical egg quality. Poult. Sci. 2013, 92 (Suppl. S1), 80. [Google Scholar]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wielen, P.W.; Biesterveld, S.; Notermans, S.; Hofstra, H.; Urlings, B.A.; van Knapen, F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef]
- Pustjens, A.M.; de Vries, S.; Schols, H.A.; Gruppen, H.; Gerrits, W.J.; Kabel, M.A. Understanding carbohydrate structures fermented or resistant to fermentation in broilers fed rapeseed (Brassica napus) meal to evaluate the effect of acid treatment and enzyme addition. Poult. Sci. 2014, 93, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Karasawa, Y. Effect of removal of caecal contents on nitrogen utilisation and nitrogen excretion in caecally ligated chickens fed on a low protein diet supplemented with urea. Br. Poult. Sci. 2000, 41, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Svihus, B.; Choct, M.; Classen, H.L. Function and nutritional roles of the avian caeca: A review. World Poult. Sci. J. 2019, 69, 249–264. [Google Scholar] [CrossRef]
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J. Neurochem. 2020, 154, 635–646. [Google Scholar] [CrossRef]
- Lobzhanidze, G.; Lordkipanidze, T.; Zhvania, M.; Japaridze, N.; MacFabe, D.F.; Pochkidze, N.; Gasimov, E.; Rzaev, F. Effect of propionic acid on the morphology of the amygdala in adolescent male rats and their behavior. Micron 2019, 125, 102732. [Google Scholar] [CrossRef]
- Crhanova, M.; Karasova, D.; Juricova, H.; Matiasovicova, J.; Jahodarova, E.; Kubasova, T.; Seidlerova, Z.; Cizek, A.; Rychlik, I. Systematic culturomics shows that half of chicken caecal microbiota members can be grown in vitro except for two lineages of Clostridiales and a single lineage of Bacteroidetes. Microorganisms 2019, 7, 496. [Google Scholar] [CrossRef]
- Hollywood, K.; Brison, D.R.; Goodacre, R. Metabolomics: Current technologies and future trends. Proteomics 2006, 6, 4716–4723. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Huergo, L.F.; Dixon, R. The emergence of 2-oxoglutarate as a master regulator metabolite. Microbiol. Mol. Biol. Rev. 2015, 79, 419–435. [Google Scholar] [CrossRef]
- Beloborodova, N.V.; Khodakova, A.S.; Bairamov, I.T.; Olenin, A.Y. Microbial origin of phenylcarboxylic acids in the human body. Biochemistry (Moscow) 2009, 74, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Turlin, E.; Sismeiro, O.; Le Caer, J.P.; Labas, V.; Danchin, A.; Biville, F. 3-Phenylpropionate catabolism and the Escherichia coli oxidative stress response. Res. Microbiol. 2005, 156, 312–321. [Google Scholar] [CrossRef]
- Gharbia, S.E.; Shah, H.N. Pathways of glutamate catabolism among Fusobacterium species. J. Gen. Microbiol. 1991, 137, 1201–1206. [Google Scholar] [CrossRef]
- Marounek, M.; Duskova, D. Metabolism of pectin in rumen bacteria Butyrivibrio fibrisolvens and Prevotella ruminicola. Lett. Appl. Microbiol. 1999, 29, 429–433. [Google Scholar] [CrossRef]
- Matthies, C.; Schink, B. Reciprocal isomerization of butyrate and isobutyrate by the strictly anaerobic bacterium strain WoG13 and methanogenic isobutyrate degradation by a defined triculture. Appl. Environ. Microbiol. 1992, 58, 1435–1439. [Google Scholar] [CrossRef]
- Pandiri, A.R. Overview of exocrine pancreatic pathobiology. Toxicol. Pathol. 2014, 42, 207–216. [Google Scholar] [CrossRef]
- Hazelwood, R.L. Avian endocrine pancreas. Am. Zool. 1973, 13, 699–709. [Google Scholar] [CrossRef]
- Morimoto, S.; Jimenez-Trejo, F.; Cerbon, M. Sex steroids effects in normal endocrine pancreatic function and diabetes. Curr. Top. Med. Chem. 2011, 11, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Parchami, A.; Kusha, S. Effect of sex on histomorphometric properties of langerhans islets in native chickens. Vet. Res. Forum 2015, 6, 327–330. [Google Scholar]
- Kui, B.; Balla, Z.; Vegh, E.T.; Pallagi, P.; Venglovecz, V.; Ivanyi, B.; Takacs, T.; Hegyi, P.; Rakonczay, Z., Jr. Recent advances in the investigation of pancreatic inflammation induced by large doses of basic amino acids in rodents. Lab. Investig. 2014, 94, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, J.; Zhang, J.; Ma, C.; Shao, C.; Hao, J.; Zheng, J.; Feng, X.; Zuo, C. High-resolution magic angle spinning (1)H magnetic resonance spectroscopy detects choline as a biomarker in a swine obstructive chronic pancreatitis model at an early stage. Mol. Biosyst. 2014, 10, 467–474. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Clow, K.; Brosnan, J.T.; Brosnan, M.E. Synthesis of guanidinoacetate and creatine from amino acids by rat pancreas. Br. J. Nutr. 2014, 111, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Rocic, B.; Lovrencic, M.V.; Poje, M.; Ashcroft, S.J. Effect of creatine on the pancreatic beta-cell. Exp. Clin. Endocrinol. Diabetes 2007, 115, 29–32. [Google Scholar] [CrossRef]
- Kui, B.; Balla, Z.; Vasas, B.; Vegh, E.T.; Pallagi, P.; Kormanyos, E.S.; Venglovecz, V.; Ivanyi, B.; Takacs, T.; Hegyi, P.; et al. New insights into the methodology of L-arginine-induced acute pancreatitis. PLoS ONE 2015, 10, e0117588. [Google Scholar] [CrossRef]
- Thomke, S.; Pettersson, H.; Neil, M.; Hakansson, J. Skeletal muscle goitrin concentration and organ weights in growing pigs fed diets containing rapeseed meal. Anim. Feed Sci. Technol. 1998, 73, 207–215. [Google Scholar] [CrossRef]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Hajiasgharzadeh, K.; Baradaran, B. Cholinergic anti-inflammatory pathway and the liver. Adv. Pharm. Bull. 2017, 7, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S; discussion 1548S. [Google Scholar] [CrossRef]
- Lopez, K.P.; Schilling, M.W.; Corzo, A. Broiler genetic strain and sex effects on meat characteristics. Poult. Sci. 2011, 90, 1105–1111. [Google Scholar] [CrossRef]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Ringuet, M.; Furness, J.B.; Dunshea, F.R. Betaine and antioxidants improve growth performance, breast muscle development and ameliorate thermoregulatory responses to cyclic heat exposure in broiler chickens. Animals (Basel) 2018, 8, 162. [Google Scholar] [CrossRef]
- Chalvatzi, S.; Papadopoulos, G.A.; Tsiouris, V.; Giannenas, I.; Karapanagiotidis, I.T.; Theodoridis, A.; Georgopoulou, I.; Fortomaris, P.D. Dimethylglycine supplementation in reduced energy broilers’ diets restores performance by improving nutrient digestibility. Animals (Basel) 2020, 10, 789. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Cartwright, S.P.; Bromley, C.; Gross, S.R.; Bill, R.M. Carnosine: Can understanding its actions on energy metabolism and protein homeostasis inform its therapeutic potential? Chem. Cent. J. 2013, 7, 38. [Google Scholar] [CrossRef]




| Ingredient | Control (%) | Canola Meal (%) | ||||
|---|---|---|---|---|---|---|
| Starter | Grower | Finisher | Starter | Grower | Finisher | |
| Corn | 49.53 | 54.68 | 59.84 | 38.02 | 44.75 | 48.35 |
| Canola meal | ‒ | ‒ | ‒ | 20.00 | 20.00 | 20.00 |
| Soybean meal | 43.06 | 37.31 | 31.57 | 34.60 | 25.40 | 23.11 |
| Canola oil | 2.39 | 3.26 | 4.12 | 2.88 | 5.52 | 4.61 |
| Salt | 0.51 | 0.52 | 0.52 | 0.49 | 0.51 | 0.50 |
| Limestone | 1.52 | 1.41 | 1.31 | 1.35 | 1.18 | 1.13 |
| Dicalcium phosphate | 1.26 | 1.09 | 0.92 | 1.12 | 1.08 | 0.78 |
| Magnesium oxide | 0.10 | 0.15 | 0.17 | 0.02 | 0.06 | 0.09 |
| L-lysine HCl | 0.11 | 0.12 | 0.13 | 0.08 | 0.14 | 0.10 |
| D,L-methionine | 0.37 | 0.33 | 0.31 | 0.30 | 0.24 | 0.24 |
| L-threonine | 0.15 | 0.13 | 0.11 | 0.14 | 0.12 | 0.09 |
| Vitamin premix | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Choline premix | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Ingredient | Control | Canola Meal | ||||
|---|---|---|---|---|---|---|
| Starter | Grower | Finisher | Starter | Grower | Finisher | |
| Metabolizable energy (Mcal/kg) | 3.00 | 3.10 | 3.20 | 3.00 | 3.10 | 3.20 |
| Dry matter (%) | 90.49 | 90.73 | 90.53 | 91.11 | 91.49 | 90.70 |
| Crude fiber (%) | 3.81 | 3.57 | 3.33 | 4.46 | 4.97 | 3.97 |
| Acid detergent fiber (%) | 5.15 | 4.86 | 4.56 | 7.29 | 7.45 | 6.70 |
| Neutral detergent fiber (%) | 8.53 | 8.53 | 8.53 | 10.70 | 12.49 | 10.69 |
| Total protein (%) | 24.20 | 21.90 | 19.60 | 26.30 | 23.26 | 21.70 |
| Digestible protein (%) | 20.96 | 19.01 | 17.06 | 21.09 | 18.65 | 17.18 |
| Fat (%) | 4.41 | 5.27 | 6.13 | 6.89 | 7.76 | 8.60 |
| Calcium (%) | 1.01 | 0.91 | 0.82 | 1.01 | 0.91 | 0.82 |
| Available phosphorus (%) | 0.50 | 0.46 | 0.41 | 0.50 | 0.46 | 0.40 |
| Magnesium (%) | 0.25 | 0.26 | 0.26 | 0.25 | 0.26 | 0.26 |
| Sodium (%) | 0.21 | 0.21 | 0.21 | 0.21 | 0.21 | 0.21 |
| Linoleic acid (%) | 1.62 | 1.92 | 2.22 | 1.97 | 2.20 | 2.58 |
| Digestible arginine (%) | 1.48 | 1.32 | 1.16 | 1.49 | 1.29 | 1.17 |
| Digestible histidine (%) | 0.56 | 0.51 | 0.46 | 0.58 | 0.53 | 0.47 |
| Digestible isoleucine (%) | 0.95 | 0.85 | 0.76 | 0.93 | 0.82 | 0.74 |
| Digestible leucine (%) | 1.75 | 1.61 | 1.48 | 1.71 | 1.55 | 1.45 |
| Digestible lysine (%) | 1.34 | 1.21 | 1.07 | 1.34 | 1.21 | 1.07 |
| Digestible methionine (%) | 0.69 | 0.63 | 0.58 | 0.66 | 0.57 | 0.54 |
| Digestible cysteine (%) | 0.31 | 0.28 | 0.26 | 0.34 | 0.34 | 0.30 |
| Digestible phenylalanine (%) | 1.07 | 0.97 | 0.86 | 1.04 | 0.92 | 0.83 |
| Digestible threonine (%) | 0.90 | 0.81 | 0.71 | 0.90 | 0.81 | 0.71 |
| Digestible tryptophan (%) | 0.29 | 0.25 | 0.22 | 0.29 | 0.26 | 0.22 |
| Digestible valine (%) | 1.01 | 0.91 | 0.82 | 1.01 | 0.91 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© Her Majesty the Queen in Right of Canada as represented by the Minister of Agriculture and Agri-Food, 2021 and the authors; Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inglis, G.D.; Wright, B.D.; Sheppard, S.A.; Abbott, D.W.; Oryschak, M.A.; Montina, T. Expeller-Pressed Canola (Brassica napus) Meal Modulates the Structure and Function of the Cecal Microbiota, and Alters the Metabolome of the Pancreas, Liver, and Breast Muscle of Broiler Chickens. Animals 2021, 11, 577. https://doi.org/10.3390/ani11020577
Inglis GD, Wright BD, Sheppard SA, Abbott DW, Oryschak MA, Montina T. Expeller-Pressed Canola (Brassica napus) Meal Modulates the Structure and Function of the Cecal Microbiota, and Alters the Metabolome of the Pancreas, Liver, and Breast Muscle of Broiler Chickens. Animals. 2021; 11(2):577. https://doi.org/10.3390/ani11020577
Chicago/Turabian StyleInglis, G. Douglas, Benjamin D. Wright, Stephanie A. Sheppard, D. Wade Abbott, Matt A. Oryschak, and Tony Montina. 2021. "Expeller-Pressed Canola (Brassica napus) Meal Modulates the Structure and Function of the Cecal Microbiota, and Alters the Metabolome of the Pancreas, Liver, and Breast Muscle of Broiler Chickens" Animals 11, no. 2: 577. https://doi.org/10.3390/ani11020577
APA StyleInglis, G. D., Wright, B. D., Sheppard, S. A., Abbott, D. W., Oryschak, M. A., & Montina, T. (2021). Expeller-Pressed Canola (Brassica napus) Meal Modulates the Structure and Function of the Cecal Microbiota, and Alters the Metabolome of the Pancreas, Liver, and Breast Muscle of Broiler Chickens. Animals, 11(2), 577. https://doi.org/10.3390/ani11020577






