1. Introduction
Animal biodiversity, the grouping of populations into breeds and domestication have evolved over the centuries, from prehistoric times to the present agricultural sedentary lifestyle, a consequence of both natural selection and human intervention. Likewise, the use of these breeds or populations, namely the poultry, for the survival of the Human species, should not be overlooked.
The characterization of animal genetic resources for food and agriculture (ANGR) comprises several types of information: phenotypic, genetic and historical, in the alignment of the Action for Animal Genetic Resources, recognizing that “A good understanding of breed characteristics is necessary to guide the taking decision-making in livestock development and breeding programs” [
1,
2].
Among avian species, chickens have by far the greatest number of breeds at risk on a global scale. The proportion of avian breeds of unknown risk status is even greater than for mammalian species and extinct breeds have mainly been reported among chickens and the regions with the greatest proportions of breeds classified as at risk for avian breeds are North America, Europe and the Caucasus (48 and 43 percent, respectively) [
3].
It is estimated that about 25% of the 1592 chicken breeds [
3] are the subject of conservation programs, 40 breeds of chicken have become extinct [
2,
3,
4,
5] and only 15% of countries have poultry conservation programs, covering 63% of local breeds and 11% of national populations of transboundary breeds [
6]. It urges to maintain programs that could protect breeds in danger of extinction and help to save and spread their genetic diversity, in particular those local breeds that are characterized by medium or low performance and maintained in small populations [
7,
8].
The breeds’ standardization, based on the morphological classification of racial importance described in the standard (qualitative classification) and its productive characteristics (quantitative classification), proves to be an important instrument for the evaluation of the animal and/or the flocks that constitute the herd existing. This evaluation should be used to identify positive and negative aspects of each animal, in order to determine and promote measures for the selection of animals or flocks [
2,
9].
The first reference to the Portuguese poultry breeds is recent, in the 30s of the last century. Manuel Véstia, in 1959 [
10], differentiated and classified the populations as “Preta Lusitânica” or “Transmontana”, “Pedrês Portuguesa”, “Amarela” or “Galinha do Minho” and “Branca” or “Raça de Pescoço Pelado”. Recent studies of the Portuguese chicken breeds were mainly related to phenotypic and productive characteristics, defining patterns and productive systems [
9,
11,
12].
Portugal, despite being a country of reduced physical dimension, is very different from the orographic, climatic and edaphic points of view. This diversity explains the variety and complexity of the vegetable landscape and has, in turn, resulted in a multiplicity of traditional farming systems in which a high number of indigenous breeds of domestic animals stands out, four of which are birds (chickens) unit [
13,
14]. Portugal is the European country with the largest number of autochthonous breeds per area unit [
14].
Autochthonous hens are produced in a smallholding context, mainly in Northwest Portugal [
11,
12,
13,
15]. This territory presents a worrisomely aging population, which resulted from the great wave of emigration during the 1960’s alongside with the continuous attraction for the coastal regions, which has economic, social and culturally shaping the rural landscape of our times.
It is in this region that autochthonous breeds of chickens have their manor, being reared in productive systems complementary to other agricultural activities, considering the production of meat and eggs as by-products of the farm, primarily for self-consumption. Indirectly, these small family farms played an important role in preventing the complete extinction of these breeds [
12,
13].
Thanks to their affection for what is genuinely Portuguese, these persistent farmers have defended, preserved and carried until today a unique and endangered genetic heritage, which requires the simultaneous intervention of technicians and breeders in its conservation and improvement.
Today, the only four autochthonous chicken breeds are produced in free-range conditions, with a simple, functional and traditional construction, adapted to the number of animals and type of production (meat or eggs) [
13,
15,
16]. The equipment is rudimentary, without much technical or technological evolution. The same rusticity is present in the nutritional aspect—farm fodder and the use of surplus or by-products of human or animal feeding complement the reduced needs of these animals, as well as the search for animal protein (insects, worms), in the very typical and leisurely act of etching the soil [
13,
14,
16].
The sustainability of production is becoming an increasingly strong consumer argument for the choice of products and producers that are part of their diet [
17,
18,
19]. The breeding system for indigenous species is balanced by the use of natural resources, land and water, making it environmentally “friendlier”, and particularly adjusted in less favored regions where these resources are scarce [
16,
20,
21,
22,
23,
24].
Traditionally, the production of local chicken breeds (breeding, fattening and then slaughtering of males) has been for gastronomic purposes, in a generally slow-growing and late-maturing period, especially when grown in systems with reduced inputs. The husbandry practices, in this artisanal system, are characterized by the use of rustic animals in free-range conditions with a low capital investment, in a very efficient productive management to develop purposes of high-biological-value protein such as meat and eggs [
25,
26,
27]. In fact, recent studies confirmed the high quality of the eggs of Portuguese native breeds, matching or superseding the quality of commercial breeds product, an interesting opportunity for the recent specialized market niches [
28].
This incalculable genetic value, comparable to any other patrimony, even monuments, needs to be characterized, preserved, promoted, disseminated and valued so that future generations can study and know it for their advantage [
2,
4,
11,
13,
14]. After a long period lacking actions related to the conservation of local genetic resources, with the Genealogical Register in an early stage, breed characterization studies began to be carried out, under a genetic conservation program for its morphological measures, egg production, growth and reproductive performance, and genetic makeup [
11].
The aim of this study was to perform the zoometric characterization of the Portuguese indigenous breeds, evaluating the large existing phenotypic variability in these populations and the different productive factors that may contribute to this variability. Zoometric traits play an important role in the live weight prediction and subsequently in the performance of animal carcasses [
29,
30], constituting a high potential economic selection criterion, with a significant impact in the paternal line of autochthonous populations.
The knowledge of the zoometric and productive traits will support the implementation of conservation strategies aimed to ensure the survival of low-efficiency local breeds. In addition to being unprecedented, this data is expected to contribute to the creation of an ICAR working group and, a basis to the implementation and validation of poultry breeding strategies.
4. Discussion
Several studies of morphometric index are performed worldwide, the vast majority directly related to the breed characterization and conformation [
10,
20,
24,
35,
36], but the use of zoometric measures as a strategy to facilitate the implementation of conservation policies aimed to ensure local resources survival, is still beginning in avian populations [
37], particularly in Portugal [
11,
13,
38].
The morphometric measurements show highly significant sexual dimorphism, due to hormonal growth effects [
39], in accordance with several authors [
17,
18,
19,
20,
22,
24,
40,
41,
42,
43,
44]. Breed had significant effect (
p ≤ 0.05) in males, with the superiority of the “Branca” roosters, being the heaviest, largest and with the highest shank diameter and the “Preta Lusitânica” roosters, the lightest, shortest and with the smallest chest circumference.
Concerning the females, no evident differentiations between breeds were observed, although the “Branca” hen presented the largest diameter and shortest shank. The “Amarela” was the shortest hen, indicating the lower potential for egg production, as body and dorsal lengths along with head length are relevant indicators to measure productivity [
45,
46].
The results observed also reflect the breeds high rusticity and the recent implementation of the selection and breeding programs. Complementary in agricultural production, breed in traditional systems well adapted to the environment, with low nutritional requirements and productivity [
20,
37,
47], these populations, naturally or due to the absence of human intervention, maintained their particular ancestral characteristics [
48,
49].
The “Branca” revealed to be a strongly built breed, mainly the roosters, and with robust legs. The dimensions of the leg have been related with the type of production, with animals presenting higher dimensions (both in width and length), being more appropriate for meat production and carnic breeds characteristic [
44]. On the other hand, “Branca” breeding program began later, only in 2014, and, for decades, its morphological and productive characteristics and color plumage, led to crossbreeding and higher genetic proximity with commercial lines, heavier and with thick hips.
The “Preta Lusitânica” breed was the smallest hen (shorter, lighter, with a smaller chest circumference) hen, reflecting the population with the least productive characteristics, meat and eggs, more used to cultural and religious practices. Without intervention of selection programs and less human action, this breed is genetically closer to the avian populations’ ancestor [
12].
Zoometric measures are, generally, stabilized during the first year of age, allowing for the biometric characterization and to contribute to the selection so that breeding programs could be carried out during this period of life. In roosters, the increase of the body weight is accomplished with the strengthening of the shank, and concerning females, beyond the body weight increase, more evident differences were verified in the stronger and robust breeds, “Branca” and “Pedrês Portuguesa”, with chest and wing enlargement. The chest circumference variable proved to be a good indicator of meatiness in most poultry species [
45,
50].
Morphological traits are essential to implement breeding programs and dimensions of certain morphological variables could make them suitable, from a productive point of view, for meat (breast measurement) or egg productions (body and dorsal lengths, head length) [
19,
22,
24,
27,
37,
51]. Autochthonous Portuguese hens present morphological traits which would made them more prone to meat production (“Branca”), although the dimensions of certain morphological variables could make them suitable for double-purpose production (“Pedrês Portuguesa” and “Amarela”) and is important to consider a breeding programs adjustment to underline their productive potential [
36,
44,
46].
The positive and significant correlations among the body measurements observed in all the groups (total, male and female) indicate high predictability among the variables [
34,
52,
53].
The positive relationship between body weight and the body measurements showed that body weight can be predicted from body measurements in fowls [
21,
33]. The values of communalities computed for all groups confirm that PCA was appropriate for the data sets and the range of communalities (0.643–0.904; 0.546–0.847; 0.360–0.743) were similar (fowl), slightly inferior (males) or inferior to those reported for body measurements of broilers [
39,
53,
54]. The lower communality observed for shank diameter (0.360) and body length (0.625) in hens interpret the body parameters’ weakness in the body measurements total variation explanation.
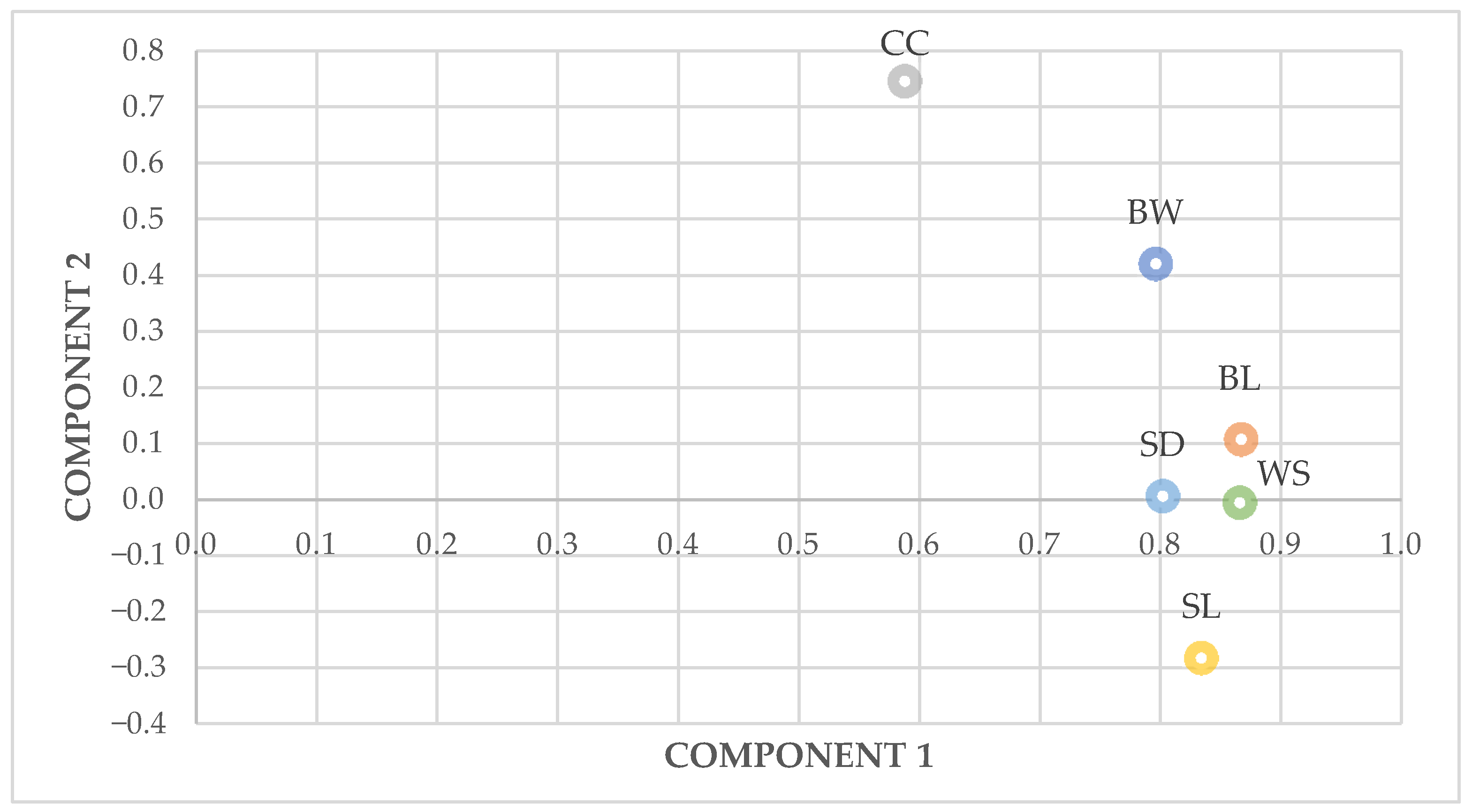
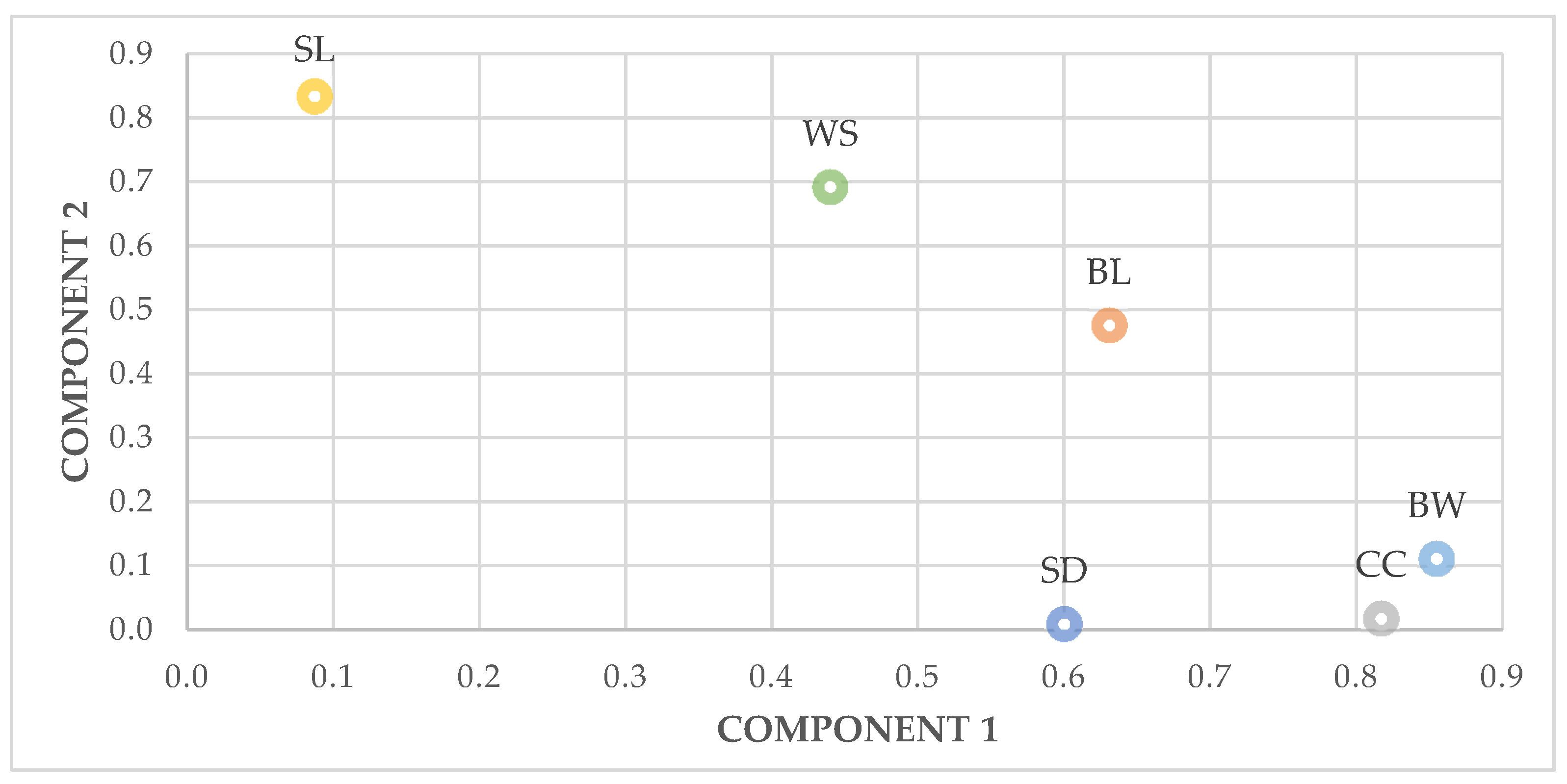
PC1 showed that the morphological traits’ variables explain the largest share of total variance, mainly in total and male groups, correlated highly with body length and wingspan, and could be described as “form factor” [
39]. In a principal component analysis of body measurements of hens, with PC1 less accounted for the largest variance in the body measurements, high positive loadings on body weight, chest circumference and body length, according to Mendes [
54] that reported PC1 high correlation with breast circumference and body weight of Ross 308 broilers. The presence of wide ranges of variation within hens could be explained to the different conditions of human intervention, whether for cultural reasons, in the productive system, or to the agroecological resources adaptation.
5. Conclusions
The results revealed the high positive correlations between morphometric measurements and its advantages to define conformation, providing a simple practical methodological framework suited for management, characterization and conservation, to be used in breeding and selection programs.
Portuguese autochthonous poultry breeds are of incalculable ancestral value. These recent selection strategies aim to conciliate the indigenous breeding resistance and adaptation to productive potential. The small size of the population and a strong directional selection may greatly affect the genetic diversity, reinforcing the need for definition and characterization.
Policies that support rural livelihoods, promote local genetic resources and value sustainable products, are a contemporary society requirement. Technical data, such as zoometric measures as a tool in biometric characterization supporting either national or international breeding programs, and scientific strategies is mandatory in the implementation of autochthonous breeds selection programs.