Approaches to Identify Pregnancy Failure in Buffalo Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Pregnancy Diagnosis
2.3. Progesterone Radioimmunoassay
2.4. PAGs Radioimmunoassay
2.5. Statistical Analysis
3. Results
3.1. Ultrasound Observations and Embryo Mortality
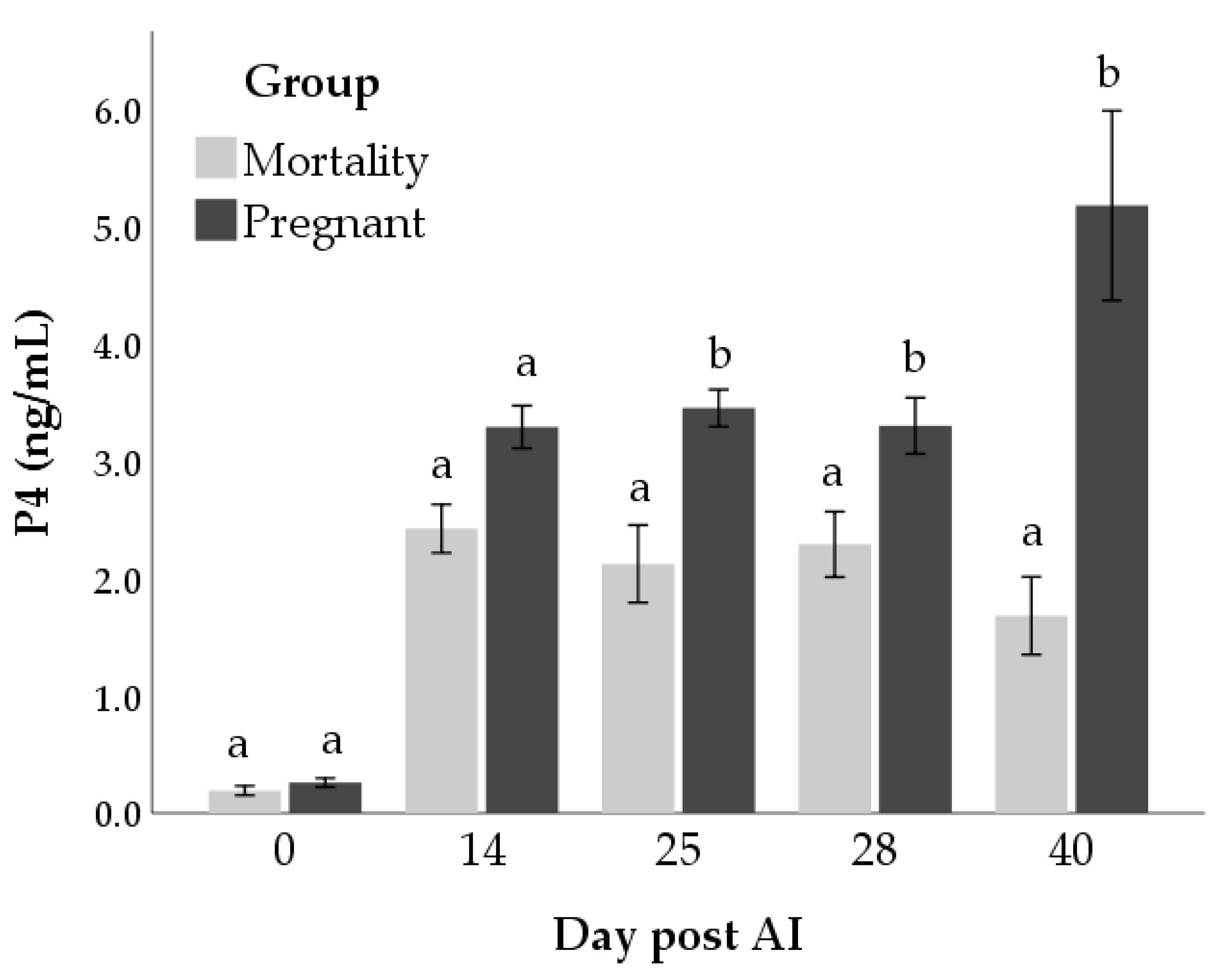
3.2. P4 Concentrations and Mortality
3.3. PAGs Concentrations and Mortality
3.4. Association between Ultrasound Outcome and PAGs Concentrations
3.5. Univariate Models Determining Predictors of Mortality at Days 25 and 28 Post-AI
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khatib, H.; Huang, W.; Mikheil, D.; Schutzkus, V.; Monson, R. Effects of signal transducer and activator of transcription (STAT) genes STAT1 and STAT3 genotypic combinations on fertilization and embryonic survival rates in Holstein cattle. J. Dairy Sci. 2009, 92, 6186–6191. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, M.C.; Baez, G.M.; Garcia-Guerra, A.; Toledo, M.Z.; Monteiro, P.L.; Melo, L.F.; Ochoa, J.C.; Santos, J.E.; Sartori, R. Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 2016, 86, 239–253. [Google Scholar] [CrossRef]
- Fricke, P.M.; Ricci, A.; Giordano, J.O.; Carvalho, P.D. Methods for and implementation of pregnancy diagnosis in dairy cows. Vet. Clin. Food Anim. Pract. 2016, 32, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Thatcher, W.; Chebel, R.; Cerri, R.; Galvao, K. The effect of embryonic death rates in cattle on the efficacy of estrus synchronization programs. Anim. Reprod. Sci. 2004, 82, 513–535. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Williams, E.; Evans, A. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Barile, V.L. Reproductive efficiency in female buffaloes. In Buffalo Production and Research; Borghese, A., Ed.; REU Technical Series 67; FAO: Roma, Italy, 2005; pp. 77–108. Available online: http://ftp.fao.org/docrep/fao/010/ah847e/ah847e.pdf (accessed on 16 December 2020).
- Barile, V.; Terzano, G.; Pacelli, C.; Todini, L.; Malfatti, A.; Barbato, O. LH peak and ovulation after two different estrus synchronization treatments in buffalo cows in the daylight-lengthening period. Theriogenology 2015, 84, 286–293. [Google Scholar] [CrossRef]
- Campanile, G.; Neglia, G.; Gasparrini, B.; Galiero, G.; Prandi, A.; Di Palo, R.; Michael, J.; Zicarelli, L. Embryonic mortality in buffaloes synchronized and mated by AI during the seasonal decline in reproductive function. Theriogenology 2005, 63, 2334–2340. [Google Scholar] [CrossRef]
- Campanile, G.; Neglia, G. Embryonic mortality in buffalo cows. Ital. J. Anim. Sci. 2007, 6, 119–129. [Google Scholar] [CrossRef]
- Baruselli, P.; Visintin, J.; Barnabe, V.; Barnabe, R.; Amaral, R.; Souza, A. Early pregnancy ultrasonography and embryonic mortality occurence in buffalo. In Proceedings of the V World Buffalo Congress, Caserta, Italy, 13–18 October 1997; pp. 13–16. [Google Scholar]
- Vale, W.; Ohashi, O.; Sousa, J.; Ribeiro, H.; Silva, A.; Nanba, S. Morte embrionaria e fetal em bufalos, Bubalus bubalis Lin. Rev. Bras. Reprod. Anim. 1989, 13, 157–165. [Google Scholar]
- Hansel, W.; Spalding, R.W.; Larson, L.L.; Laster, D.B.; Wagner, J.F.; Braun, R.K. Influence of human chorionic gonadotropin on pregnancy rates in lactating dairy and beef cows. J. Dairy Sci. 1976, 59, 751–754. [Google Scholar] [CrossRef]
- Morris, D.G.; Grealy, M.; Leese, H.; Diskin, M.G.; Sreenan, J. Cattle Embryo Growth Development and Viabilty; Teagasc: Dublin, Ireland, 2001; ISBN 1-84170-224-2. [Google Scholar]
- Fricke, P. Scanning the future—Ultrasonography as a reproductive management tool for dairy cattle. J. Dairy Sci. 2002, 85, 1918–1926. [Google Scholar] [CrossRef]
- Balhara, A.K.; Gupta, M.; Singh, S.; Mohanty, A.K.; Singh, I. Early Pregnancy Diagnosis in Bovines: Current Status and Future Directions. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Pohler, K.G.; Pereira, M.H.C.; Lopes, F.R.; Lawrence, J.C.; Keisler, D.H.; Smith, M.F.; Vasconcelos, J.L.M.; Green, J.A. Circulating concentrations of bovine pregnancy-associated glycoproteins and late embryonic mortality in lactating dairy herds. J. Dairy Sci. 2016, 99, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Pawshe, C.H.; Appa Rao, K.B.C.; Totey, S.M. Ultrasonographic imaging to monitor early pregnancy and embryonic development in the buffalo (Bubalus bubalis). Theriogenology 1994, 41, 697–709. [Google Scholar] [CrossRef]
- Ali, A.; Fahmy, S. Ultrasonographic fetometry and determination of fetal sex in buffaloes (Bubalus bubalis). Anim. Reprod. Sci. 2008, 106, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bhosreker, M.R.; Hangarge, I.M. Ultra sonography for early pregnancy diagnosis in buffaloes. Indian J. Anim. Reprod. 2000, 21, 143–144. [Google Scholar]
- Pinheiro Ferreira, J.C.P.; Martin, I.; Irikura, C.R.; Gimenes, L.U.; Fujihara, C.J.; Jorge, A.M.; Oba, E. Ultrasonographic monitoring of early pregnancy development in Murrah buffalo heifers (Bubalus bubalis). Livest. Sci. 2011, 138, 174–179. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, J.; Khanna, S.; Phulia, S.; Sarkar, S.K.; Singh, I. Fetal age determination in Murrah buffaloes from days 22 through 60 with ultrasonography. Indian J. Anim. Sci. 2012, 82, 374–376. [Google Scholar]
- Karen, A.; Darwish, S.; Ramoun, A.; Tawfeek, K.; Van Hanh, N.; De Sousa, N.; Sulon, J.; Szenci, O.; Beckers, J.-F. Accuracy of ultrasonography and pregnancy-associated glycoprotein test for pregnancy diagnosis in buffaloes. Theriogenology 2007, 68, 1150–1155. [Google Scholar] [CrossRef]
- López-Gatius, F.; García-Ispierto, I. Ultrasound and Endocrine Findings that Help to Assess the Risk of Late Embryo/Early Foetal Loss by Non-Infectious Cause in Dairy Cattle. Reprod. Domest. Anim. 2010, 45, 15–24. [Google Scholar] [CrossRef]
- Barbato, O.; Barile, V.L. The Pregnancy Diagnosis in Buffalo Species: Laboratory Methods. J. Buffalo Sci. 2012, 1, 157–162. [Google Scholar] [CrossRef]
- Arora, R.; Pandey, R. Changes in peripheral plasma concentrations of progesterone, estradiol-17β, and luteinizing hormone during pregnancy and around parturition in the buffalo (Bubalus bubalis). Gen. Comp. Endocrinol. 1982, 48, 403–410. [Google Scholar] [CrossRef]
- Kaul, V.; Prakash, B.S. Accuracy of pregnancy/non pregnancy diagnosis in zebu and crossbred cattle and Murrah buffaloes by milk progesterone determination post insemination. Trop. Anim. Health Prod. 1994, 26, 187–192. [Google Scholar] [CrossRef]
- Batra, S.K.; Prakash, B.S.; Madan, M.L. Relationship of progesterone. Trop. Anim. Health Prod. 1993, 25, 185–192. [Google Scholar]
- Starbuck, M.J.; Dailey, R.A.; Inskeep, E.K. Factors affecting retention of early pregnancy in dairy cattle. Anim. Reprod. Sci. 2004, 84, 27–39. [Google Scholar] [CrossRef]
- Zoli, A.P.; Beckers, J.F.; Wouters-Ballman, P.; Closset, J.; Falmagne, P.; Ectors, F. Purification and Characterization of a Bovine Pregnancy-Associated Glycoprotein. Biol. Reprod. 1991, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, J.M.; Serrano, B.; Lopez-Gatius, F. Identification of novel pregnancy-associated glycoproteins (PAG) expressed by the peri-implantation conceptus of domestic ruminants. Anim. Reprod. Sci. 2008, 103, 120–134. [Google Scholar] [CrossRef]
- Wallace, R.M.; Pohler, K.G.; Smith, M.F.; Green, J.A. Placental PAGs: Gene origins, expression patterns, and use as markers of pregnancy. Reproduction 2015, 149, R115–R126. [Google Scholar] [CrossRef]
- Zoli, A.P.; Guilbault, L.A.; Delahaut, P.; Ortiz, W.B.; Beckers, J.-F. Radioimmunoassay of a Bovine Pregnancy-Associated Glycoprotein in Serum: Its Application for Pregnancy Diagnosis. Biol. Reprod. 1992, 46, 83–92. [Google Scholar] [CrossRef]
- Touzard, E.; Reinaud, P.; Dubois, O.; Guyader-Joly, C.; Humblot, P.; Ponsart, C.; Charpigny, G. Specific expression patterns and cell distribution of ancient and modern PAG in bovine placenta during pregnancy. Reproduction 2013, 146, 347–362. [Google Scholar] [CrossRef]
- Szafranska, B.; Xie, S.; Green, J.; Roberts, R.M. Porcine pregnancy-associated glycoproteins: New members of the aspartic proteinase gene family expressed in trophectoderm. Biol. Reprod. 1995, 53, 21–28. [Google Scholar] [CrossRef]
- Xie, S.; Green, J.; Bixby, J.B.; Szafranska, B.; DeMartini, J.C.; Hecht, S.; Roberts, R.M. The diversity and evolutionary relationships of the pregnancy-associated glycoproteins, an aspartic proteinase subfamily consisting of many trophoblast-expressed genes. Proc. Natl. Acad. Sci. USA 1997, 94, 12809–12816. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, J.M.; Remy, B.; Alabart, J.L.; Folch, J.; Wattiez, R.; Falmagne, P.; Beckers, J.F. Isolation and Partial Characterization of a Pregnancy-Associated Glycoprotein Family from the Goat Placenta. Biol. Reprod. 1998, 58, 109–115. [Google Scholar] [CrossRef]
- Green, J.A.; Xie, S.; Quan, X.; Bao, B.; Gan, X.; Mathialagan, N.; Beckers, J.-F.; Roberts, R.M. Pregnancy-Associated Bovine and Ovine Glycoproteins Exhibit Spatially and Temporally Distinct Expression Patterns During Pregnancy1. Biol. Reprod. 2000, 62, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- El Amiri, B.; Sousa, N.M.; Alvarez Oxiley, A.; Hadarbach, D.; Beckers, J.-F. Pregnancy-associated glycoprotein (PAG) concentration in plasma and milk samples for early pregnancy diagnosis in Lacaune dairy sheep. Res. Vet. Sci. 2015, 99, 30–36. [Google Scholar] [CrossRef]
- Sousa, N.M.; Zongo, M.; Pitala, W.; Boly, H.; Sawadogo, L.; Sanon, M.; de Figueiredo, J.R.; Gonçalves, P.B.D.; El Amiri, B.; Perènyi, Z.; et al. Pregnancy-associated glycoprotein concentrations during pregnancy and the postpartum period in Azawak Zebu cattle. Theriogenology 2003, 59, 1131–1142. [Google Scholar] [CrossRef]
- Majewska, M.; Panasiewicz, G.; Majewski, M.; Szafranska, B. Localization of chorionic pregnancy-associated glycoprotein family in the pig. Reprod. Biol. 2006, 6, 205–230. [Google Scholar]
- Brandt, G.A.; Parks, T.E.; Killian, G.; Ealy, A.D.; Green, J.A. A cloning and expression analysis of pregnancy-associated glycoproteins expressed in trophoblasts of the white-tail deer placenta. Mol. Reprod. Dev. 2007, 74, 1355–1362. [Google Scholar] [CrossRef]
- Bériot, M.; Tchimbou, A.F.; Barbato, O.; Beckers, J.-F.; de Sousa, N.M. Identification of pregnancy-associated glycoproteins and alpha-fetoprotein in fallow deer (Dama dama) placenta. Acta Vet. Scand. 2014, 56, 4. [Google Scholar] [CrossRef]
- De Carolis, M.; Barbato, O.; Acuti, G.; Trabalza Marinucci, M.; de Sousa, N.M.; Canali, C.; Moscati, L. Plasmatic Profile of Pregnancy-Associated Glycoprotein (PAG) during Gestation and Postpartum in Sarda and Lacaune Sheep Determined with Two Radioimmunoassay Systems. Animals 2020, 10, 1502. [Google Scholar] [CrossRef] [PubMed]
- Barbato, O.; Sousa, N.M.; Klisch, K.; Clerget, E.; Debenedetti, A.; Barile, V.L.; Malfatti, A.; Beckers, J.F. Isolation of new pregnancy-associated glycoproteins from water buffalo (Bubalus bubalis) placenta by Vicia villosa affinity chromatography. Res. Vet. Sci. 2008, 85, 457–466. [Google Scholar] [CrossRef]
- Barbato, O.; Melo de Sousa, N.; Barile, V.L.; Canali, C.; Beckers, J.-F. Purification of pregnancy-associated glycoproteins from late-pregnancy Bubalus bubalis placentas and development of a radioimmunoassay for pregnancy diagnosis in water buffalo females. BMC Vet. Res. 2013, 9, 89. [Google Scholar] [CrossRef]
- Zoli, A.P.; Demez, P.; Beckers, J.-F.; Reznik, M.; Beckers, A. Light and Electron Microscopic Immunolocalization of Bovine Pregnancy-Associated Glycoprotein in the Bovine Placentome. Biol. Reprod. 1992, 46, 623–629. [Google Scholar] [CrossRef]
- Barbato, O.; Sousa, N.M.; Debenedetti, A.; Canali, C.; Todini, L.; Beckers, J.F. Validation of a new pregnancy-associated glycoprotein radioimmunoassay method for the detection of early pregnancy in ewes. Theriogenology 2009, 72, 993–1000. [Google Scholar] [CrossRef]
- Barbato, O.; Chiaradia, E.; Barile, V.L.; Pierri, F.; de Sousa, N.M.; Terracina, L.; Canali, C.; Avellini, L. Investigation into omocysteine, vitamin E and malondialdehyde as indicators of successful artificial insemination in synchronized buffalo cows (Bubalus bubalis). Res. Vet. Sci. 2016, 104, 100–105. [Google Scholar] [CrossRef]
- Barbato, O.; Menchetti, L.; Sousa, N.M.; Malfatti, A.; Brecchia, G.; Canali, C.; Beckers, J.F.; Barile, V.L. Pregnancy-associated glycoproteins (PAGs) concentrations in water buffaloes (Bubalus bubalis) during gestation and the postpartum period. Theriogenology 2017, 97, 73–77. [Google Scholar] [CrossRef]
- El-Battawy, K.A.; Sousa, N.M.; Szenci, O.; Beckers, J.F. Pregnancy-associated glycoprotein profile during the first trimester of pregnancy in Egyptian buffalo cows. Reprod. Domest. Anim. Zuchthyg. 2009, 44, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Barbato, O.; Bui, X.N.; Beckers, J.-F.; de Sousa, N.M. Assessment of pregnancy-associated glycoprotein (PAG) concentrations in swamp buffalo samples from fetal and maternal origins by using interspecies antisera. Anim. Sci. J. 2012, 83, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Guelfi, G.; Stefanetti, V.; De Luca, S.; Giontella, A.; Barile, V.L.; Barbato, O. Serum microRNAs in buffalo cows: Potential biomarkers of pregnancy. Res. Vet. Sci. 2017, 115, 294–300. [Google Scholar] [CrossRef]
- Barbato, O.; Guelfi, G.; Barile, V.; Menchetti, L.; Tortiello, C.; Canali, C.; Brecchia, G.; Traina, G.; Beckers, J.-F.; de Sousa, N.M. Using real-time PCR to identify pregnancy-associated glycoprotein 2 (PAG-2) in water buffalo (Bubalus bubalis) blood in early pregnancy. Theriogenology 2017, 89, 106–113. [Google Scholar] [CrossRef]
- Barbato, O.; Guelfi, G.; Menchetti, L.; Brecchia, G.; Melo de Sousa, N.; Canali, C.; Grandoni, F.; Scatà, M.C.; De Matteis, G.; Casano, A.B.; et al. Investigation of PAG2 mRNA Expression in Water Buffalo Peripheral Blood Mononuclear Cells and Polymorphonuclear Leukocytes from Maternal Blood at the Peri-Implantation Period. Vet. Sci. 2019, 6, 8. [Google Scholar] [CrossRef]
- Boiti, C.; Ceccarelli, P.; Beghelli, V.; Daniotti, P.; Pennisi, F. Messa a punto di un metodo per la determinazione radioimmunologica del progesterone plasmatico (A RIA method for plasma progesterone determination). Proc. Soc. Ital. Sci. Vet. 1974, 26, 366–371. [Google Scholar]
- Todini, L.; Malfatti, A.; Barbato, O.; Costarelli, S.; Debenedetti, A. Progesterone Plus PMSG Priming in Seasonally Anovulatory Lactating Sarda Ewes Exposed to the Ram Effect. J. Reprod. Dev. 2007, 53, 437–441. [Google Scholar] [CrossRef][Green Version]
- Greenwood, F.C.; Hunter, W.M.; Glover, J.S. The preparation of 131I-labelled human growth hormone of high specific radioactivity. Biochem. J. 1963, 89, 114–123. [Google Scholar] [CrossRef]
- Skelley, D.S.; Brown, L.P.; Besch, P.K. Radioimmunoassay. Clin. Chem. 1973, 19, 146–186. [Google Scholar] [CrossRef] [PubMed]
- Greiner, M.; Pfeiffer, D.; Smith, R.D. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 2000, 45, 23–41. [Google Scholar] [CrossRef]
- Pohler, K.G.; Reese, S.T.; Franco, G.A.; Oliveira, R.V.; Paiva, R.; Fernandez, L.; de Melo, G.; Vasconcelos, J.L.M.; Cooke, R.; Poole, R.K. New approaches to diagnose and target reproductive failure in cattle. Anim. Reprod. 2020, 17. [Google Scholar] [CrossRef]
- Pope, W.F. Uterine Asynchrony: A Cause of Embryonic Loss12. Biol. Reprod. 1988, 39, 999–1003. [Google Scholar] [CrossRef]
- Kastelic, J.P.; Curran, S.; Ginther, O.J. Accuracy of ultrasonography for pregnancy diagnosis on days 10 to 22 in heifers. Theriogenology 1989, 31, 813–820. [Google Scholar] [CrossRef]
- Pierson, R.A.; Ginther, O.J. Ultrasonography for detection of pregnancy and study of embryonic development in heifers. Theriogenology 1984, 22, 225–233. [Google Scholar] [CrossRef]
- Curran, S.; Pierson, R.; Ginther, O. Ultrasonographic appearance of the bovine conceptus from days 10 through 20. J. Am. Vet. Med. Assoc. 1986, 189, 1295–1302. [Google Scholar]
- Kastelic, J.; Curran, S.; Pierson, R.; Ginther, O. Ultrasonic evaluation of the bovine conceptus. Theriogenology 1988, 29, 39–54. [Google Scholar] [CrossRef]
- Vecchio, D.; Di Palo, R.; Zicarelli, L.; Grassi, C.; Cammarano, A.; D‘Occhio, M.; Campanile, G. Embryonic mortality in buffalo naturally mated. Ital. J. Anim. Sci. 2007, 6, 677–679. [Google Scholar] [CrossRef]
- Naikoo, M.; Patel, D.; Derashri, H. Early pregnancy diagnosis by transrectal ultrasonography in Mehsana buffaloes (Bubalus bubalis). Buffalo Bull. 2013, 32, 120–125. [Google Scholar]
- Reese, S.T.; Geary, T.W.; Franco, G.A.; Moraes, J.G.N.; Spencer, T.E.; Pohler, K.G. Pregnancy associated glycoproteins (PAGs) and pregnancy loss in high vs sub fertility heifers. Theriogenology 2019, 135, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms Controlling the Function and Life Span of the Corpus Luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef]
- Inskeep, E.K. Preovulatory, postovulatory, and postmaternal recognition effects of concentrations of progesterone on embryonic survival in the cow1,2. J. Anim. Sci. 2004, 82, E24–E39. [Google Scholar] [CrossRef] [PubMed]
- Barile, V.; Terzano, G.; Allegrini, S.; Maschio, M.; Razzano, M.; Neglia, G.; Pacelli, C. Relationship among preovulatory follicle, corpus luteum and progesterone in oestrus synchronized buffaloes. Ital. J. Anim. Sci. 2007, 6, 663–666. [Google Scholar] [CrossRef]
- Campanile, G.; Neglia, G.; Michael, J. Embryonic and fetal mortality in river buffalo (Bubalus bubalis). Theriogenology 2016, 86, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, M.C.; Szenci, O.; Willemse, A.H.; Bajcsy, C.S.A.; Dieleman, S.J.; Taverne, M.A.M. Early pregnancy diagnosis in cattle by means of linear-array real-time ultrasound scanning of the uterus and a qualitative and quantitative milk progesterone test. Theriogenology 1990, 33, 697–707. [Google Scholar] [CrossRef]
- Humblot, F.; Camous, S.; Martal, J.; Charlery, J.; Jeanguyot, N.; Thibier, M.; Sasser, R.G. Pregnancy-specific protein B, progesterone concentrations and embryonic mortality during early pregnancy in dairy cows. J. Reprod. Fertil. 1988, 83, 215–223. [Google Scholar] [CrossRef]
- Pohler, K.G.; Geary, T.W.; Johnson, C.L.; Atkins, J.A.; Jinks, E.M.; Busch, D.C.; Green, J.A.; MacNeil, M.D.; Smith, M.F. Circulating bovine pregnancy associated glycoproteins are associated with late embryonic/fetal survival but not ovulatory follicle size in suckled beef cows. J. Anim. Sci. 2013, 91, 4158–4167. [Google Scholar] [CrossRef]
- Pohler, K.G.; Green, J.A.; Geary, T.W.; Peres, R.F.G.; Pereira, M.H.C.; Vasconcelos, J.L.M.; Smith, M.F. Predicting Embryo Presence and Viability. In Regulation of Implantation and Establishment of Pregnancy in Mammals: Tribute to 45 Year Anniversary of Roger V. Short’s “Maternal Recognition of Pregnancy”; Geisert, R.D., Bazer, F.W., Eds.; Advances in Anatomy, Embryology and Cell Biology; Springer International Publishing: Cham, Switzerland, 2015; pp. 253–270. ISBN 978-3-319-15856-3. [Google Scholar]
- Kindahl, H.; Kornmatitsuk, B.; Königsson, K.; Gustafsson, H. Endocrine changes in late bovine pregnancy with special emphasis on fetal well-being. Domest. Anim. Endocrinol. 2002, 23, 321–328. [Google Scholar] [CrossRef]
- Kornmatitsuk, B.; Veronesi, M.C.; Madej, A.; Dahl, E.; Ropstad, E.; Beckers, J.F.; Forsberg, M.; Gustafsson, H.; Kindahl, H. Hormonal measurements in late pregnancy and parturition in dairy cows—Possible tools to monitor foetal well being. Anim. Reprod. Sci. 2002, 72, 153–164. [Google Scholar] [CrossRef]
- Dobson, H.; Rowan, T.G.; Kippax, I.S.; Humblot, P. Assessment of fetal number, and fetal and placental viability throughout pregnancy in cattle. Theriogenology 1993, 40, 411–425. [Google Scholar] [CrossRef]
- Patel, O.V.; Sulon, J.; Beckers, J.F.; Takahashi, T.; Hirako, M.; Sasaki, N.; Domeki, I. Plasma bovine pregnancy-associated glycoprotein concentrations throughout gestation in relationship to fetal number in the cow. Eur. J. Endocrinol. 1997, 137, 423–428. [Google Scholar] [CrossRef]
- Beckers, J.F.; Drion, P.V.; Garbayo, J.M.; Perény, Z.S.; Zarrouk, A.; Sulon, J.; Remy, B.; Szenci, O. Pregnancy Associated Glycoproteins in ruminants: Inctive members of the aspartic proteinase family. Acta Vet. Hung. 1999, 47, 461–469. [Google Scholar] [PubMed]
- Wooding, F. Structure and function of placental binucleate (giant) cells. Bibl. Anat. 1982, 22, 134–139. [Google Scholar]
- Klisch, K.; Jeanrond, E.; Pang, P.-C.; Pich, A.; Schuler, G.; Dantzer, V.; Kowalewski, M.P.; Dell, A. A Tetraantennary Glycan with Bisecting N-Acetylglucosamine and the Sda Antigen is the Predominant N-Glycan on Bovine Pregnancy-Associated Glycoproteins. Glycobiology 2007, 18, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Klisch, K.; Miglino, M.A.; Pereira, F.T.V.; Bevilacqua, E. Binucleate trophoblast giant cells in the water buffalo (Bubalus bubalis) placenta. J. Morphol. 2006, 267, 50–56. [Google Scholar] [CrossRef]
- Austin, K.J.; King, C.P.; Vierk, J.E.; Sasser, R.G.; Hansen, T.R. Pregnancy-Specific Protein B Induces Release of an Alpha Chemokine in Bovine Endometrium. Endocrinology 1999, 140, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, D.; Monfardini, E.; Opsomer, G.; Burvenich, C.; Dosogne, H.; de Kruif, A.; Beckers, J.-F. Chemiluminescence of bovine polymorphonuclear leucocytes during the periparturient period and relation with metabolic markers and bovine pregnancy-associated glycoprotein. J. Dairy Res. 2000, 67, 249–259. [Google Scholar] [CrossRef]
- Silva, E.; Sterry, R.A.; Kolb, D.; Mathialagan, N.; McGrath, M.F.; Ballam, J.M.; Fricke, P.M. Accuracy of a Pregnancy-Associated Glycoprotein ELISA to Determine Pregnancy Status of Lactating Dairy Cows Twenty-Seven Days After Timed Artificial Insemination. J. Dairy Sci. 2007, 90, 4612–4622. [Google Scholar] [CrossRef]
- Romano, J.E.; Larson, J.E. Accuracy of pregnancy specific protein-B test for early pregnancy diagnosis in dairy cattle. Theriogenology 2010, 74, 932–939. [Google Scholar] [CrossRef]
- Oliveira Filho, R.; Franco, G.; Reese, S.; Dantas, F.; Fontes, P.; Cooke, R.; Rhinehart, J.; Thompson, K.; Pohler, K. Using pregnancy associated glycoproteins (PAG) for pregnancy detection at day 24 of gestation in beef cattle. Theriogenology 2020, 141, 128–133. [Google Scholar] [CrossRef]
- Barbato, O.; Menchetti, L.; Sousa, N.M.; Brecchia, G.; Malfatti, A.; Canali, C.; Beckers, J.-F.; Barile, V.L. Correlation of two radioimmunoassay systems for measuring plasma pregnancy-associated glycoproteins concentrations during early pregnancy and postpartum periods in water buffalo. Reprod. Domest. Anim. 2018, 53, 1483–1490. [Google Scholar] [CrossRef]
- Pohler, K.G.; Peres, R.F.G.; Green, J.A.; Graff, H.; Martins, T.; Vasconcelos, J.L.M.; Smith, M.F. Use of bovine pregnancy-associated glycoproteins to predict late embryonic mortality in postpartum Nelore beef cows. Theriogenology 2016, 85, 1652–1659. [Google Scholar] [CrossRef]
- Gatea, A.O.; Smith, M.F.; Pohler, K.G.; Egen, T.; Pereira, M.H.C.; Vasconselos, J.L.M.; Lawrence, J.C.; Green, J.A. The ability to predict pregnancy loss in cattle with ELISAs that detect pregnancy associated glycoproteins is antibody dependent. Theriogenology 2018, 108, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Szenci, O.; Humblot, P.; Beckers, J.F.; Sasser, G.; Sulon, J.; Baltusen, R.; Varga, J.; Bajcsy, C.S.A.; Taverne, M.A.M. Plasma Profiles of Progesterone and Conceptus Proteins in Cows with Spontaneous Embryonic/Fetal Mortality as Diagnosed by Ultrasonography. Vet. J. 2000, 159, 287–290. [Google Scholar] [CrossRef]
- Thompson, I.M.; Cerri, R.L.A.; Kim, I.H.; Green, J.A.; Santos, J.E.P.; Thatcher, W.W. Effects of resynchronization programs on pregnancy per artificial insemination, progesterone, and pregnancy-associated glycoproteins in plasma of lactating dairy cows. J. Dairy Sci. 2010, 93, 4006–4018. [Google Scholar] [CrossRef]


| Day Post-AI | Ultrasound Observation | Outcome | Significance | |
|---|---|---|---|---|
| Mortality | Pregnant | |||
| 25 | No vesicle | 8 a (66.7%) | 4 b (8.0%) | 0.0001 |
| Vesicle | 1 a (8.3%) | 22 b (44.0%) | ||
| Vesicle + embryo | 1 a (8.3%) | 3 a (6.0%) | ||
| Vesicle + embryo + beat | 2 a (16.7%) | 21 b (42.0%) | ||
| 28 | No vesicle | 1 a (8.3%) | 0 a (0.0%) | 0.0001 |
| Vesicle | 2 a (16.7%) | 3 a (6.0%) | ||
| Vesicle + embryo | 3 b (25.0%) | 0 a (0.0%) | ||
| Vesicle + embryo + beat | 6 a (50.0%) | 47 b (94.0%) | ||
| 40 | No vesicle | 11 b (91.7%) | 0 a (0.0%) | 0.0001 |
| Vesicle + embryo | 1 a (8.3%) | 0 a (0.0%) | ||
| Vesicle + embryo + beat | 0 a (0.0%) | 50 b (100.0%) | ||
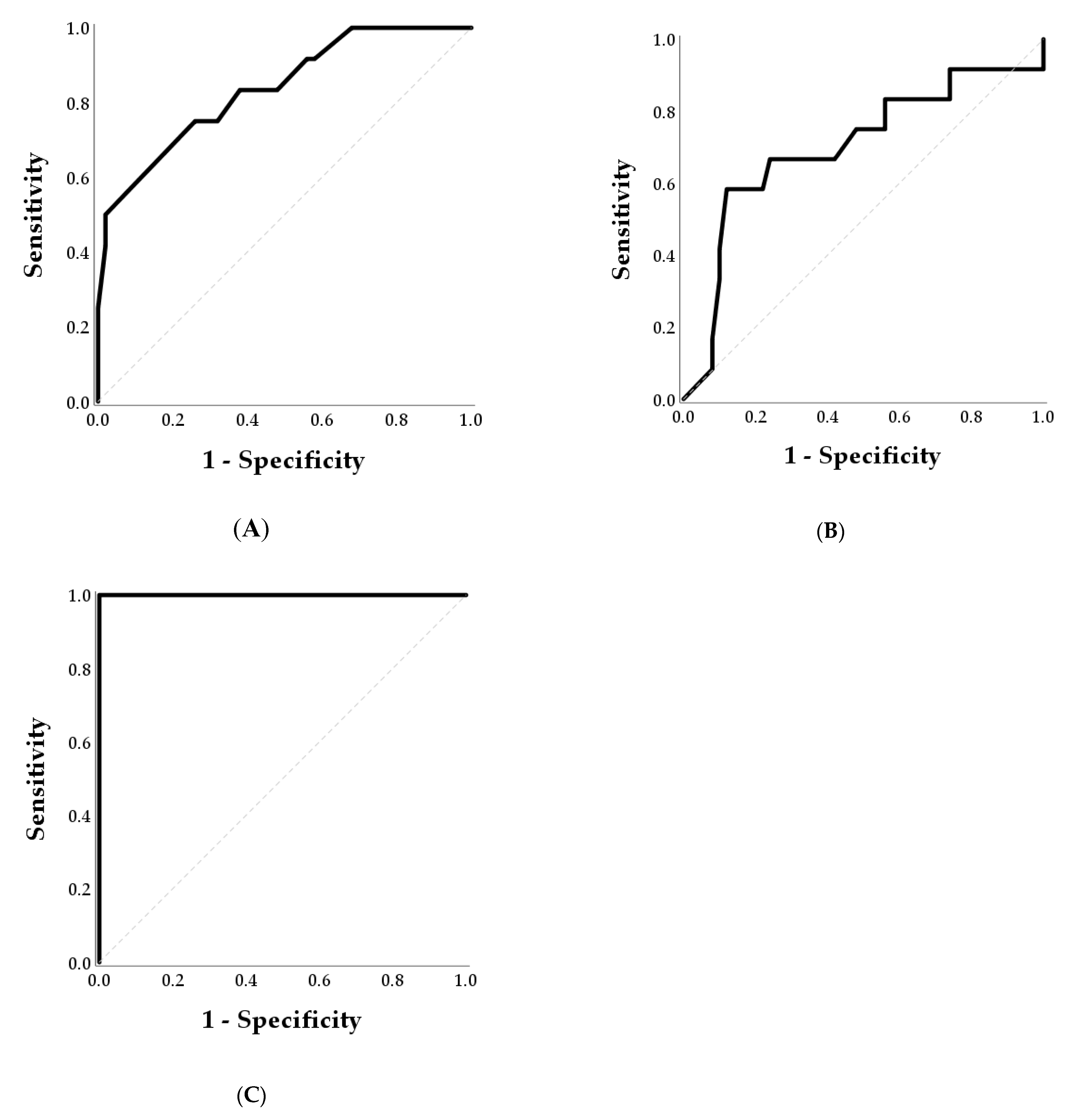
| Day | Parameter | AUC | 95% CI | p-Value | Cut-off (ng/mL) | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| 25 | PAG | 0.837 | 0.706–0.967 | <0.001 | 1.1 | 75 | 74 | 74 |
| P4 | 0.793 | 0.639–0.946 | 0.002 | 2.6 | 67 | 76 | 74 | |
| 28 | PAG | 0.700 | 0.516–0.884 | 0.033 | 2.2 | 67 | 76 | 74 |
| P4 | 0.722 | 0.537–0.906 | 0.018 | 2.6 | 75 | 66 | 68 | |
| 40 | PAG | 1.000 | 1.000–1.000 | <0.001 | 2.7 | 100 | 100 | 100 |
| P4 | 0.883 | 0.758–1000 | <0.001 | 2.4 | 83 | 86 | 85 |
| Day Post-AI | Predictor | OR | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| 25 | Ultrasound outcome | ||||||
| No embryo + beat vs. Embryo + beat | 3.621 | 0.717 | 18.272 | 0.119 | |||
| PAG concentrations ≤1.1 ng/mL vs. >1.1 ng/mL | 6.375 | 1.517 | 26.784 | 0.011 | |||
| P4 concentration ≤2.6 ng/mL vs. >2.6 ng/mL | 4.667 | 1.217 | 17.894 | 0.025 | |||
| 28 | Ultrasound outcome | ||||||
| No Embryo + beat vs. Embryo + beat | 15.667 | 3.083 | 79.613 | 0.001 | |||
| PAG concentrations ≤2.2 ng/mL vs. >2.2 ng/mL | 6.333 | 1.618 | 24.786 | 0.008 | |||
| P4 concentrations ≤2.6 ng/mL vs. >2.6 ng/mL | 4.895 | 1.176 | 20.372 | 0.029 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barile, V.L.; Menchetti, L.; Casano, A.B.; Brecchia, G.; Melo de Sousa, N.; Zelli, R.; Canali, C.; Beckers, J.F.; Barbato, O. Approaches to Identify Pregnancy Failure in Buffalo Cows. Animals 2021, 11, 487. https://doi.org/10.3390/ani11020487
Barile VL, Menchetti L, Casano AB, Brecchia G, Melo de Sousa N, Zelli R, Canali C, Beckers JF, Barbato O. Approaches to Identify Pregnancy Failure in Buffalo Cows. Animals. 2021; 11(2):487. https://doi.org/10.3390/ani11020487
Chicago/Turabian StyleBarile, Vittoria Lucia, Laura Menchetti, Anna Beatrice Casano, Gabriele Brecchia, Noelita Melo de Sousa, Riccardo Zelli, Claudio Canali, Jean François Beckers, and Olimpia Barbato. 2021. "Approaches to Identify Pregnancy Failure in Buffalo Cows" Animals 11, no. 2: 487. https://doi.org/10.3390/ani11020487
APA StyleBarile, V. L., Menchetti, L., Casano, A. B., Brecchia, G., Melo de Sousa, N., Zelli, R., Canali, C., Beckers, J. F., & Barbato, O. (2021). Approaches to Identify Pregnancy Failure in Buffalo Cows. Animals, 11(2), 487. https://doi.org/10.3390/ani11020487








