Effect of First Long-Term Training on Whole Blood Count and Blood Clotting Parameters in Thoroughbreds
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Animal Research
2.2. Animals
2.3. Sample Collection
2.4. Laboratory Analysis
2.4.1. Clotting Parameters
2.4.2. TAT Complex
2.5. Statistical Analysis
3. Results
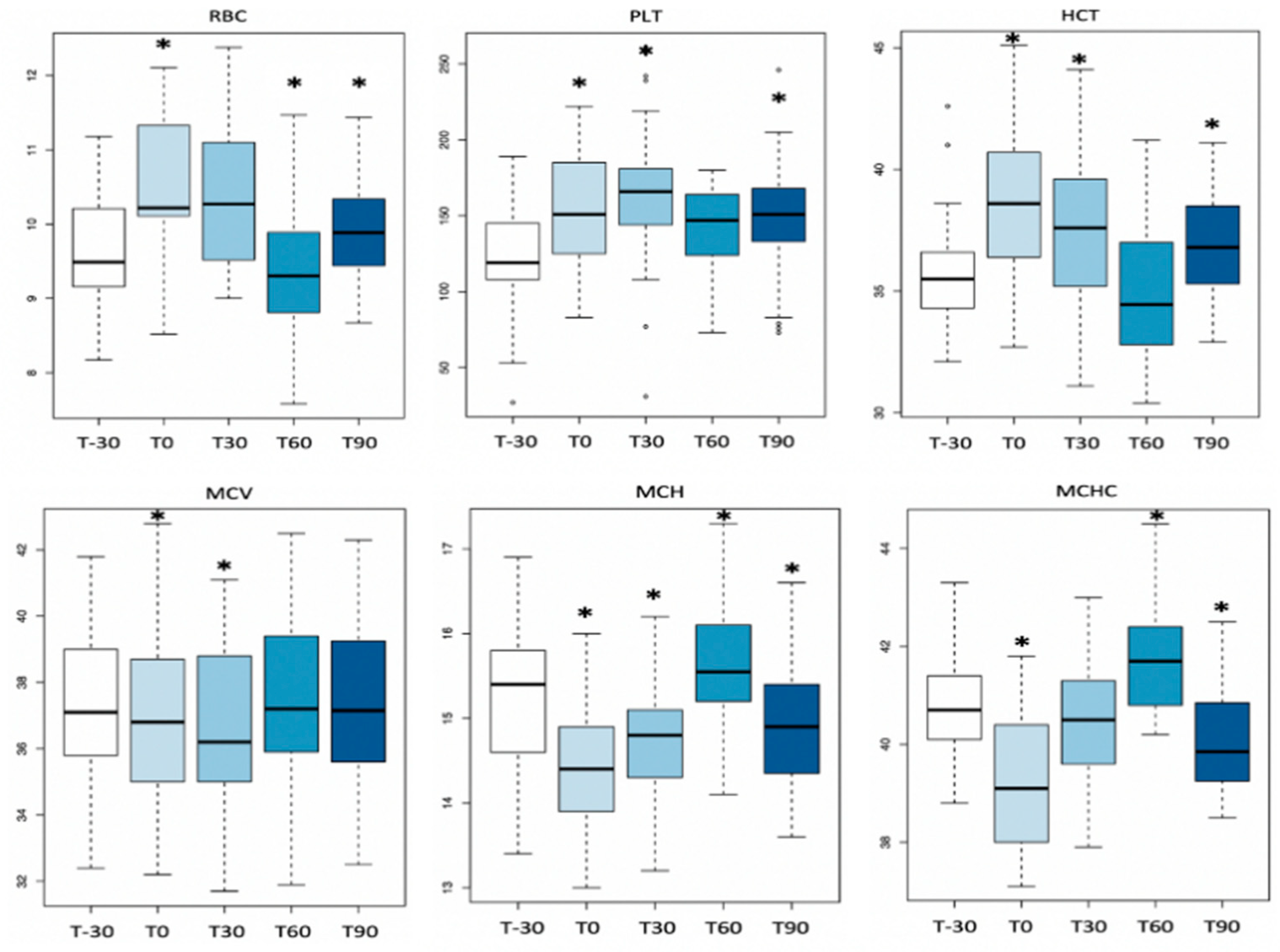
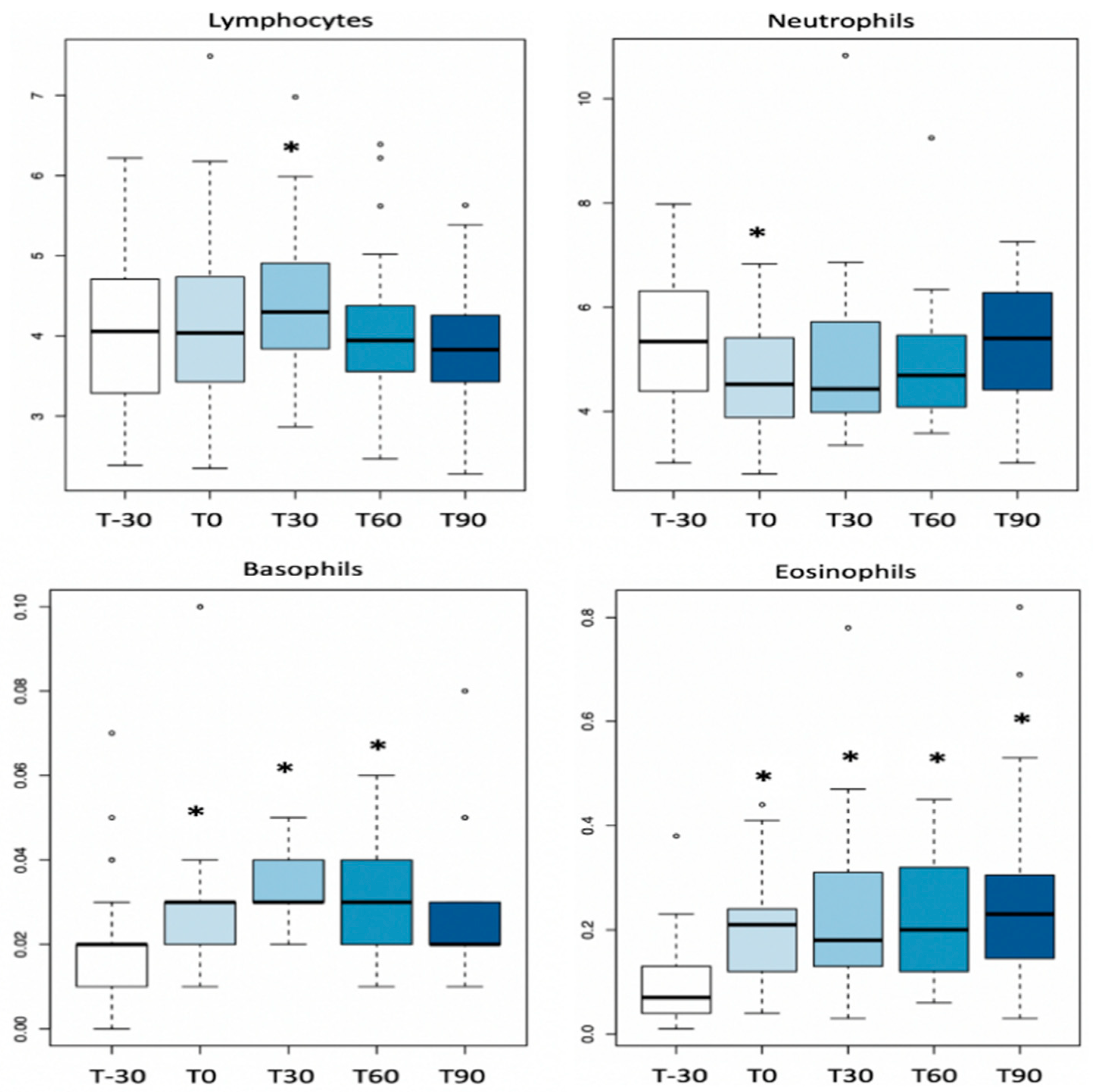
3.1. Complete Blood Count
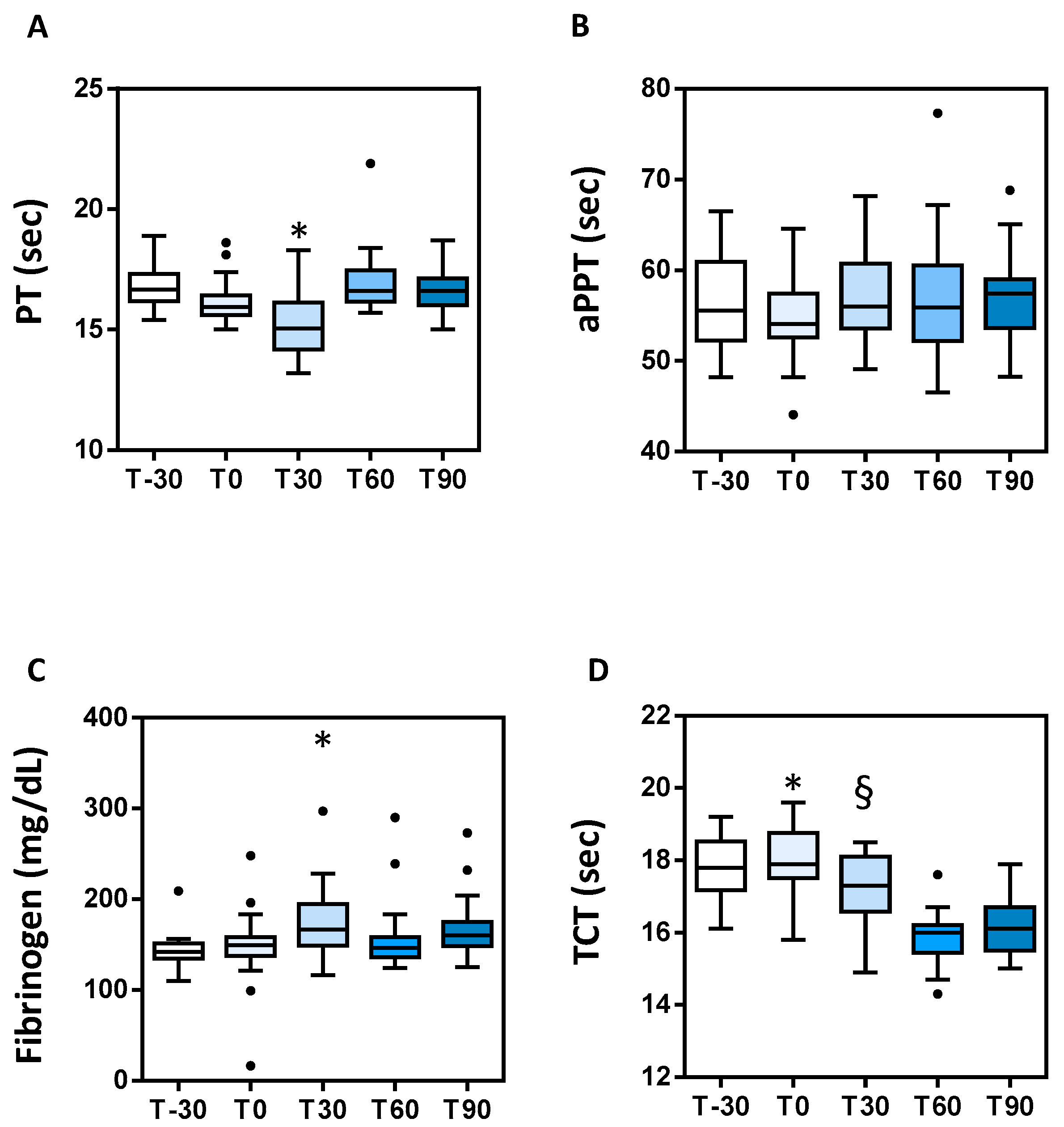
3.2. Clotting Parameters
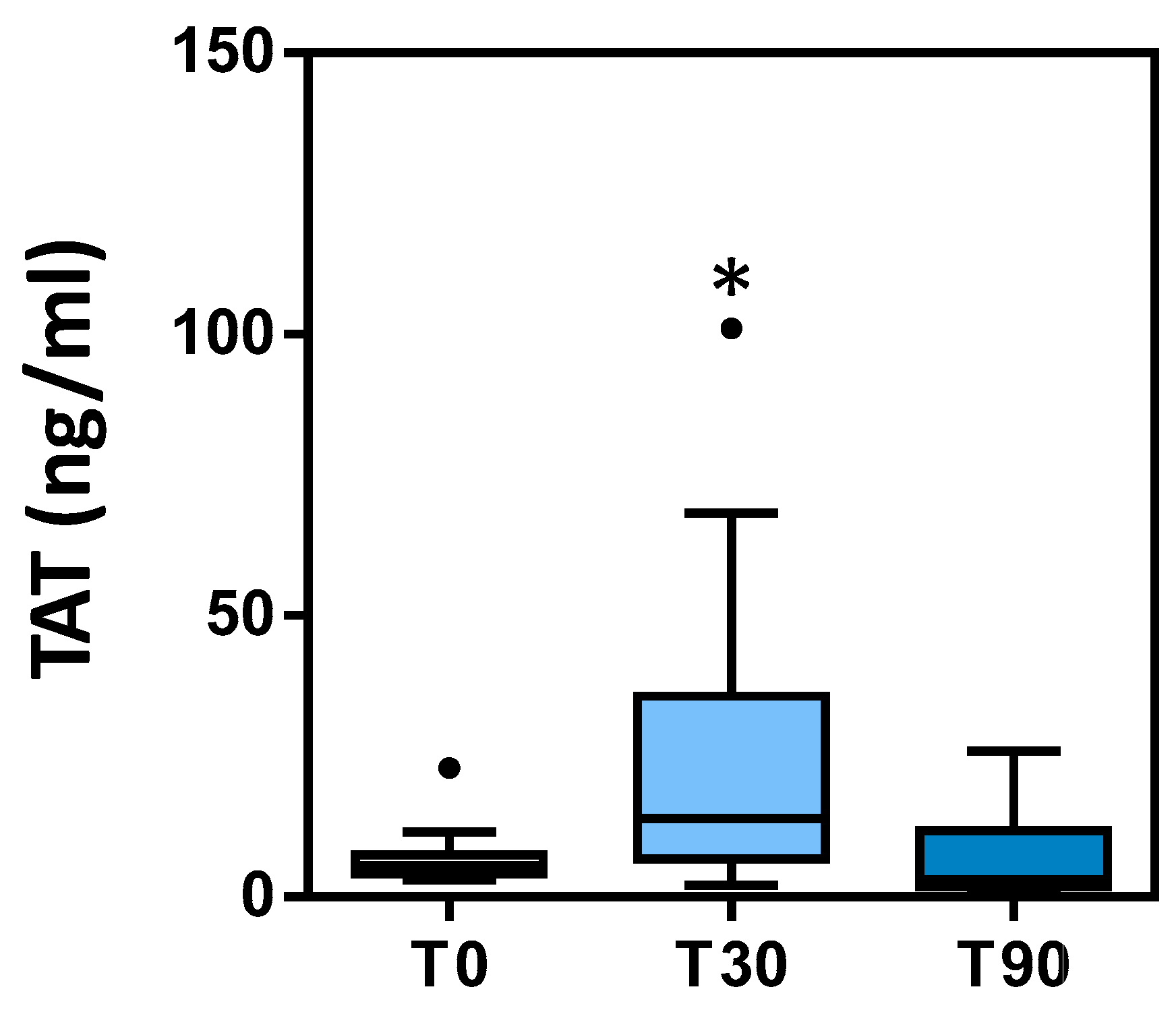
3.3. TAT Complex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Soroko, M.; Śpitalniak-Bajerska, K.; Zaborski, D.; Poźniak, B.; Dudek, K.; Janczarek, I. Exercise-Induced Changes in Skin Temperature and Blood Parameters in Horses. Arch. Anim. Breed. 2019, 62, 205–213. [Google Scholar] [CrossRef]
- Piccione, G.; Casella, S.; Giannetto, C.; Messina, V.; Monteverde, V.; Caola, G.; Guttadauro, S. Haematological and haematochemical responses to training and competition in standardbred horses. Comp. Haematol. Int. 2009, 19, 95–101. [Google Scholar] [CrossRef]
- Fazio, F.; Assenza, A.; Tosto, F.; Casella, S.; Piccione, G.; Caola, G. Modifications of some acute phase proteins and the white blood cell count in thoroughbreds during training. Veter. Rec. 2010, 167, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Miglio, A.; Cappelli, K.; Capomaccio, S.; Mecocci, S.; Silvestrelli, M.; Antognoni, M.T. Metabolic and Biomolecular Changes Induced by Incremental Long-Term Training in Young Thoroughbred Racehorses during First Workout Season. Animals 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.H.; Ricketts, S.W.; Mason, D.K. Haematological Response to Racing and Training Exercise in Thoroughbred Horses, with Particular Reference to the Leucocyte Response. Equine Vet. J. 1983, 15, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.J.; Allen, J.R. Hematologic Responses to Exercise and Training. Vet. Clin. N. Am. Equine Pract. 1985, 1, 461–476. [Google Scholar] [CrossRef]
- Assenza, A.; Tosto, F.; Casella, S.; Fazio, F.; Giannetto, C.; Piccione, G. Changes in blood coagulation induced by exercise training in young athletic horses. Res. Veter. Sci. 2013, 95, 1151–1154. [Google Scholar] [CrossRef]
- El-Sayed, M.S.; Ali, N.; El-Sayed Ali, Z. Aggregation and Activation of Blood Platelets in Exercise and Training. Sports Med. 2005, 35, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Arfuso, F.; Fazio, F.; Giudice, E.; Di Pietro, S.; Bruschetta, D.; Piccione, G. Different training schedules influence platelet aggregation in show jumping horses. Pol. J. Veter. Sci. 2017, 20, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Bazzano, M.; Giannetto, C.; Marafioti, S.; Fazio, F. Training-Induced Changes in Clotting Parameters of Athletic Horses. J. Vet. Sci. 2014, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Fazio, F.; Giudice, E.; Grasso, F.; Caola, G. Exercise-Induced Changes in the Clotting Times and Fibrinolytic Activity during Official 1600 and 2 000 Meters Trot Races in the Standardbred Horses. Acta Vet. Brno 2005, 74, 509–514. [Google Scholar] [CrossRef][Green Version]
- Tikhomirova, S.V.; Vikulov, A.D.; Baranov, A.A.; Osetrov, I.A. Plasma-Coagulation Hemostasis in Physically Active Subjects during Adaptation to Physical Exercise. Hum. Physiol. 2007, 33, 736–741. [Google Scholar] [CrossRef]
- Piccione, G.; Marafioti, S.; Giannetto, C.; Panzera, M.; Fazio, F. Effect of Dietary Supplementation with Omega 3 on Clotting Time, Fibrinogen Concentration and Platelet Aggregation in the Athletic Horse. Livest. Sci. 2014, 161, 109–113. [Google Scholar] [CrossRef]
- Giordano, A.; Meazza, C.; Salvadori, M.; Paltrinieri, S. Thromboelastometric Profiles of Horses Affected by Exercise-Induced Pulmonary Hemorrhages. Vet. Med. Int. 2010, 2010, 945789. [Google Scholar] [CrossRef] [PubMed]
- McKeever, K.H.; Hinchcliff, K.W.; Kociba, G.J.; Reed, S.M.; Muir, W.W. Changes in Coagulation and Fibrinolysis in Horses during Exercise. Am. J. Vet. Res. 1990, 51, 1335–1339. [Google Scholar] [PubMed]
- Monreal, L.; Angles, A.M.; Espada, Y.; Monasterio, J.; Monreal, M. Changes in haemostasis in endurance horses: Detection by highly sensitive ELISA-tests. Equine Veter. J. 2010, 27, 120–123. [Google Scholar] [CrossRef]
- Casella, S.; Giannetto, C.; Fazio, F.; Assenza, A.; Piccione, G. Nictemeral Profile of Platelet Aggregation and Clotting Parameters in Horses during Training. Bull Vet. Inst. Pulawy 2009, 53, 801–806. [Google Scholar]
- Kingston, J.K.; Sampson, S.N.; Beard, L.A.; Meyers, K.M.; Sellon, D.C.; Bayly, W.M. The Effect of Supramaximal Exercise on Equine Platelet Function. Equine Vet. J. Suppl. 2010, 31, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Assenza, A.; Casella, S.; Giannetto, C.; Tosto, F.; Caola, G. Modifications of Platelet Aggregation during Treadmill Section and Obstacle Course in Athletic Horse. Acta Veter. 2010, 60, 165–172. [Google Scholar] [CrossRef]
- Piccione, G.; Grasso, F.; Fazio, F.; Giudice, E. The effect of physical exercise on the daily rhythm of platelet aggregation and body temperature in horses. Veter. J. 2008, 176, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Karampour, S.; Gaeini, A. Response of coagulation and anti-coagulant factors of elite athletes following acute resistance and high-intensity interval training. J. Sports Med. Phys. Fit. 2017, 58, 120–126. [Google Scholar] [CrossRef]
- Zaar, M.; Herzig, M.C.; Fedyk, C.G.; Montgomery, R.K.; Prat, N.; Parida, B.K.; Hinojosa-Laborde, C.; Muniz, G.W.; Shade, R.E.; Bauer, C.; et al. Similar Hemostatic Responses to Hypovolemia Induced by Hemorrhage and Lower Body Negative Pressure Reveal a Hyperfibrinolytic Subset of Non-Human Primates. PLoS ONE 2020, 15, e0234844. [Google Scholar] [CrossRef]
- Wolf, M.F.; Girdhar, G.; Anderson, A.A.; Ubl, S.R.; Thinamany, S.; Jeffers, H.N.; DeRusha, C.E.; Rodriguez-Fernandez, J.; Hoffmann, S.; Strief, C.A. In vitro methodology for medical device material thrombogenicity assessments: A use condition and bioanalytical proof-of-concept approach. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Carlström, M.; Weitzberg, E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Rimpo, K.; Tanaka, A.; Ukai, M.; Ishikawa, Y.; Hirabayashi, M.; Shoyama, T. Thrombin-Antithrombin Complex Measurement Using a Point-of-Care Testing Device for Diagnosis of Disseminated Intravascular Coagulation in Dogs. PLoS ONE 2018, 13, e0205511. [Google Scholar] [CrossRef]
- Ravanat, C.; Freund, M.; Dol, F.; Cadroy, Y.; Roussi, J.; Incardona, F.; Maffrand, J.-P.; Boneu, B.; Drouet, L.; Legrand, C.; et al. Cross-reactivity of human molecular markers for detection of prethrombotic states in various animal species. Blood Coagul. Fibrinolysis 1995, 6, 446–455. [Google Scholar] [CrossRef]
- Maruyama, H.; Watari, T.; Miura, T.; Sakai, M.; Takahashi, T.; Koie, H.; Yamaya, Y.; Asano, K.; Edamura, K.; Sato, T.; et al. Plasma Thrombin-Antithrombin Complex Concentrations in Dogs with Malignant Tumours. Vet. Rec. 2005, 156, 839–840. [Google Scholar] [CrossRef]
- Topper, M.J.; Prasse, K.W.; Morris, M.J.; Duncan, A.; Crowe, N.A. Enzyme-Linked Immunosorbent Assay for Thrombin-Antithrombin III Complexes in Horses. Am. J. Vet. Res. 1996, 57, 427–431. [Google Scholar]
- Miglio, A.; Morelli, C.; Maresca, C.; Felici, A.; Di Giambattista, A.; Antognoni, M.T. Hematologic Reference Intervals for the Italian Heavy Draft Horse. Comp. Clin. Pathol. 2019, 28, 833–840. [Google Scholar] [CrossRef]
- Miglio, A.; Moscati, L.; Scoccia, E.; Maresca, C.; Antognoni, M.T.; Felici, A. Reference Values for Serum Amyloid A, Haptoglobin, Lysozyme, Zinc and Iron in Healthy Lactating Lacaune Sheep. Acta Vet. Scand 2018, 60, 46. [Google Scholar] [CrossRef] [PubMed]
- Epstein, K.L. Coagulopathies in Horses. Vet. Clin. N. Am. Equine Pract. 2014, 30, 437–452. [Google Scholar] [CrossRef]
- Nlme: Linear and Nonlinear Mixed Effects Models Version 3.1-147 from CRAN. Available online: https://rdrr.io/cran/nlme/ (accessed on 18 April 2020).
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research. 2020. Available online: https://cran.r-project.org/web/packages/psych/index.html (accessed on 18 October 2020).
- Miglio, A.; Antognoni, M.T.; Maresca, C.; Moncada, C.; Riondato, F.; Scoccia, E.; Mangili, V. Serum protein concentration and protein fractions in clinically healthly Lacaune and Sarda sheep using agarose gel electrophoresis. Vet. Clin. Pathol. 2015, 44, 564–569. [Google Scholar] [CrossRef]
- THRALL-WEISER-ALLISON-CAMPBELL-Veterinary Hematology and Clinical Chemistry-2° Ed.-EV-Editoria Scientifica-Libri Veterinaria. Available online: https://distribuzione.evsrl.it/ArticleDetail.aspx?lang=it&from=HP&id=4402 (accessed on 2 December 2020).
- Clinical Examination of Horses by Victor C. Speirs (1997, Hardcover) for Sale Online. Available online: https://www.ebay.com/p/801086 (accessed on 2 December 2020).
- Topper, M.J.; Prasse, K.W. Use of Enzyme-Linked Immunosorbent Assay to Measure Thrombin-Antithrombin III Complexes in Horses with Colic. Am. J. Vet. Res. 1996, 57, 456–462. [Google Scholar]
- Lai, S.; Tsai, K.; Lin, Y.; Liu, P.; Lin, Y.; Chang, P.; Dai, M.; Chao, T.; Han, C.; Lin, G. Association of red blood cell size and physical fitness in a military male cohort: The CHIEF study. Scand. J. Med. Sci. Sports 2021, 31, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Andriichuk, A.; Tkachenko, H. Effect of gender and exercise on haematological and biochemical parameters in Holsteiner horses. J. Anim. Physiol. Anim. Nutr. 2017, 101, e404–e413. [Google Scholar] [CrossRef] [PubMed]
- Hilberg, T.; Schmidt, V.; Lösche, W.; Gabriel, H.H.W. Platelet Activity and Sensitivity to Agonists after Exhaustive Treadmill Exercise. J. Sports Sci. Med. 2003, 2, 15–22. [Google Scholar]
- Baria, M.; Miller, M.M.; Borchers, J.; Desmond, S.; Onate, J.; Magnussen, R.; Vasileff, W.K.; Flanigan, D.; Kaeding, C.; Durgam, S. High Intensity Interval Exercise Increases Platelet and Transforming Growth Factor-β Yield in Platelet-Rich Plasma. PM&R 2020, 4, 129–135. [Google Scholar] [CrossRef]
- Heber, S.; Volf, I. Effects of Physical (In)Activity on Platelet Function. Biomed. Res. Int. 2015, 2015, 165078. [Google Scholar] [CrossRef]
- Cappelli, K.; Amadori, M.; Mecocci, S.; Miglio, A.; Antognoni, M.T.; Razzuoli, E. Immune Response in Young Thoroughbred Racehorses under Training. Animals 2020, 10, 1809. [Google Scholar] [CrossRef]
- Dos Santos, C.M.M.; Diniz, V.L.S.; Bachi, A.L.L.; dos Santos de Oliveira, L.C.; Ghazal, T.; Passos, M.E.P.; de Oliveira, H.H.; Murata, G.; Masi, L.N.; Martins, A.R.; et al. Moderate Physical Exercise Improves Lymphocyte Function in Melanoma-Bearing Mice on a High-Fat Diet. Nutr. Metab. 2019, 16, 63. [Google Scholar] [CrossRef]
- Sand, K.L.; Flatebo, T.; Andersen, M.B.; Maghazachi, A.A. Effects of Exercise on Leukocytosis and Blood Hemostasis in 800 Healthy Young Females and Males. World J. Exp. Med. 2013, 3, 11–20. [Google Scholar] [CrossRef]
- Witkowska-Pilaszewicz, O.; Pingwara, R.; Winnicka, A. The Effect of Physical Training on Peripheral Blood Mononuclear Cell Ex Vivo Proliferation, Differentiation, Activity, and Reactive Oxygen Species Production in Racehorses. Antioxidants 2020, 9, 1155. [Google Scholar] [CrossRef]
- Adamu, L.; Adzahan, N.M.; Abdullah, R.; Ahmad, B. Effects of Race Distance on Physical, Hematological and Biochemical Parameters of Endurance Horses. Am. J. Anim. Veter. Sci. 2010, 5, 244–248. [Google Scholar] [CrossRef][Green Version]
- Todoroki, N.; Watanabe, Y.; Akaike, T.; Katagiri, Y.; Tanoue, K.; Yamazaki, H.; Tsuji, T.; Toyoshima, S.; Osawa, T. Enhancement by IL-1 Beta and IFN-Gamma of Platelet Activation: Adhesion to Leukocytes via GMP-140/PADGEM Protein (CD62). Biochem. Biophys. Res. Commun. 1991, 179, 756–761. [Google Scholar] [CrossRef]
- Pitchford, S.C.; Yano, H.; Lever, R.; Riffo-Vasquez, Y.; Ciferri, S.; Rose, M.J.; Giannini, S.; Momi, S.; Spina, D.; O’connor, B.; et al. Platelets Are Essential for Leukocyte Recruitment in Allergic Inflammation. J. Allergy Clin. Immunol. 2003, 112, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ulfman, L.H.; Joosten, D.P.H.; Van Aalst, C.; Lammers, J.-W.; Van De Graaf, E.A.; Koenderman, L.; Zwaginga, J.J. Platelets Promote Eosinophil Adhesion of Patients with Asthma to Endothelium under Flow Conditions. Am. J. Respir. Cell Mol. Biol. 2003, 28, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Lin, H.-Y.; Cheng, M.-L.; Wong, A.M.-K. Chronic intermittent hypoxia modulates eosinophil- and neutrophil-platelet aggregation and inflammatory cytokine secretion caused by strenuous exercise in men. J. Appl. Physiol. 2007, 103, 305–314. [Google Scholar] [CrossRef]
- Petito, E.; Falcinelli, E.; Paliani, U.; Cesari, E.; Vaudo, G.; Sebastiano, M.; Cerotto, V.; Guglielmini, G.; Gori, F.; Malvestiti, M.; et al. Neutrophil More than Platelet Activation Associates with Thrombotic Complications in COVID-19 Patients. J. Infect. Dis 2020, 10, 261–269. [Google Scholar] [CrossRef]
- Riegger, J.; Byrne, R.A.; Joner, M.; Chandraratne, S.; Gershlick, A.H.; Ten Berg, J.M.; Adriaenssens, T.; Guagliumi, G.; Godschalk, T.C.; Neumann, F.-J.; et al. Histopathological Evaluation of Thrombus in Patients Presenting with Stent Thrombosis. A Multicenter European Study: A Report of the Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort Consortium. Eur. Heart J. 2016, 37, 1538–1549. [Google Scholar] [CrossRef]
- Moosbauer, C.; Morgenstern, E.; Cuvelier, S.L.; Manukyan, D.; Bidzhekov, K.; Albrecht, S.; Lohse, P.; Patel, K.D.; Engelmann, B. Eosinophils Are a Major Intravascular Location for Tissue Factor Storage and Exposure. Blood 2007, 109, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, O.J.T.; Tien, N.; Bochner, B.S.; Konstantopoulos, K. Exogenous eosinophil activation converts PSGL-1-dependent binding to CD18-dependent stable adhesion to platelets in shear flow. Am. J. Physiol. Physiol. 2003, 284, C1223–C1234. [Google Scholar] [CrossRef]
- Todd, S.; Hemmaway, C.; Nagy, Z. Catastrophic thrombosis in idiopathic hypereosinophilic syndrome. Br. J. Haematol. 2014, 165, 425. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, S.; Marr, K.; Lees, P.; Cunningham, F.; Page, C. Effects of platelet activating factor on the distribution of radiolabelled leucocytes and platelets in normal horses and asymptomatic horses with chronic obstructive pulmonary disease. Res. Veter. Sci. 1996, 61, 107–113. [Google Scholar] [CrossRef]
- Pitchford, S.C.; Momi, S.; Giannini, S.; Casali, L.; Spina, D.; Page, C.P.; Gresele, P. Platelet P-Selectin Is Required for Pulmonary Eosinophil and Lymphocyte Recruitment in a Murine Model of Allergic Inflammation. Blood 2005, 105, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Pitchford, S.C.; Riffo-Vasquez, Y.; Sousa, A.; Momi, S.; Gresele, P.; Spina, D.; Page, C.P. Platelets Are Necessary for Airway Wall Remodeling in a Murine Model of Chronic Allergic Inflammation. Blood 2004, 103, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Boisclair, M.D.; Ireland, H.; Lane, D.A. Assessment of Hypercoagulable States by Measurement of Activation Fragments and Peptides. Blood Rev. 1990, 4, 25–40. [Google Scholar] [CrossRef]
- Herren, T.; Bartsch, P.; Haeberli, A.; Straub, P.W. Increased thrombin-antithrombin III complexes after 1 h of physical exercise. J. Appl. Physiol. 1992, 73, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- Bärtsch, P.; Haeberli, A.; Straub, P.W. Blood Coagulation after Long Distance Running: Antithrombin III Prevents Fibrin Formation. Thromb. Haemost. 1990, 63, 430–434. [Google Scholar] [CrossRef]
- Miglio, A.; Antognoni, M.T.; Miniscalco, B.; Caivano, D.; Lepri, E.; Birettoni, F.; Mangili, V. Acute undifferentiated leukemia in a dog. Aust. Vet. J. 2014, 92, 499–503. [Google Scholar] [CrossRef]
- Weiss, D.; Monreal, L.; Angles, A.; Monasterio, J. Evaluation of thrombin-antithrombin complexes and fibrin fragment D in carbohydrate-induced acute laminitis. Res. Veter. Sci. 1996, 61, 157–159. [Google Scholar] [CrossRef]
| March (T-30) | April (T0) | May (T30) | June (T60) | July (T90) |
|---|---|---|---|---|
| 15 min Walk | 15 min Walk | 15 min Walk | 15 min Walk | 15 min Walk |
| 10 min Trot | 10 min Trot | 10 min Trot | 10 min Trot | 10 min Trot |
| Rest | 6 min Canter | 6 min Canter | 6 min Canter | 6 min Canter |
| 10 min Trot | Tuesday: | Tuesday: | Tuesday: | Tuesday: |
| Walk | 1 min Gallop | 2 min Gallop | 3 min Gallop | 4 min Gallop |
| Parameters | T-30 | T0 | T30 | T60 | T90 | RIs of 2-Year-Old Thoroughbred Horses in Training [29,31,34] |
|---|---|---|---|---|---|---|
| RBC (×1012/L) | 9.6 ± 0.7 | 10.4 ± 0.9 | 10.3 ± 0.9 | 9.4 ± 0.8 | 9.9 ± 0.70 | 8.7–11.7 |
| Hgb (g/dL) | 14.5 ± 1.1 | 15.0 ± 1.3 | 15.1 ± 1.3 | 14.6 ± 1.2 | 14.3 ± 2.0 | 12.8–16.6 |
| Hct (%) | 35 ± 2.0 | 38 ± 3.0 | 37 ± 3.0 | 35 ± 3.0 | 36 ± 2.0 | 34–45 |
| MCV (fL) | 37.2 ± 2.4 | 36.8 ± 2.6 | 36.3 ± 2.5 | 37.4 ± 2.5 | 37.0 ± 2.5 | 37.0–42.1 |
| MCH (pg) | 15.1 ± 0.8 | 14.4 ± 0.7 | 14.7 ± 0.7 | 15.5 ± 0.8 | 14.8 ± 0.7 | 13.7–15.7 |
| MCHC (mg/dL) | 40.7 ± 1.1 | 39.2 ± 1.3 | 40.5 ± 1.3 | 41.7 ± 1.0 | 40.1 ± 1.1 | 35.9–37.9 |
| PLT (×109/L) | 123 ± 37 | 151 ± 35 | 166 ± 52 | 142 ± 25 | 149 ± 42 | 127–206 |
| WBC (×109/L) | 9.9 ± 1.9 | 9.5 ± 1.6 | 10.2 ± 1.9 | 9.7 ± 1.5 | 9.9 ± 1.5 | 7.3–12.7 |
| Neutrophils (×109/L) | 5.3± 1.3 | 4.7 ± 1.1 | 5.0± 1.6 | 4.9 ± 1.2 | 5.2 ± 1.2 | 4.0–6.0 |
| Lymphocyte (×109/L) | 4.01 ± 0.92 | 4.16 ± 1.09 | 4.42 ± 0.97 | 4.06 ± 1.03 | 3.90 ± 0.78 | 2.7–4.4 |
| Monocytes (×109/L) | 0.45 ± 0.10 | 0.46 ± 0.11 | 0.49 ± 0.10 | 0.49 ± 0.12 | 0.49 ± 0.09 | 0.26–0.56 |
| Eosinophyls (×109/L) | 0.09 ± 0.07 | 0.19 ± 0.09 | 0.22 ± 0.16 | 0.22 ± 0.11 | 0.27 ± 0.18 | 0–0.3 |
| Basophyls (×109/L) | 0.02 ± 0.01 | 0.03± 0.01 | 0.03± 0.01 | 0.03± 0.01 | 0.02 ± 0.01 | 0–0.2 |
| Parameters | T-30 | T0 | T30 | T60 | T90 | Ris [34,35,36,37] |
|---|---|---|---|---|---|---|
| PT (s) | 16.8 ± 0.16 | 16.1 ± 0.14 | 15.26 ± 0.27 a | 16.91 ± 0.25 | 16.63 ± 0.18 | 10–17 |
| APTT (s) | 56.76 ± 0.90 | 54.97 ± 0.79 | 56.80 ± 0.91 | 56.84 ± 1.29 | 56.73 ± 1.09 | 27–58 |
| TCT (s) | 17.83 ± 0.14 | 18.08 ± 0.16 b | 17.26 ± 0.17 c | 15.86 ± 0.13 | 16.13 ± 0.14 | 15–19 |
| Fbg (mg/dL) | 142 ± 3.1 | 148.8 ± 6.5 | 172.8 ± 7.5 d | 155 ± 6.9 | 166.9 ± 6.4 | 100–400 |
| TAT (ng/mL) | ------- | 6.33 ± 0.5 | 23.44 ± 3.7 e | ------- | 7.03 ± 1.6 | 1.95–9.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miglio, A.; Falcinelli, E.; Mezzasoma, A.M.; Cappelli, K.; Mecocci, S.; Gresele, P.; Antognoni, M.T. Effect of First Long-Term Training on Whole Blood Count and Blood Clotting Parameters in Thoroughbreds. Animals 2021, 11, 447. https://doi.org/10.3390/ani11020447
Miglio A, Falcinelli E, Mezzasoma AM, Cappelli K, Mecocci S, Gresele P, Antognoni MT. Effect of First Long-Term Training on Whole Blood Count and Blood Clotting Parameters in Thoroughbreds. Animals. 2021; 11(2):447. https://doi.org/10.3390/ani11020447
Chicago/Turabian StyleMiglio, Arianna, Emanuela Falcinelli, Anna Maria Mezzasoma, Katia Cappelli, Samanta Mecocci, Paolo Gresele, and Maria Teresa Antognoni. 2021. "Effect of First Long-Term Training on Whole Blood Count and Blood Clotting Parameters in Thoroughbreds" Animals 11, no. 2: 447. https://doi.org/10.3390/ani11020447
APA StyleMiglio, A., Falcinelli, E., Mezzasoma, A. M., Cappelli, K., Mecocci, S., Gresele, P., & Antognoni, M. T. (2021). Effect of First Long-Term Training on Whole Blood Count and Blood Clotting Parameters in Thoroughbreds. Animals, 11(2), 447. https://doi.org/10.3390/ani11020447








