Efficiency of GnRH–Loaded Chitosan Nanoparticles for Inducing LH Secretion and Fertile Ovulations in Protocols for Artificial Insemination in Rabbit Does
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Manufacturing of Gonadotropin-Releasing Hormone (GnRH)-Loaded Chitosan Nanoparticles
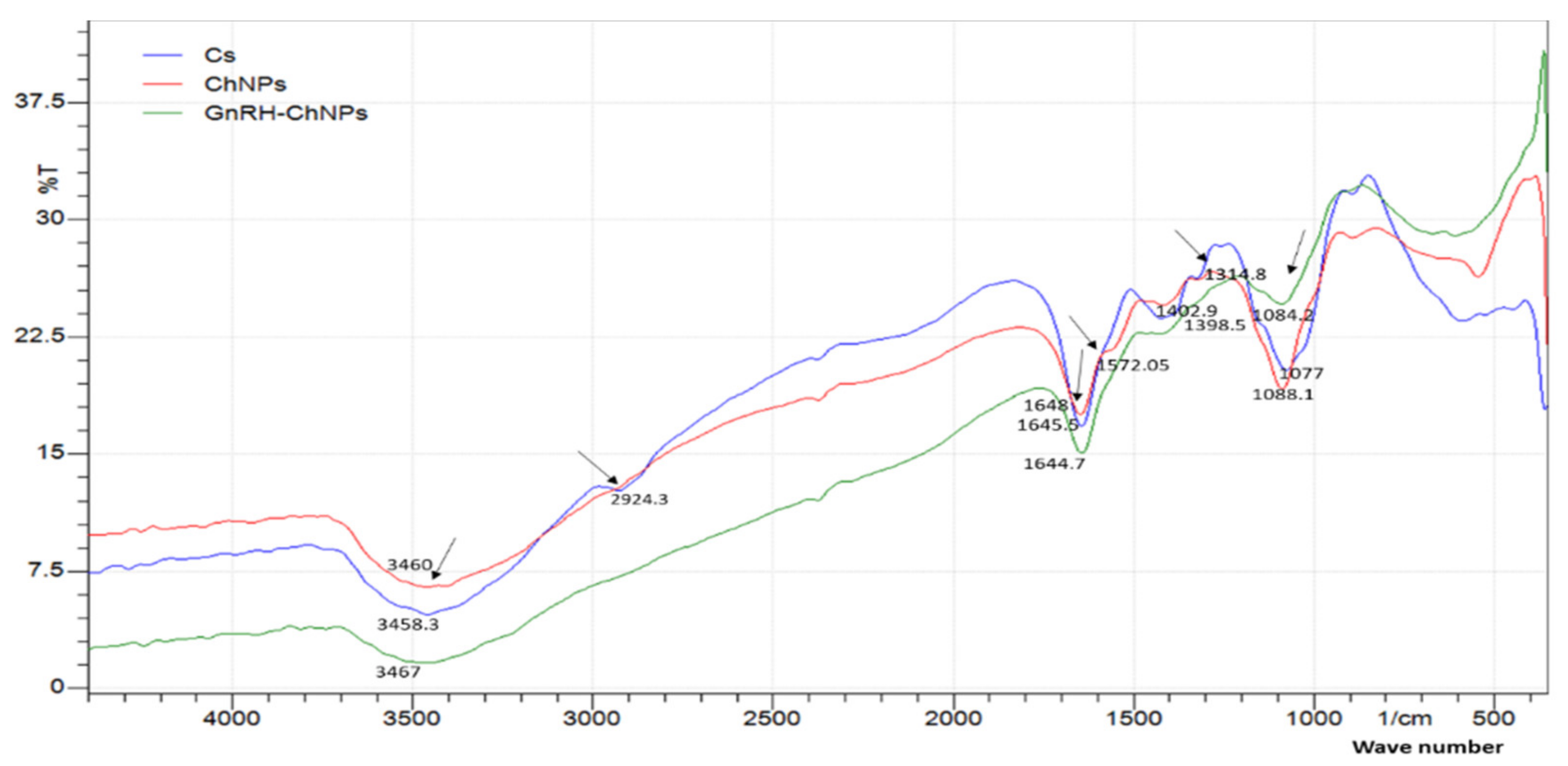
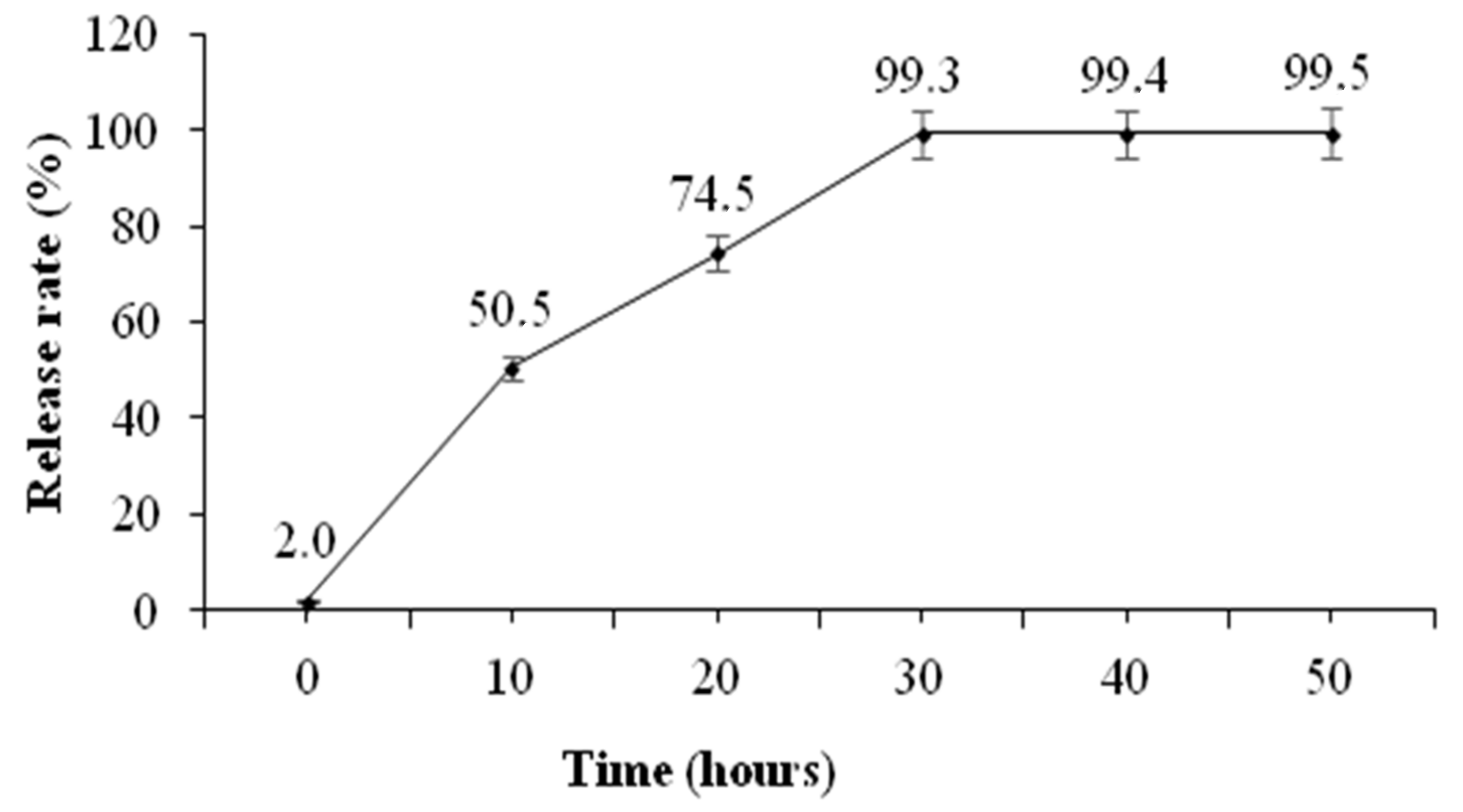
2.2. In Vitro Assessment of the Physicochemical Properties and Release Patterns of GnRH-Loaded Nanoparticles
2.3. In vivo Assessment of the Reproductive Response of Does Treated with GnRH–Loaded Nanoparticles
2.3.1. Animals and Ethic Statement
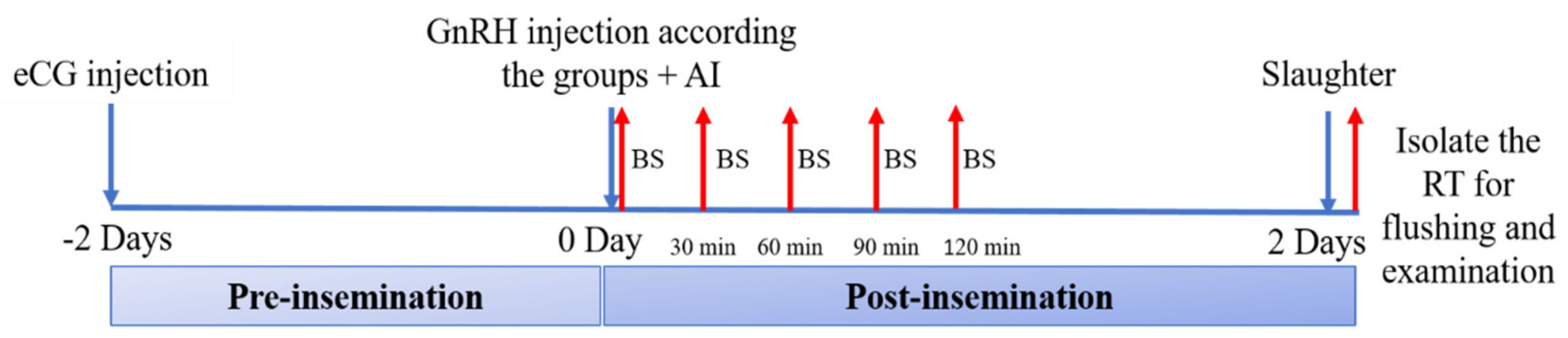
2.3.2. Trial 1. Patterns of LH Secretion, Ovulatory Efficiency, and Early Fertility
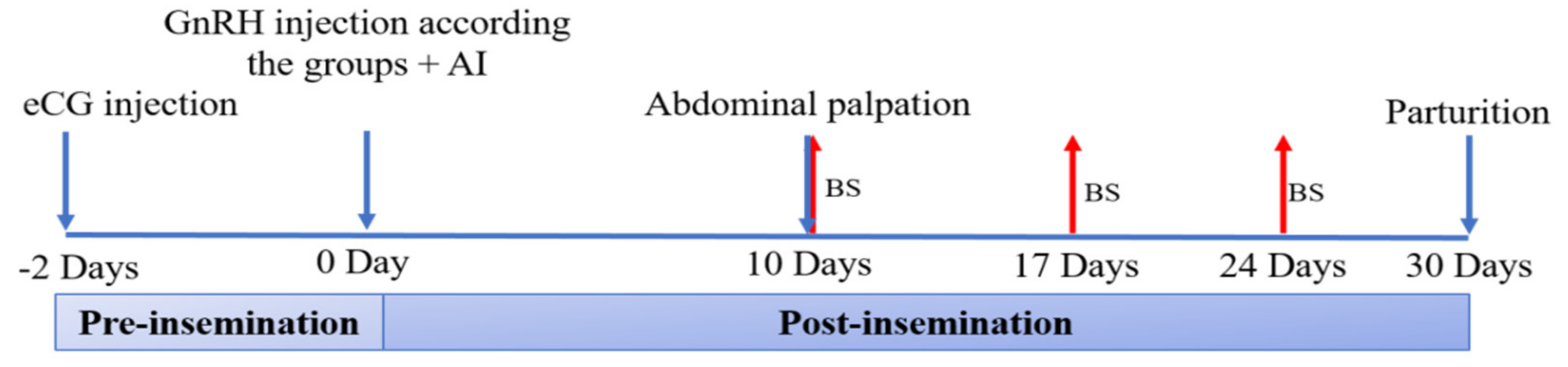
2.3.3. Trial 2. Fertility and Productive Traits
2.4. Statistical Analysis
3. Results
3.1. In Vitro Assessment of the Physicochemical Properties and Release Patterns of GnRH–Loaded Nanoparticles
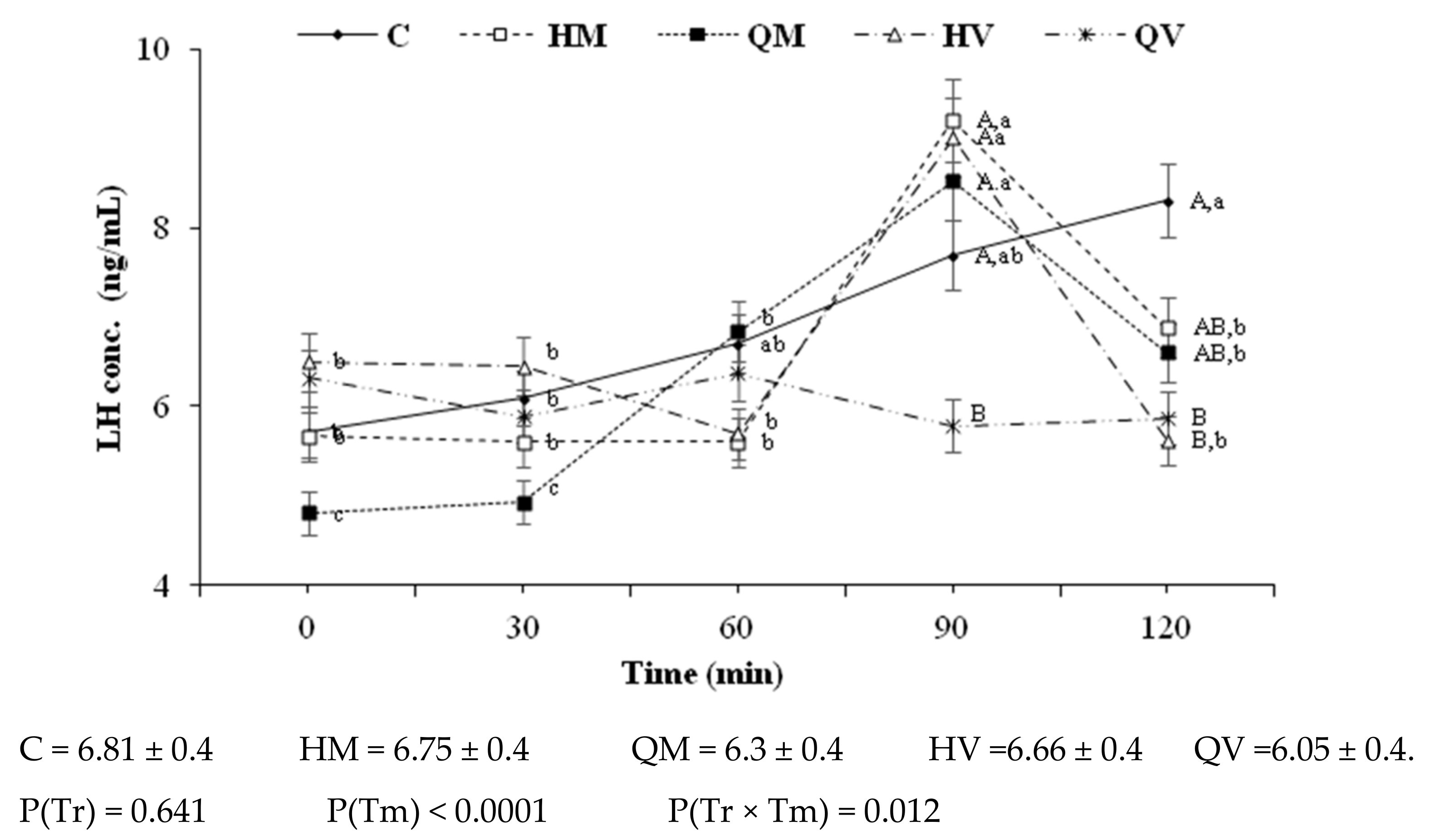
3.2. In Vivo Assessment of Patterns of LH Secretion, Ovulatory Efficiency, and Early Fertility in Does Treated with GnRH-Loaded Nanoparticles
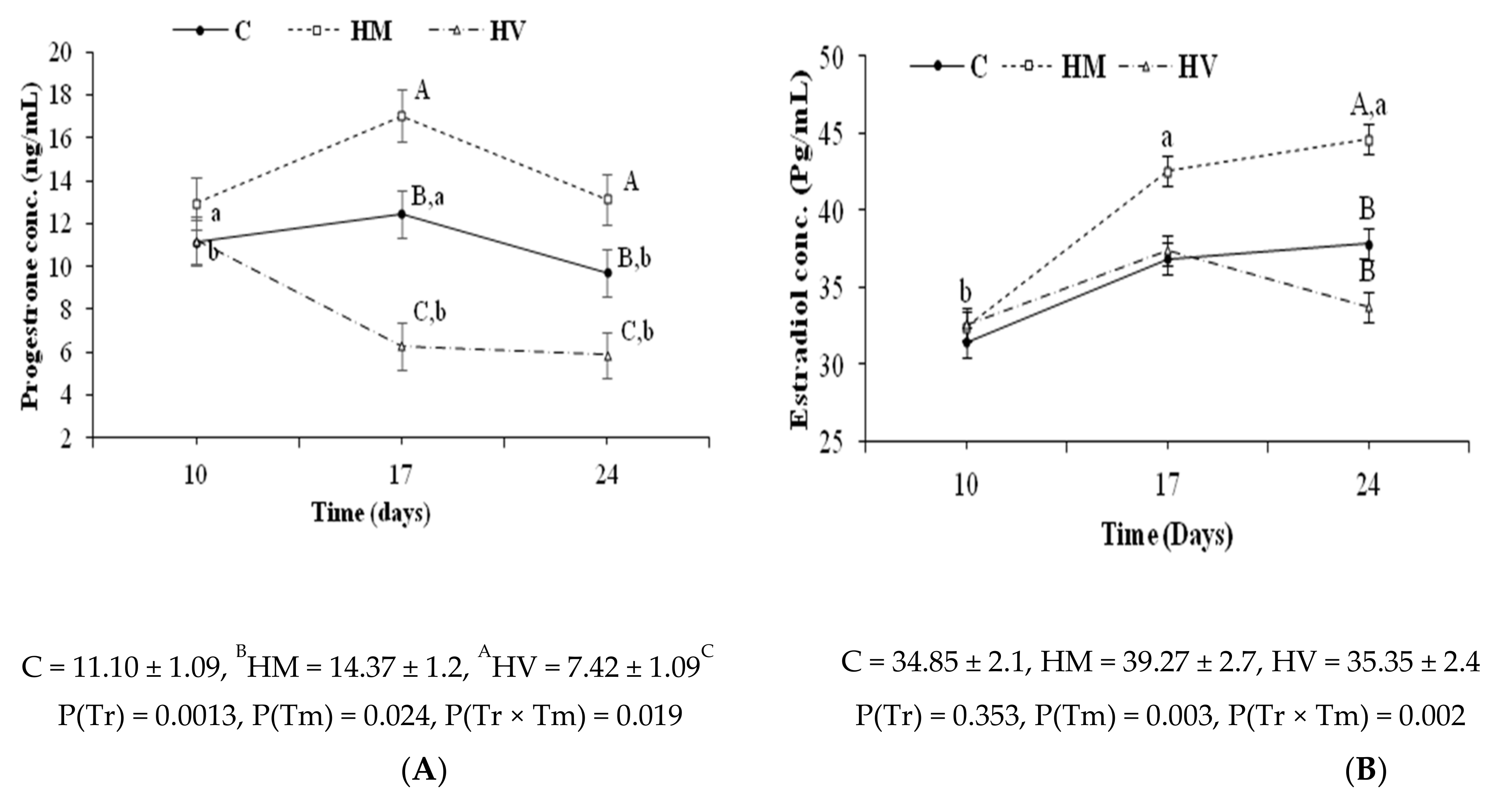
3.3. In Vivo Assessment of Fertility and Productive Traits in Does Treated with GnRH-Loaded Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Boiti, C. Underlying physiological mechanisms controlling the reproductive axis of rabbit does. In Proceedings of the Proc.: 8th World Rabbit Congress, Puebla, Mexico, 7–10 September 2004; pp. 7–10. [Google Scholar]
- Quintela, L.A.; Peña, A.I.; Vega, M.D.; Gullón, J.; Prieto, C.; Barrio, M.; Becerra, J.J.; García-Herradón, P.J. Reproductive Performance of Rabbit Does Artificially Inseminated via Intravaginal Administration of [des-Gly 10, d-Ala6]-LHRH Ethylamide as Ovulation Inductor. Reprod. Domest. Anim. 2009, 44, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, A. Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. J. Hum. Reprod. Sci. 2014, 7, 170. [Google Scholar] [CrossRef]
- Quintela, L.A.; Peña, A.I.; Vega, M.D.; Gullón, J.; Prieto, M.C.; Barrio, M.; Becerra, J.J.; Maseda, F.; García-Herradón, P.J. Ovulation induction in rabbit does submitted to artificial insemination by adding buserelin to the seminal dose. Reprod. Nutr. Dev. 2004, 44, 79–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashem, N.M.; Gonzalez-Bulnes, A. State-of-the-Art and Prospective of Nanotechnologies for Smart Reproductive Management of Farm Animals. Anim. 2020, 10, 840. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, Z.; Afroz, H. Prospects and applications of nanobiotechnology: A medical perspective. J. Nanobiotechnology 2012, 10, 31. [Google Scholar] [CrossRef]
- Rather, M.A.; Sharma, R.; Gupta, S.; Ferosekhan, S.; Ramya, V.L.; Jadhao, S.B. Chitosan-Nanoconjugated Hormone Nanoparticles for Sustained Surge of Gonadotropins and Enhanced Reproductive Output in Female Fish. PLoS One 2013, 8, e57094. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2008, 3, 16–20. [Google Scholar] [CrossRef]
- Esquivel, R.; Juárez, J.; Almada, M.; Ibarra, J.; Valdez, M.A. Synthesis and Characterization of New Thiolated Chitosan Nanoparticles Obtained by Ionic Gelation Method. Int. J. Polym. Sci. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Casares-Crespo, L.; Fernández-Serrano, P.; Viudes-De-Castro, M.P. Protection of GnRH analogue by chitosan-dextran sulfate nanoparticles for intravaginal application in rabbit artificial insemination. Theriogenology 2018, 116, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; Sallam, S. Reproductive performance of goats treated with free gonadorelin or nanoconjugated gonadorelin at estrus. Domest. Anim. Endocrinol. 2020, 71, 106390. [Google Scholar] [CrossRef]
- Hassanein, E.M.; Hashem, N.M.; El-Azrak, K.M.; Hassan, G.A.; Salem, M.H. Effect of GnRH–loaded chitosan-TPP nanoparticles on ovulation induction and embryo recovery rate using artificial insemination in. In Proceedings of the 10th International Poultry Conference, Sharm El-Sheikh, Egypt, 26–29 November 2018. [Google Scholar]
- Dounighi, N.M.; Eskandari, R.; Avadi; Zolfagharian, H.; Sadeghi, A.M.M.; Rezayat, M. Preparation and in vitro characterization of chitosan nanoparticles containing Mesobuthus eupeus scorpion venom as an antigen delivery system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 44–52. [Google Scholar] [CrossRef]
- Hashem, N.M.; Aboul-Ezz, Z.R. Effects of a single administration of different gonadotropins on day 7 post-insemination on pregnancy outcomes of rabbit does. Theriogenology 2018, 105, 1–6. [Google Scholar] [CrossRef]
- Hashem, N.M.; Abo-Elsoud, M.; El-Din, A.N.; Kamel, K.; Hassan, G. Prolonged exposure of dietary phytoestrogens on semen characteristics and reproductive performance of rabbit bucks. Domest. Anim. Endocrinol. 2018, 64, 84–92. [Google Scholar] [CrossRef]
- Statistical Analysis Systems, version 9.1; SAS Institute Inc.: Cary, NC, USA, 2004.
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2012, 64, 61–71. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharm. 2018, 10, 57. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surfaces B: Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Kumari, R.; Gupta, S.; Singh, A.R.; Ferosekhan, S.; Kothari, D.C.; Pal, A.K.; Jadhao, S.B. Chitosan Nanoencapsulated Exogenous Trypsin Biomimics Zymogen-Like Enzyme in Fish Gastrointestinal Tract. PLoS ONE 2013, 8, e74743. [Google Scholar] [CrossRef]
- Agarwal, M.; Agarwal, M.K.; Shrivastav, N.; Pandey, S.; Das, R.; Gaur, P. Preparation of Chitosan Nanoparticles and their In-vitro Characterization. Int. J. Life-Sciences Sci. Res. 2018, 4, 1713–1720. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Barbari, G.R.; Dorkoosh, F.A.; Amini, M.; Sharifzadeh, M.; Atyabi, F.; Balalaie, S.; Tehrani, N.R.; Tehrani, M.R. A novel nanoemulsion-based method to produce ultrasmall, water-dispersible nanoparticles from chitosan, surface modified with cell-penetrating peptide for oral delivery of proteins and peptides. Int. J. Nanomed. 2017, 12. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Alam El-Din, H.M.; Elberry, M.H.; Allam, N.G.; Hasanin, M.T.M.; Abdellah, A.M. Synthesis, characterization and cytotoxic evaluation of chitosan nanoparticles: In vitro liver cancer model. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035008. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Marei, A.E.-S.M. Development of a Solid-Phase Extraction (SPE) Cartridge Based on Chitosan-Metal Oxide Nanoparticles (Ch-MO NPs) for Extraction of Pesticides from Water and Determination by HPLC. Int. J. Anal. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Amidi, M.; Romeijn, S.G.; Borchard, G.; Junginger, H.E.; Hennink, W.E.; Jiskoot, W. Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J. Control. Release 2006, 111, 107–116. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mumper, R.J.; Khan, M.A.; Allen, D.D. Nanoparticle Technology for Drug Delivery Across the Blood-Brain Barrier. Drug Dev. Ind. Pharm. 2002, 28, 1–13. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification–diffusion methods: Critical comparison. Adv. Colloid Interface Sci. 2011, 163, 90–122. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Herrler, A.; Von Rango, U.; Beier, H.M. Embryo-maternal signalling: How the embryo starts talking to its mother to accomplish implantation. Reprod. Biomed. Online 2003, 6, 244–256. [Google Scholar] [CrossRef]
- Nam, D.H.; Lee, S.H.; Kim, H.S.; Lee, G.S.; Jeong, Y.W.; Kim, S.; Kim, J.H.; Kang, S.K.; Lee, B.C.; Hwang, W.S. The role of gonadotropin-releasing hormone (GnRH) and its receptor in development of porcine preimplantation embryos derived from in vitro fertilization. Theriogenology 2005, 63, 190–201. [Google Scholar] [CrossRef]
- Keyes, P.L.; Wiltbank, M.C. Endocrine Regulation of the Corpus Luteum. Annu. Rev. Physiol. 1988, 50, 465–482. [Google Scholar] [CrossRef]
- Khan-Dawood, F.S.; Dawood, M. Chorionic gonadotropin receptors and immunoreactive chorionic gonadotropin in implantation of the rabbit blastocyst. Am. J. Obstet. Gynecol. 1984, 148, 359–365. [Google Scholar] [CrossRef]
- Inskeep, E.K. Time-Dependent Embryotoxicity of the Endogenous Luteolysin Prostaglandin F2a in Ruminants. In Endocrine Toxicology, 3rd ed.; Informa UK Limited: London, UK, 2016; pp. 254–269. [Google Scholar]

| Nanoparticles. | Particle Size (nm) | PdI | Zeta Potential (mѴ) | Loading Efficiency (%) |
|---|---|---|---|---|
| ChNPs | 95.19 ± 1.90 | 0.165 | +34.0 | - |
| GnRH–ChNPs | 212 ± 2.69 | 0.295 | +8.0 | 90 |
| Variables | Groups (n = 4 Does/Group) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| C | HM | QM | HV | QV | ||||
| Ovarian structure (mean ± S.E.M.) | ||||||||
| Total ovarian follicles | 15.5 ± 1.0 c | 33.0 ± 3.6 b | 36.5 ± 0.9 ab | 40.2 ± 0.2 a | 36.5 ± 2.0 ab | 0.0001 | ||
| Ovulation points | 6.3 ± 0.9 a | 9.3 ± 1.2 a | 6.0±1.2 a | 8.8 ± 1.2 a | 0.00 ± 0.00 b | 0.001 | ||
| Total embryos | 4.0 ± 0.7 b | 6.2 ± 0.9 a | 5.2±1.4 ab | 8.0 ± 0.9 a | 0.00 ± 0.00 c | 0.001 | ||
| Non-fertilized ova | 0.25 ± 0.3 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.00 | 0.438 | ||
| Embryo developmental stages (%) | ||||||||
| Morula | 50 b(8/16) | 48 b(12/25) | 76.2 a(16/21) | 25 c(8/32) | - | 0.004 | ||
| Early blastocyst | 25(4/16) | 40(10/25) | 23.8(5/21) | 50(16/32) | - | 0.175 | ||
| Blastocyst | 0(0/16) | 12(3/25) | 0(0/21) | 0(0/32) | - | 0.361 | ||
| Expanded blastocyst | 0 b(0/16) | 0 b(0/16) | 0 b(0/21) | 15.6 a(5/32) | - | 0.009 | ||
| Hatching blastocyst | 0 b(0/16) | 0 b(0/16) | 0 b(0/21) | 9.4 a(3/32) | - | 0.044 | ||
| Variables | Treatments (n = 14 Does/Group) | p-Value | ||
|---|---|---|---|---|
| C | HM | HV | ||
| Fertility evaluation parameters1 | ||||
| Conception rate (%) | 78.5 a (11/14) | 71.5 a (10/14) | 50 b (7/14) | 0.019 |
| Parturition rate (%) | 78.5 a (11/14) | 64.2 a (9/14) | 35.7 b (5/14) | 0.050 |
| Abortion rate (%) | 0.0 (0/11) | 10.0 (1/10) | 28.5 (2/7) | 0.136 |
| Pregnancy outcomes parameters (mean ± S.E.M.)2 | ||||
| Litter size at birth | 6.08 ± 0.70 a | 5.47 ± 0.59 a | 2.50 ± 0.54 b | 0.001 |
| No. of live litter | 6.08 ± 0.70 a | 5.47 ± 0.59 a | 1.92 ± 0.63 b | 0.001 |
| No. of dead litter | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.54 ± 0.29 a | 0.034 |
| Viability rate at birth (%) | 100 a | 100 a | 76.67 b | 0.017 |
| Litter weight at birth (g) | 60.5 ± 3.4 b | 66.0 ± 4.3 ab | 76.0 ± 6.8 a | 0.036 |
| Total litter weight at birth (g) | 344.2 ± 29.8 a | 347.33 ± 39.2 a | 177.3 ± 33.9 b | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanein, E.M.; Hashem, N.M.; El-Azrak, K.E.-D.M.; Gonzalez-Bulnes, A.; Hassan, G.A.; Salem, M.H. Efficiency of GnRH–Loaded Chitosan Nanoparticles for Inducing LH Secretion and Fertile Ovulations in Protocols for Artificial Insemination in Rabbit Does. Animals 2021, 11, 440. https://doi.org/10.3390/ani11020440
Hassanein EM, Hashem NM, El-Azrak KE-DM, Gonzalez-Bulnes A, Hassan GA, Salem MH. Efficiency of GnRH–Loaded Chitosan Nanoparticles for Inducing LH Secretion and Fertile Ovulations in Protocols for Artificial Insemination in Rabbit Does. Animals. 2021; 11(2):440. https://doi.org/10.3390/ani11020440
Chicago/Turabian StyleHassanein, Eman M., Nesrein M. Hashem, Kheir El-Din M. El-Azrak, Antonio Gonzalez-Bulnes, Gamal A. Hassan, and Mohamed H. Salem. 2021. "Efficiency of GnRH–Loaded Chitosan Nanoparticles for Inducing LH Secretion and Fertile Ovulations in Protocols for Artificial Insemination in Rabbit Does" Animals 11, no. 2: 440. https://doi.org/10.3390/ani11020440
APA StyleHassanein, E. M., Hashem, N. M., El-Azrak, K. E.-D. M., Gonzalez-Bulnes, A., Hassan, G. A., & Salem, M. H. (2021). Efficiency of GnRH–Loaded Chitosan Nanoparticles for Inducing LH Secretion and Fertile Ovulations in Protocols for Artificial Insemination in Rabbit Does. Animals, 11(2), 440. https://doi.org/10.3390/ani11020440







