Effects of Food Changes on Intestinal Bacterial Diversity of Wintering Hooded Cranes (Grus monacha)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Site and Sample Collection
2.3. Sample Pretreatment
2.4. Sequence Data Processing
2.5. Determination of Potentially Pathogenic Species
2.6. Testing Food Composition
- Drying: The faecal samples were placed in an oven at 65 °C until the weight was constant.
- Grinding: Dried faecal samples were ground into powder and placed in self-sealing bags.
- Screening: The ground faecal samples were screened with a net screen of 40 mesh (0.40 mm) and 100 mesh (0.15 mm) to make the size of sample fragments between 0.15 mm and 0.40 mm.
- Concentrated nitric acid treatment: Sieving substances on a 100 mesh net screen were placed into a 50 mL beaker. Then, 2–3 mL of concentrated nitric acid was added to the small beaker. The mixture was kept quiescent for 3 min before being heated in a water bath at 90 °C for 2–3 min. The mixture was diluted with water, poured through a sieve, rinsed with distilled water until the colour was constant, and placed into a petri dish.
- Slide generation: A small amount of sample fragment was placed on a glass slide with a glue head dropper. A drop of distilled water was placed on the sample, which was unfolded with tweezers, before it was covered with a coverslip; excess water was removed.
2.7. Data Analysis
3. Results
3.1. Food Composition
3.2. Intestinal Bacterial Composition
3.3. Alpha Diversity and Beta Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohl, K.D. Diversity and function of the avian gut microbiota. J. Comp. Physiol. B 2012, 182, 591–602. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. Targeting the gut microbiota by dietary nutrients: A new avenue for human health. Crit. Rev. Food Sci. Nutr. 2019, 59, 181–195. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Q.; Dai, J.; Zhang, S.; Wei, F. Evidence of cellulose metabolism by the giant panda gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 17714–17719. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nat. Cell Biol. 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Roggenbuck, M.; Schnell, I.B.; Blom, N.; Bælum, J.; Bertelsen, M.F.; Sicheritz-Pontén, T.; Sørensen, S.J.; Gilbert, M.T.P.; Graves, G.R.; Hansen, L.H. The microbiome of New World vultures. Nat. Commun. 2014, 5, 5498. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species 2016. Hooded Crane (Grus monacha). Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22692151A93337861.en (accessed on 20 January 2021).
- Song, Y.W.; Zhou, L.Z. Effects of habitat changes on spatio-temporal pattern of the wintering waterbrid community at Shengjin Lake. J. Anhui Agric. Univ. 2019, 46, 610–617. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Y.M. Diet of Hooded Crane (Grus monacha) in autumn, Lindian, China. Chin. J. Wildl. 2015, 36, 76–79. [Google Scholar] [CrossRef]
- Hou, J.J. Diet Niche Partitioning by Four Wintering Cranes in Poyang Lake. Master’s Thesis, Nanchang University, Nanchang, China, 2019. [Google Scholar]
- Zhou, B.; Zhou, L.; Chen, J.; Cheng, Y.; Xu, W. Diurnal time-activity budgets of wintering hooded cranes (Grus monacha) in Shengjin Lake, China. Waterbirds 2010, 33, 110–115. [Google Scholar] [CrossRef]
- Zhou, L.L. Seasonal Shifts of Food Habit of the Hooded Crane (Grus monacha) Wintering in the Lakes of Yangtze River Floodplain in Anhui Province. Master’s Thesis, Anhui University, Hefei, China, 2013. [Google Scholar]
- Wu, L.; Sun, Y.; Li, J.; Li, Y.; Wu, Y.; Li, D. A phylogeny of the Passerida (Aves: Passeriformes) based on mitochondrial 12S ribosomal RNA gene. Avian Res. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Knutie, S.A.; Gotanda, K.M. A non-invasive method to collect fecal samples from wild birds for microbiome studies. Microb. Ecol. 2018, 76, 851–855. [Google Scholar] [CrossRef]
- Zhang, F.; Xiang, X.; Dong, Y.; Yan, S.; Song, Y.; Zhou, L. Significant differences in the gut bacterial communities of hooded crane (Grus monacha) in different seasons at a stopover site on the flyway. Animals 2020, 10, 701. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Xu, Z.Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef]
- Xiang, X.; Jin, L.; Yang, Z.; Zhang, N.; Zhang, F. Dramatic shifts in intestinal fungal community between wintering hooded crane and domestic goose. Avian Res. 2021, 12, 1–6. [Google Scholar] [CrossRef]
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Gibert, C.; Escarguel, G. PER-SIMPER-A new tool for inferring community assembly processes from taxon occurrences. Glob. Ecol. Biogeogr. 2018, 28, 374–385. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, L.; Dong, Y.; Cheng, Y.; Song, Y. The gut microbiome of hooded cranes (Grus monacha) wintering at Shengjin Lake, China. Microbiology 2017, 6, e00447. [Google Scholar] [CrossRef]
- Wan, W.; Zhou, L.; Song, Y. Shifts in foraging behavior of wintering hooded cranes (Grus monacha) in three different habitats at Shengjin Lake, China. Avian Res. 2016, 7, 13. [Google Scholar] [CrossRef]
- Wei, Z.; Zheng, M.; Zhou, L.; Xu, W. Flexible foraging response of wintering hooded cranes (Grus monacha) to food availability in the lakes of the Yangtze River floodplain, China. Animals 2020, 10, 568. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y. Study on Vegetation Community Characteristics in Wetland of Shengjin Lake in Anhui Province. Master’s Thesis, Anhui University, Hefei, China, 2011. [Google Scholar]
- Zou, H.F.; Feng, X.D.; Wu, Q.M.; Wu, Y.N.; Hao, M.; Ma, J.Z. Diet component and preference of white-naped crane during courtship period in Zhalong Nature Reserve. J. Northeast For. Univ. 2012, 40, 69–76. [Google Scholar]
- Jiao, S.W.; Jiang, K.Y.; Zuo, A.J.; Wu, M.; Lei, G.C.; Zhou, Y. Foraging behavior and food resources of wintering Grus monacha in China. Sichuan Dong Wu 2017, 36, 392–397. [Google Scholar] [CrossRef]
- Barbosa, A.; Balagué, V.; Valera, F.; Martínez, A.; Benzal, J.; Motas, M.; Diaz, J.I.; Mira, A.; Pedrós-Alió, C. Age-related differences in the gastrointestinal microbiota of chinstrap penguins (Pygoscelis antarctica). PLoS ONE 2016, 11, e0153215. [Google Scholar] [CrossRef] [PubMed]
- Dewar, M.L.; Arnould, J.P.Y.; Dann, P.; Trathan, P.; Groscolas, R.; Smith, S. Interspecific variations in the gastrointestinal microbiota in penguins. Microbiology 2013, 2, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Xiang, X.; Dong, Y.; Cheng, L.; Zhou, L. Comparing the intestinal bacterial communities of sympatric wintering hooded crane (Grus monacha) and domestic goose (Anser anser domesticus). Avian Res. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Hird, S.M.; Sanchez, C.; Carstens, B.C.; Brumfield, R.T. Comparative gut microbiota of 59 neotropical bird species. Front. Microbiol. 2015, 6, 1403. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Genet. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Altaher, Y.; Jahromi, M.; Ebrahim, R.; Zulkifli, I.; Liang, J.B. Lactobacillus Pentosus Ita23 and L. Acidipiscis Ita44 enhance feed conversion efficiency and beneficial gut microbiota in broiler chickens. Braz. J. Poult. Sci. 2015, 17, 159–164. [Google Scholar] [CrossRef]
- Mikaelyan, A.; Dietrich, C.; Köhler, T.; Poulsen, M.; Sillam-Dussès, D.; Brune, A. Diet is the primary determinant of bacterial community structure in the guts of higher termites. Mol. Ecol. 2015, 24, 5284–5295. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Pardal, S.; Proença, D.N.; Lopes, R.J.; Ramos, J.A.; Mendes, L.; Morais, P.V. Diversity of cloacal microbial community in migratory shorebirds that use the Tagus estuary as stopover habitat and their potential to harbor and disperse pathogenic microorganisms. FEMS Microbiol. Ecol. 2012, 82, 63–74. [Google Scholar] [CrossRef] [PubMed]
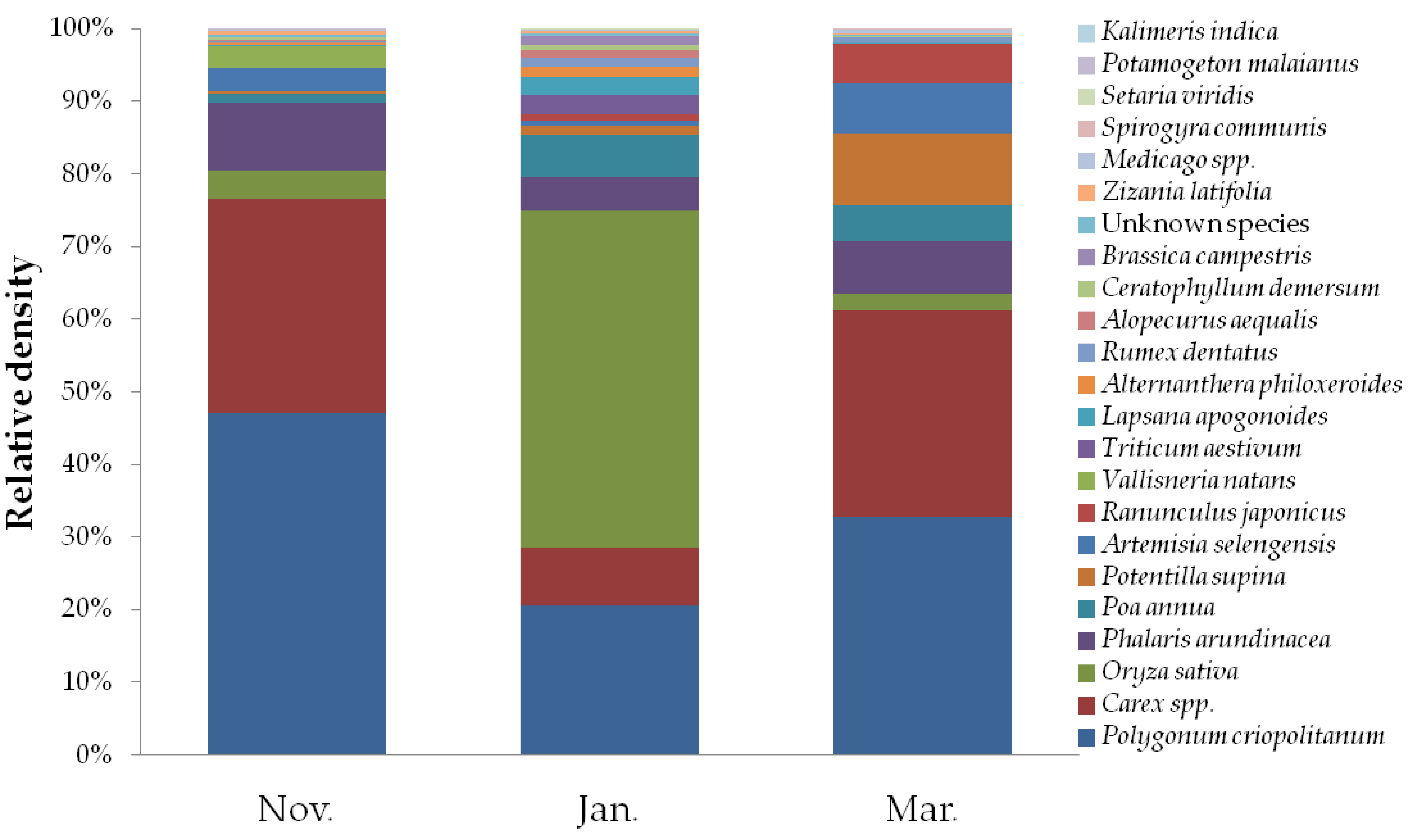

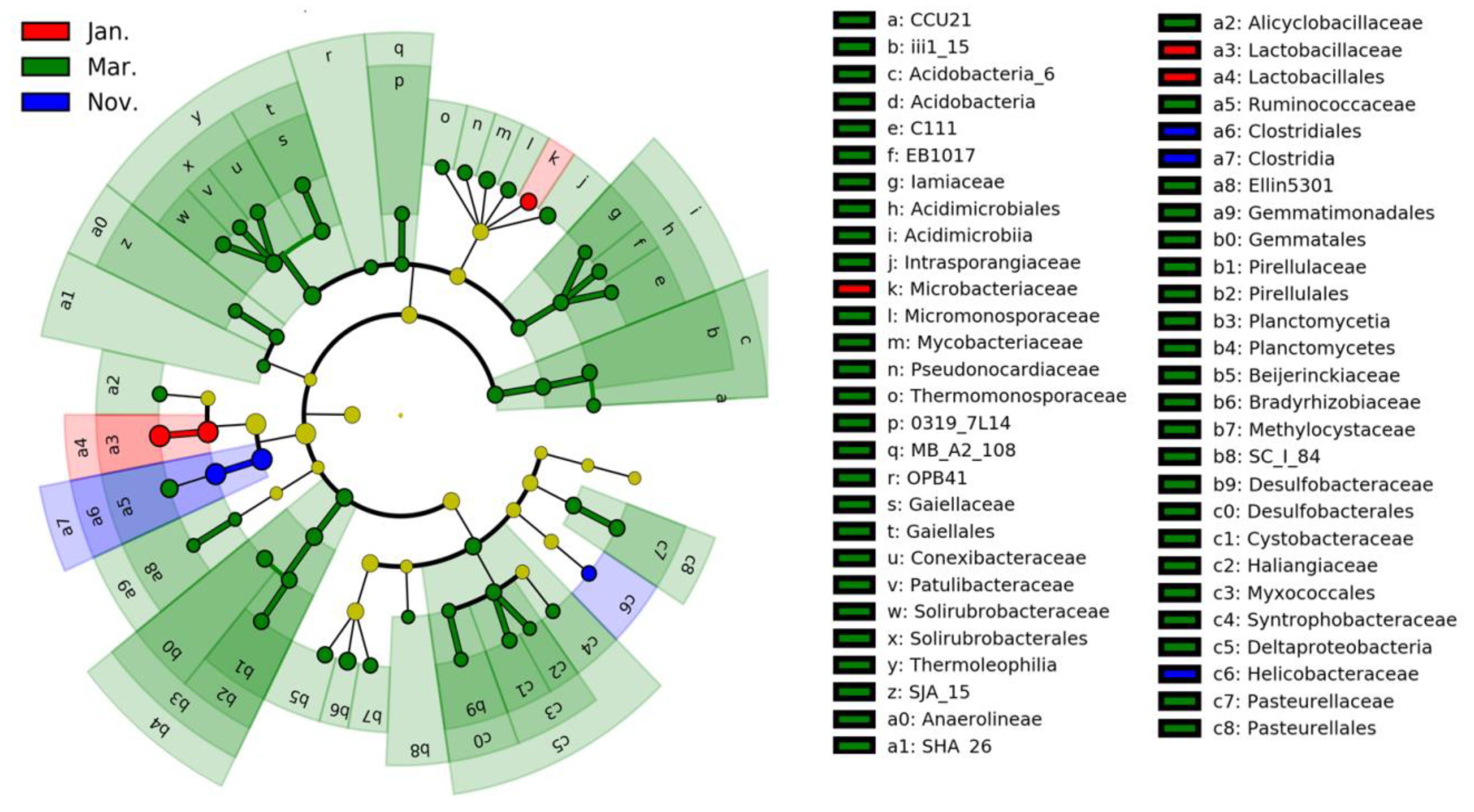


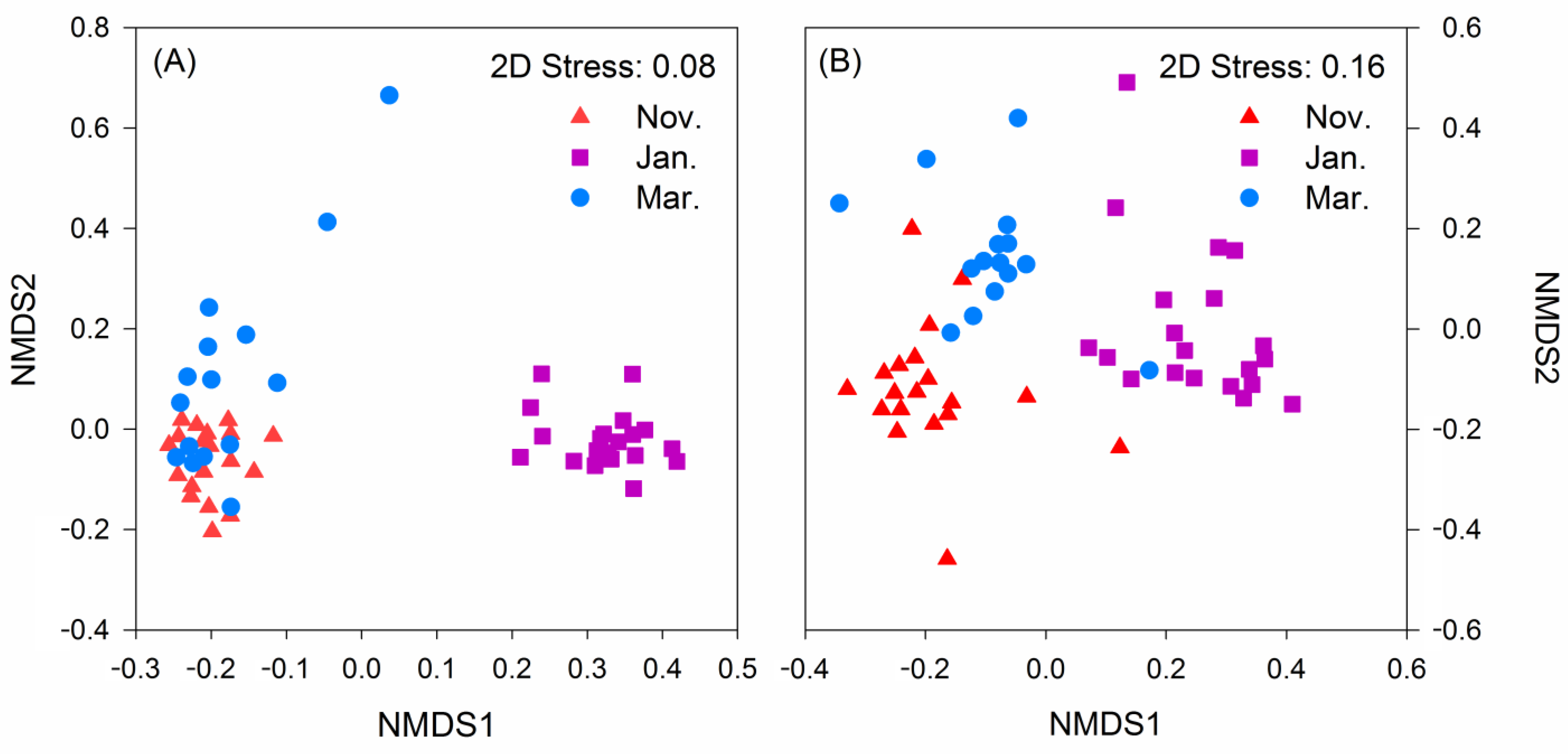
| ASVs | Taxa | Contribution (%) | ||
|---|---|---|---|---|
| November vs. January | November vs. March | January vs. March | ||
| 0360 | Lactobacillus acidipiscis | 21.65 | - | 22.93 |
| 0001 | Clostridium metallolevans | 11.23 | 11.92 | - |
| 0003 | o__Bacillales | 7.49 | 8.10 | 3.62 |
| 0005 | g__Paenibacillus | 3.30 | 3.28 | - |
| 0543 | Escherichia coli | 2.75 | 3.16 | - |
| 0002 | Lactobacillus acidipiscis | - | 12.76 | 13.25 |
| 0015 | Bacillus coahuilensis | - | - | 3.39 |
| 1329 | Lactobacillus acidipiscis | - | - | 2.18 |
| Foods | R | p-Value |
|---|---|---|
| Oryza sativa | 0.448 | 0.001 |
| Polygonum criopolitanum | 0.364 | 0.001 |
| Carex spp. | 0.272 | 0.001 |
| Phalaris arundinacea | 0.241 | 0.001 |
| Lapsana apogonoides | 0.226 | 0.002 |
| Poa annua | 0.127 | 0.015 |
| Ranunculus japonicus | 0.121 | 0.024 |
| Potentilla supina | 0.116 | 0.033 |
| Vallisneria natans | 0.110 | 0.036 |
| Foods | R | p-Value |
|---|---|---|
| Oryza sativa | 0.293 | 0.001 |
| Phalaris arundinacea | 0.197 | 0.001 |
| Lapsana apogonoides | 0.180 | 0.015 |
| Polygonum criopolitanum | 0.177 | 0.001 |
| Carex spp. | 0.174 | 0.001 |
| Vallisneria natans | 0.143 | 0.033 |
| Alternanthera philoxeroides | 0.111 | 0.048 |
| Treatment | ANOSIM | |
|---|---|---|
| R | p-Value | |
| November vs. January | 0.999 | 0.001 |
| November vs. March | 0.307 | 0.001 |
| January vs. March | 0.929 | 0.001 |
| Treatment | ANOSIM | |
|---|---|---|
| R | p-Value | |
| November vs. January | 0.788 | 0.001 |
| November vs. March | 0.503 | 0.001 |
| January vs. March | 0.654 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Zhou, L.; Yang, Z.; Gu, J. Effects of Food Changes on Intestinal Bacterial Diversity of Wintering Hooded Cranes (Grus monacha). Animals 2021, 11, 433. https://doi.org/10.3390/ani11020433
Zhang N, Zhou L, Yang Z, Gu J. Effects of Food Changes on Intestinal Bacterial Diversity of Wintering Hooded Cranes (Grus monacha). Animals. 2021; 11(2):433. https://doi.org/10.3390/ani11020433
Chicago/Turabian StyleZhang, Nazhong, Lizhi Zhou, Zhuqing Yang, and Jingjing Gu. 2021. "Effects of Food Changes on Intestinal Bacterial Diversity of Wintering Hooded Cranes (Grus monacha)" Animals 11, no. 2: 433. https://doi.org/10.3390/ani11020433
APA StyleZhang, N., Zhou, L., Yang, Z., & Gu, J. (2021). Effects of Food Changes on Intestinal Bacterial Diversity of Wintering Hooded Cranes (Grus monacha). Animals, 11(2), 433. https://doi.org/10.3390/ani11020433








