Handling Associated with Drenching Does Not Impact Survival and General Health of Low Birth Weight Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals
2.3. Piglet Selection
2.4. Experimental Treatments
2.5. Data Collection
2.5.1. Skin Lesion Scoring
2.5.2. Blood Sampling
2.5.3. IgG and IGF-1 Analysis
2.6. Statistical Analysis
3. Results
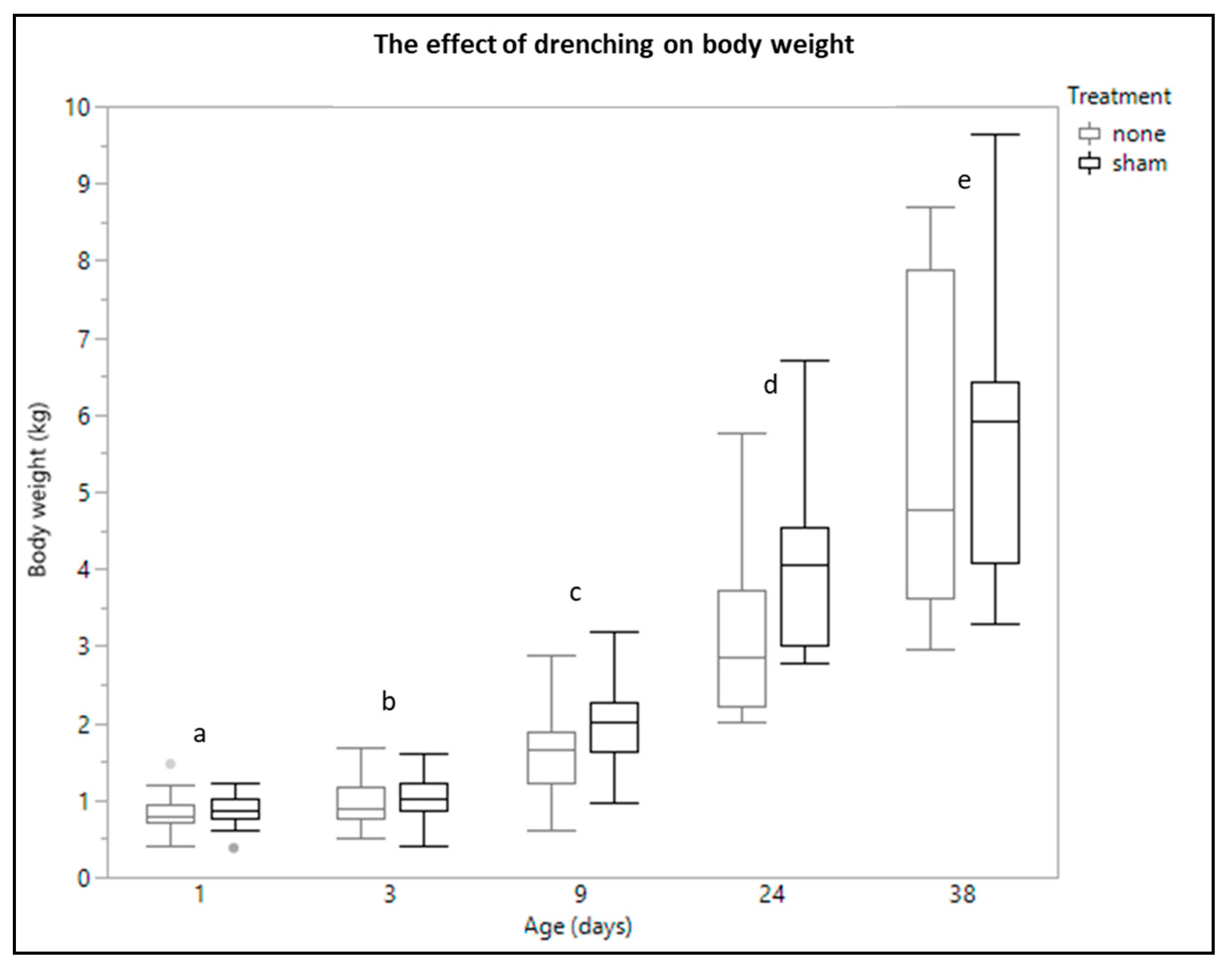
3.1. Body Weight
3.2. Glucose Levels
3.3. NEFA Levels
3.4. Urea Levels
3.5. IgG Levels
3.6. IGF-1 Levels
3.7. Haematological Analysis
3.8. Skin Lesion Scores
3.9. Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemp, B.; Da Silva, C.L.A.; Soede, N.M. Recent advances in pig reproduction: Focus on impact of genetic selection for female fertility. Reprod. Domest. Anim. 2018, 53, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, K.; Baxter, E.; Ask, B.; Berg, P.; D’Eath, R.; Jarvis, S.; Jensen, K.K.; Lawrence, A.; Moustsen, V.; Robson, S.K.; et al. The ethical and welfare implications of large litter size in domestic pig-challenges and solutions. Proj. Rep. 2011, 17, 1–148. [Google Scholar]
- Beaulieu, A.D.; Aalhus, J.L.; Williams, N.H.; Patience, J.F. Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork1. J. Anim. Sci. 2010, 88, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Lund, M.S.; Sorensen, D. Selection for litter size at day five to improve litter size at weaning and piglet survival rate. J. Anim. Sci. 2007, 85, 1385–1392. [Google Scholar] [CrossRef]
- Rutherford, K.; Baxter, E.; D’Eath, R.; Turner, S.P.; Arnott, G.; Roehe, R.; Ask, B.; Sandøe, P.; Moustsen, V.; Thorup, F.; et al. The welfare implications of large litter size in the domestic pig I: Biological factors. Anim. Welf. 2013, 22, 199–218. [Google Scholar] [CrossRef]
- Pandolfi, F.; Edwards, S.A.; Robert, F.; Kyriazakis, I. Risk factors associated with the different categories of piglet perinatal mortality in French farms. Prev. Vet. Med. 2017, 137. [Google Scholar] [CrossRef] [PubMed]
- Nuntapaitoon, M.; Tummaruk, P. Factors influencing piglet pre-weaning mortality in 47 commercial swine herds in Thailand. Trop. Anim. Health Prod. 2018, 50, 129–135. [Google Scholar] [CrossRef]
- Quesnel, H.; Brossard, L.; Valancogne, A.; Quiniou, N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal 2008, 2, 1842–1849. [Google Scholar] [CrossRef]
- Milligan, B.N.; Dewey, C.E.; de Grau, A.F. Neonatal-piglet weight variation and its relation to pre-weaning mortality and weight gain on commercial farms. Prev. Vet. Med. 2002, 56, 119–127. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Vallet, J.L.; Miles, J.R. Comparison of myelination between large and small pig fetuses during late gestation. Anim. Reprod. Sci. 2012, 132, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Herpin, P.; Damon, M.; Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 2002, 78, 25–45. [Google Scholar] [CrossRef]
- Theil, P.; Nielsen, M.; Sorensen, M.; Lauridsen, C. Nutritional Physiology of Pigs: With Emphasis on Danish Production Conditions—Lactation, Milk and Suckling; Knudsen, K.E.B., Kjeldsen, N.J., Poulsen, H.D., Jensen, B.B., Eds.; Videncenter for Svineproduktion, Landbrug & Fødevarer: Copenhagen, Denmark, 2012; pp. 1–49. [Google Scholar]
- Ferrari, C.V.; Sbardella, P.E.; Bernardi, M.L.; Coutinho, M.L.; Vaz, I.S., Jr.; Wentz, I.; Bortolozzo, F.P. Effect of birth weight and colostrum intake on mortality and performance of piglets after cross-fostering in sows of different parities. Prev. Vet. Med. 2014, 114, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Baxter, E.M.; Jarvis, S.; D’Eath, R.B.; Ross, D.W.; Robson, S.K.; Farish, M.; Nevison, I.M.; Lawrence, A.B.; Edwards, S.A. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 2008, 69, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.; Rutherford, K.; d’Eath, R.; Arnott, G.; Turner, S.; Sandøe, P.; Moustsen, V.; Thorup, F.; Edwards, S.; Lawrence, A. The welfare implications of large litter size in the domestic pig II: Management factors. Anim. Welf. 2013, 22, 219–238. [Google Scholar] [CrossRef]
- Alexopoulos, J.G.; Lines, D.S.; Hallett, S.; Plush, K.J. A review of success factors for piglet fostering in lactation. Animals 2018, 8, 38. [Google Scholar] [CrossRef]
- Calderon Diaz, J.A.; Garcia Manzanilla, E.; Diana, A.; Boyle, L.A. Cross-fostering implications for pig mortality, welfare and performance. Front. Vet. Sci. 2018, 5, 123. [Google Scholar] [CrossRef]
- Schmitt, O.; Baxter, E.M.; Lawlor, P.G.; Boyle, L.A.; O'Driscoll, K. A single dose of fat-based energy supplement to light birth weight pigs shortly after birth does not increase their survival and growth. Animals 2019, 9, 227. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Effect of oral supplementation with different energy boosters in newborn piglets on pre-weaning mortality, growth and serological levels of IGF-I and IgG. J. Anim. Sci. 2017, 95, 353–360. [Google Scholar] [CrossRef]
- de Greeff, A.; Resink, J.W.; van Hees, H.M.; Ruuls, L.; Klaassen, G.J.; Rouwers, S.M.; Stockhofe-Zurwieden, N. Supplementation of piglets with nutrient-dense complex milk replacer improves intestinal development and microbial fermentation. J. Anim. Sci. 2016, 94, 1012–1019. [Google Scholar] [CrossRef]
- de Vos, M.; Che, L.; Huygelen, V.; Willemen, S.; Michiels, J.; Van Cruchten, S.; Van Ginneken, C. Nutritional interventions to prevent and rear low-birthweight piglets. J. Anim. Physiol. Anim. Nutr. 2014, 98, 609–619. [Google Scholar] [CrossRef]
- Madsen, J.G.; Mueller, S.; Kreuzer, M.; Bigler, M.B.; Silacci, P.; Bee, G. Milk replacers supplemented with either L-arginine or L-carnitine potentially improve muscle maturation of early reared low birth weight piglets from hyperprolific sows. Animal 2018, 12, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Le Bourgot, C.; Ferret-Bernard, S.; Le Normand, L.; Savary, G.; Menendez-Aparicio, E.; Blat, S.; Appert-Bossard, E.; Respondek, F.; Le Huerou-Luron, I. Maternal short-chain fructooligosaccharide supplementation influences intestinal immune system maturation in piglets. PLoS ONE 2014, 9, e107508. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wei, H.K.; Xiang, Q.H.; Wang, J.; Zhou, Y.F.; Peng, J. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation. J. Vet. Med. Sci. 2016, 78, 1487–1494. [Google Scholar] [CrossRef]
- Douglas, S.L.; Edwards, S.A.; Kyriazakis, I. Management strategies to improve the performance of low birth weight pigs to weaning and their long-term consequences. J. Anim. Sci. 2014, 92, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.R.; de Jonge, N.; Sugiharto, S.; Nielsen, J.L.; Lauridsen, C.; Canibe, N. The microbial community of the gut differs between piglets fed sow milk, milk replacer or bovine colostrum. Br. J. Nutr. 2017, 117, 964–978. [Google Scholar] [CrossRef]
- Milligan, B.; Fraser, D.; Kramer, D. The effect of littermate weight on survival, weight gain, and suckling behavior of low-birth-weight piglets in cross-fostered. J. Swine Health Prod. 2001, 9, 161–168. [Google Scholar]
- Schokker, D.; Fledderus, J.; Jansen, R.; Vastenhouw, S.A.; de Bree, F.M.; Smits, M.A.; Jansman, A.A.J.M. Supplementation of fructooligosaccharides to suckling piglets affects intestinal microbiota colonization and immune development. J. Anim. Sci. 2018, 96, 2139–2153. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.P.; Farnworth, M.J.; White, I.M.S.; Brotherstone, S.; Mendl, M.; Knap, P.; Penny, P.; Lawrence, A.B. The accumulation of skin lesions and their use as a predictor of individual aggressiveness in pigs. Appl. Anim. Behav. Sci. 2006, 96, 245–259. [Google Scholar] [CrossRef]
- Bernardino, T.; Tatemoto, P.; Morrone, B.; Mazza Rodrigues, P.H.; Zanella, A.J. Piglets born from sows fed high fibre diets during pregnancy are less aggressive prior to weaning. PLoS ONE 2016, 11, e0167363. [Google Scholar] [CrossRef]
- Gardner, J.M.; de Lange, C.F.; Widowski, T.M. Belly-nosing in early-weaned piglets is not influenced by diet quality or the presence of milk in the diet. J. Anim. Sci. 2001, 79, 73–80. [Google Scholar] [CrossRef][Green Version]
- Rundgren, M.; Löfquist, I. Effects on performance and behaviour of mixing 20-kg pigs fed individually. Anim. Sci. 1989, 49, 311–315. [Google Scholar] [CrossRef]
- Pluske, J.R.; Williams, I.H. Reducing stress in piglets as a means of increasing production after weaning: Administration of amperozide or co-mingling of piglets during lactation? Anim. Sci. 1996, 62, 121–130. [Google Scholar] [CrossRef]
- Parratt, C.A.; Chapman, K.J.; Turner, C.; Jones, P.H.; Mendl, M.T.; Miller, B.G. The fighting behaviour of piglets mixed before and after weaning in the presence or absence of a sow. Appl. Anim. Behav. Sci. 2006, 101, 54–67. [Google Scholar] [CrossRef]
- Declerck, I.; Dewulf, J.; Sarrazin, S.; Maes, D. Long-term effects of colostrum intake in piglet mortality and performance. J. Anim. Sci. 2016, 94, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- van Wettere, W.H.; Willson, N.L.; Pain, S.J.; Forder, R.E. Effect of oral polyamine supplementation pre-weaning on piglet growth and intestinal characteristics. Animal 2016, 10, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Declerck, I.; Dewulf, J.; Decaluwé, R.; Maes, D. Effects of energy supplementation to neonatal (very) low birth weight piglets on mortality, weaning weight, daily weight gain and colostrum intake. Livest. Sci. 2016, 183, 48–53. [Google Scholar] [CrossRef]
- Santos, L.S.; Caldara, F.R.; Machado, S.T.; Nääs, I.A.; Foppa, L.; Garcia, R.G.; Moura, R.; Machado, S.P. Sows' parity and coconut oil postnatal supplement on piglets performance. Rev. MVZ Córdoba 2015, 20, 4513–4521. [Google Scholar] [CrossRef][Green Version]
- Manzke, N.E.; Gomes, B.K.; Xavier, E.G.; de Lima, G. Efficacy of energy supplementation on growth performance and immune response of suckling pigs. J. Anim. Sci. 2018, 96, 4723–4730. [Google Scholar] [CrossRef]
- de Oliveira, D.; Paranhos da Costa, M.J.R.; Zupan, M.; Rehn, T.; Keeling, L.J. Early human handling in non-weaned piglets: Effects on behaviour and body weight. Appl. Anim. Behav. Sci. 2015, 164, 56–63. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Barnett, J.L. The effects of aversively handling pigs, either individually or in groups, on their behaviour, growth and corticosteroids. Appl. Anim. Behav. Sci. 1991, 30, 61–72. [Google Scholar] [CrossRef]
- Grandin, T.; Shivley, C. How farm animals react and perceive stressful situations such as handling, restraint, and transport. Animals 2015, 5, 1233–1251. [Google Scholar] [CrossRef]
- Brajon, S.; Laforest, J.-P.; Bergeron, R.; Tallet, C.; Hötzel, M.-J.; Devillers, N. Persistency of the piglet’s reactivity to the handler following a previous positive or negative experience. Appl. Anim. Behav. Sci. 2015, 162, 9–19. [Google Scholar] [CrossRef]
- Ward, S.A.; Kirkwood, R.N.; Plush, K.J. Are larger litters a concern for piglet survival or an effectively manageable trait? Animals 2020, 10, 309. [Google Scholar] [CrossRef]
- Bonastre, C.; Mitjana, O.; Tejedor, M.T.; Calavia, M.; Yuste, A.G.; Úbeda, J.L.; Falceto, M.V. Acute physiological responses to castration-related pain in piglets: The effect of two local anesthetics with or without meloxicam. Animal 2016, 10, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Spilsbury, M.; Mota-Rojas, D.; Villanueva-Garcia, D.; Martinez-Burnes, J.; Orozco, H.; Ramirez-Necoechea, R.; Mayagoitia, A.L.; Trujillo, M.E. Perinatal asphyxia pathophysiology in pig and human: A review. Anim. Reprod. Sci. 2005, 90. [Google Scholar] [CrossRef]
- Panzardi, A.; Bernardi, M.L.; Mellagi, A.P.; Bierhals, T.; Bortolozzo, F.P.; Wentz, I. Newborn piglet traits associated with survival and growth performance until weaning. Prev. Vet. Med. 2013, 110, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.L.; Hemsworth, P.H.; Hand, A.M. Effects of chronic stress on some blood parameters in the pig. Appl. Anim. Ethol. 1983, 9, 273–277. [Google Scholar] [CrossRef]
- Christiansen, J.J.; Djurhuus, C.B.; Gravholt, C.H.; Iversen, P.; Christiansen, J.S.; Schmitz, O.; Weeke, J.; Jorgensen, J.O.; Moller, N. Effects of cortisol on carbohydrate, lipid, and protein metabolism: Studies of acute cortisol withdrawal in adrenocortical failure. J. Clin. Endocrinol. Metab. 2007, 92, 3553–3559. [Google Scholar] [CrossRef]
- Spencer, G.S. Relationship between plasma somatomedin activity and levels of cortisol and free fatty acids following stress in pigs. J. Endocrinol. 1980, 84, 109–114. [Google Scholar] [CrossRef]
- Vanden Hole, C.; Ayuso, M.; Aerts, P.; Prims, S.; van Cruchten, S.; van Ginneken, C. Glucose and glycogen levels in piglets that differ in birth weight and vitality. Heliyon 2019, 5, e02510. [Google Scholar] [CrossRef] [PubMed]
- Amdi, C.; Krogh, U.; Flummer, C.; Oksbjerg, N.; Hansen, C.F.; Theil, P.K. Intrauterine growth restricted piglets defined by their head shape ingest insufficient amounts of colostrum. J. Anim. Sci. 2013, 91, 5605–5613. [Google Scholar] [CrossRef]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Milligan, B.N.; Fraser, D.; Kramer, D.L. Within-litter birth weight variation in the domestic pig and its relation to pre-weaning survival, weight gain, and variation in weaning weights. Livest. Prod. Sci. 2002, 76, 181–191. [Google Scholar] [CrossRef]
- Devillers, N.; Le Dividich, J.; Prunier, A. Influence of colostrum intake on piglet survival and immunity. Animal 2011, 5, 1605–1612. [Google Scholar] [CrossRef]
- Numberger, J.; Ritzmann, M.; Übel, N.; Eddicks, M.; Reese, S.; Zoels, S. Ear tagging in piglets: The cortisol response with and without analgesia in comparison with castration and tail docking. Animal 2016, 10, 1864–1870. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Davis, B.L.; Brooks, T.A.; McGlone, J.J. Physiology and behavior of pigs before and after castration: Effects of two topical anesthetics. Animal 2010, 4, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.A.; Bryer, P.J.; Krebs, N.; McGlone, J.J. Tail docking in pigs: Acute physiological and behavioural responses. Animal 2008, 2, 292–297. [Google Scholar] [CrossRef]
- Marchant-Forde, J.N.; Lay, D.C., Jr.; McMunn, K.A.; Cheng, H.W.; Pajor, E.A.; Marchant-Forde, R.M. Postnatal piglet husbandry practices and well-being: The effects of alternative techniques delivered separately1,2. J. Anim. Sci. 2009, 87, 1479–1492. [Google Scholar] [CrossRef]
- Klobasa, F.; Werhahn, E.; Butler, J.E. Composition of sow milk during lactation. J. Anim. Sci. 1987, 64, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.J.; Wang, F.; Zhang, S.H. Postnatal adaptation of the gastrointestinal tract in neonatal pigs: A possible role of milk-borne growth factors. Livest. Prod. Sci. 2000, 66, 95–107. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Stress-induced augmentation of immune function—The role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav. Immun. 2002, 16, 785–798. [Google Scholar] [CrossRef]
- Mkwanazi, M.V.; Ncobela, C.N.; Kanengoni, A.T.; Chimonyo, M. Effects of environmental enrichment on behaviour, physiology and performance of pigs—A review. Asian Australas J. Anim. Sci. 2019, 32. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.M.; Jarvis, S.; Palarea-Albaladejo, J.; Edwards, S.A. The weaker sex? The propensity for male-biased piglet mortality. PLoS ONE 2012, 7, e30318. [Google Scholar] [CrossRef] [PubMed]

| Composition | Gestation Diet | Lactation Diet |
|---|---|---|
| Crude protein (%) | 13.9 | 15.0 |
| Crude fat (%) | 3.8 | 3.8 |
| Crude fiber (%) | 8.3 | 7.4 |
| Crude ash (%) | 5.1 | 5.4 |
| Total sugars and starch (%) | 40.0 | 41.2 |
| Lysine (%) | 0.7 | 0.8 |
| Methionine (%) | 0.3 | 0.3 |
| Phosphor (%) | 0.5 | 0.6 |
| Calcium (%) | 0.7 | 0.8 |
| Vitamin E (mg/kg) | 150.0 | 150.0 |
| Vitamin A (IU/kg) | 10,000 | 10,000 |
| Vitamin D3 (IU/kg) | 2,000 | 2,000 |
| Iron (mg/kg) | 53.0 | 53.0 |
| Iodine (mg/kg) | 2.0 | 2.0 |
| Copper (mg/kg) | 5.0 | 5.0 |
| Manganese (mg/kg) | 43.0 | 43.0 |
| Zinc (mg/kg) | 15.0 | 15.0 |
| Selenium (mg/kg) | 0.4 | 0.4 |
| Dependent Variable | Age | Sex | Treatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 9 | Day 38 | Female | Male | No treatment | Sham | ||||||||||
| n | Median ± SD | n | Median ± SD | p-Value | n | Median ± SD | n | Median ± SD | p-value | n | Median ± SD | n | Median ± SD | p-Value | |
| Glucose (mmol/L) | 20 | 6.87 ± 1.64 | 16 | 6.40 ± 1.70 | 0.300 | 24 | 6.40 ± 1.92 | 12 | 6.75 ± 1.03 | 0.960 | 16 | 6.55 ± 2.02 | 20 | 6.56 ± 1.32 | 0.341 |
| NEFA (mmol/L) | 20 | 0.58 ± 0.79 | 14 | 0.17 ± 0.18 | <0.001 | 23 | 0.46 ± 0.47 | 11 | 0.45 ± 1.05 | 0.925 | 15 | 0.47 ± 0.52 | 19 | 0.33 ± 0.82 | 0.125 |
| Urea (mmol/L) | 19 | 3.66 ± 0.88 | 15 | 1.66 ± 0.69 | <0.001 | 22 | 2.84 ± 1.41 | 12 | 2.32 ± 1.13 | 0.479 | 14 | 3.24 ± 1.13 | 20 | 2.32 ± 1.40 | 0.081 |
| Ig G (mg/mL) | 11 | 3.31 ± 4.93 | 11 | 2.97 ± 1.02 | 0.344 | 16 | 3.24 ± 4.27 | 6 | 2.55 ± 1.13 | - | 10 | 3.39 ± 5.50 | 12 | 2.55 ± 1.79 | 0.560 |
| IGF-1 (ng/mL) | 11 | 8.95 ± 15.99 | 11 | 38.61 ± 21.02 | 0.008 | 16 | 23.48 ± 25.05 | 6 | 15.03 ± 14.48 | - | 10 | 23.48 ± 27.05 | 12 | 20.17 ± 17.62 | 0.175 |
| RBC (1012/L) | 10 | 4.27 ± 0.42 | 15 | 5.69 ± 0.56 | <0.001 | 17 | 5.23 ± 0.96 | 8 | 5.33 ± 0.89 | 0.687 | 9 | 5.11 ± 1.04 | 16 | 5.46 ± 0.88 | 0.769 |
| HCT (%) | 10 | 30.45 ± 2.50 | 15 | 35.9 ± 3.51 | 0.005 | 17 | 33.5 ± 3.58 | 8 | 32.30 ± 4.96 | 0.600 | 9 | 32.60 ± 3.88 | 16 | 33.20 ± 4.15 | 0.759 |
| HGB (g/dL) | 10 | 8.40 ± 0.71 | 15 | 10.40 ± 1.12 | 0.001 | 17 | 9.20 ± 1.43 | 8 | 9.35 ± 1.26 | 0.814 | 9 | 9.20 ± 1.70 | 16 | 9.65 ± 1.17 | 0.418 |
| WBC (103/µL) | 10 | 10.73 ± 5.10 | 15 | 17.95 ± 4.20 | 0.021 | 17 | 13.18 ± 5.61 | 8 | 18.24 ± 4.11 | 0.039 | 9 | 12.66 ± 6.58 | 16 | 17.81 ± 4.39 | 0.908 |
| Lymphocytes (103/µL) | 10 | 4.18 ± 1.75 | 15 | 7.96 ± 1.62 | 0.001 | 17 | 6.15 ± 2.42 | 8 | 7.06 ± 1.87 | 0.616 | 9 | 4.94 ± 2.82 | 16 | 6.93 ± 1.90 | 0.950 |
| Monocytes (103/µL) | 10 | 0.89 ± 0.37 | 15 | 1.55 ± 0.57 | <0.001 | 17 | 0.94 ± 0.52 | 8 | 1.47 ± 0.70 | 0.086 | 9 | 1.08 ± 0.51 | 16 | 1.12 ± 0.64 | 0.952 |
| Neutrophils (103/µL) | 10 | 5.83 ± 3.15 | 15 | 8.33 ± 2.90 | 0.172 | 17 | 6.14 ± 3.22 | 8 | 9.70 ± 2.24 | 0.005 | 9 | 5.49 ± 3.77 | 16 | 8.40 ± 2.52 | 0.612 |
| Eosinophils (103/µL) | 10 | 0.10 ± 0.20 | 15 | 0.19 ± 0.13 | 0.817 | 17 | 0.13 ± 0.11 | 8 | 0.27 ± 0.20 | 0.038 | 9 | 0.12 ± 0.12 | 16 | 0.19 ± 0.17 | 0.913 |
| Basophils (103/µL) | 10 | 0.02 ± 0.06 | 15 | 0.01 ± 0.02 | 0.177 | 17 | 0.01 ± 0.05 | 8 | 0.02 ± 0.01 | 0.552 | 9 | 0.01 ± 0.06 | 16 | 0.02 ± 0.01 | 0.440 |
| Thrombocytes (103/µL) | 10 | 1087.50 ± 374.48 | 15 | 444.00 ± 173.61 | <0.001 | 17 | 646.00 ± 458.02 | 8 | 560.00 ± 283.45 | 0.461 | 9 | 764.00 ± 535.69 | 16 | 596.00 ± 334.41 | 0.689 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Tichelen, K.; Prims, S.; Ayuso, M.; Van Kerschaver, C.; Vandaele, M.; Degroote, J.; Van Cruchten, S.; Michiels, J.; Van Ginneken, C. Handling Associated with Drenching Does Not Impact Survival and General Health of Low Birth Weight Piglets. Animals 2021, 11, 404. https://doi.org/10.3390/ani11020404
Van Tichelen K, Prims S, Ayuso M, Van Kerschaver C, Vandaele M, Degroote J, Van Cruchten S, Michiels J, Van Ginneken C. Handling Associated with Drenching Does Not Impact Survival and General Health of Low Birth Weight Piglets. Animals. 2021; 11(2):404. https://doi.org/10.3390/ani11020404
Chicago/Turabian StyleVan Tichelen, Kevin, Sara Prims, Miriam Ayuso, Céline Van Kerschaver, Mario Vandaele, Jeroen Degroote, Steven Van Cruchten, Joris Michiels, and Chris Van Ginneken. 2021. "Handling Associated with Drenching Does Not Impact Survival and General Health of Low Birth Weight Piglets" Animals 11, no. 2: 404. https://doi.org/10.3390/ani11020404
APA StyleVan Tichelen, K., Prims, S., Ayuso, M., Van Kerschaver, C., Vandaele, M., Degroote, J., Van Cruchten, S., Michiels, J., & Van Ginneken, C. (2021). Handling Associated with Drenching Does Not Impact Survival and General Health of Low Birth Weight Piglets. Animals, 11(2), 404. https://doi.org/10.3390/ani11020404








