Establishing Cell Lines from Fresh or Cryopreserved Tissue from the Great Crested Newt (Triturus cristatus): A Preliminary Protocol
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Biopsy and Culture Media
2.2. Pre-Treatment and Primary Cell Culture
2.3. Cryopreservation and Recovery
2.4. Evaluation of Proliferation and Growth Conditions
2.5. Animals
2.6. Statistical Analysis
3. Results
Development of Cell Lines
4. Discussion and Conclusion
4.1. Parameters Affecting the Success Rate of Amphibian Cell Lines
4.2. Testing Optimal Growth Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Rannap, V.; Rannap, R.; Vuorio, V. Protection of the Great Crested Newt—Best Practice Guidelines—The Experiences of LIFE-Nature Project “Protection of Triturus cristatus in the Eastern Baltic Region”; Life Nature Project: Tallinn, Estonia, 2009. [Google Scholar]
- Clulow j Upton, R.; Trudeau, V.L.; Clulow, S. Amphibian assisted reproductive technologies: Moving from technology to application. In Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology, Switzerland; Comizzoli, P., Brown, J., Holt, W., Eds.; Springer: Berlin, Germany, 2019; pp. 413–463. [Google Scholar]
- Strand, J.; Thomsen, H.; Jensen, J.B.; Marcussen, C.; Nicolajsen, T.B.; Skriver, M.B.; Søgaard, I.M.; Ezaz, T.; Purup, S.; Callesen, H.; et al. Biobanking in amphibian and reptilian conservation and management: Opportunities and challenges. Conserv. Genet. Resour. 2020, 143, 44–58. [Google Scholar] [CrossRef]
- Zimkus, B.M.; Hassapakis, C.L.; Houck, M.L. Integrating current methods for the preservation of amphibian genetic resources and viable tissues to achieve best practices for species conservation. Amphib. Reptile Conserv. 2018, 12, e165. [Google Scholar]
- Chemnick, L.G.; Houck, M.L.; Ryder, O.A. Banking of genetic resources: The Frozen Zoo® at the San Diego Zoo. In Conservation Genetics in the Age of Genomics; Amato, G., Desalle, R., Rosenblum, H.C., Ryder, O.A., Eds.; Columbio University Press: New York, NY, USA, 2009; pp. 124–130. [Google Scholar]
- San Diego Zoo Institute for Conservation Research; Frozen Zoo®: San Diego, CA, USA, 2018; Available online: https://institute.sandiegozoo.org/resources/frozen-zoo (accessed on 5 June 2020).
- Saragusty, J.; Diecke, S.; Drukker, M.; Durrant, B.; Friedrich Ben-Nun, I.; Galli, C.; Göritz, F.; Hayashi, K.; Hermes, R.; Holtze, S.; et al. Rewinding the process of mammalian extinction. Zoo Biol. 2016, 35, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Clothier, R.H.; Balls, M.; Hostry, G.S.; Robertson, N.J.; Horner, S.A. Amphibian organ culture in experimental toxicology: The effects of paracetamol and phenacetin on cultured tissues from urodele and anuran amphibians. Toxicology 1982, 25, 31–40. [Google Scholar] [CrossRef]
- Slack, J.M.W.; Darlington, B.G.; Gillespie, L.L.; Godsave, S.F.; Isaacs, H.V.; Paterno, G.D. Mesoderm induction by fibroblast growth factor in early Xenopus development. Philos. Trans. Royal Soc. Lond. 1990, 327, 75–84. [Google Scholar]
- Tata, J.R.; Kawahara, A.; Baker, B.S. Prolactin inhibits both thyroid hormone-induced morphogenesis and cell death in cultured amphibian larval tissues. Dev. Biol. 1991, 146, 72–80. [Google Scholar] [CrossRef]
- Okumoto, H. Establishment of three cell lines derived from frog melanophores establishment of three cell lines derived from frog melanophores. Zool. Sci. 2001, 18, 483–496. [Google Scholar] [CrossRef]
- Groot, H.; Munuz-Carmargo, C.; Moscoso, J.; Riveros, G.; Salazar, V.; Florez, F.K.; Mitrani, E. Skin micro-organs from several frog species secrete a repertoire of powerful antimicrobials in culture. J. Antibiot. 2012, 65, 461–467. [Google Scholar] [CrossRef]
- Houck, M.L.; Lear, T.L.; Charter, S.J. Animal cytogenetics. In The AGT Cytogenetics Laboratory Manual, 4th; Arsham, M., Barch, M., Lawce, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2017; pp. 1055–1102. [Google Scholar]
- Raker, S.; Klinger, M.; Kruse, C.; Gebert, M. Pros and cons of fish skin cells in culture: Long-term full skin and short-term scale cell culture from rainbow trout, Oncorhynchus mykiss. Eur. J. Cell Biol. 2011, 90, 1041–105115. [Google Scholar] [CrossRef]
- Kho, D.; MacDonald, C.; Johnson, R.; Unsworth, C.P.; O’Carroll, S.J.; du Mez, E.; Angel, C.E.; Graham, E.S. Application of xCELLigence RTCA biosensor technology for revealing the profile and window of drug responsiveness in real time. Biosensors 2015, 5, 199–222. [Google Scholar] [CrossRef]
- Mollard, R. Culture, cryobanking and passaging of karyotypically validated native australian amphibian cells. Cryobiology 2018, 81, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, Z.; Huang, X.; Gao, X.; Zhang, Q. Establishment of three cell lines from chinese giant salamander and their sensitivities to the wild-type and recombinant ranavirus. Vet. Res. 2015, 46, 58. [Google Scholar] [CrossRef] [PubMed]
- Sinzinelle, L.; Thuret, R.; Hwang, H.; Herszberg, B.; Paillard, E.; Bronchain, O.J.; Stemple, D.L.; Dhorne-Pollet, S.; Pollet, N. Characterization of a novel xenopus tropicalis cell line as a model for in vitro studies. Genesis 2012, 50, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Stanchfield, J.E.; Yager, J.D. An estrogen responsive primary amphibian liver cell culture system. Exp. Cell Res. 1978, 116, 239–252. [Google Scholar] [CrossRef]
- Nishikawa, A.; Yoshizato, K. Hormonal regulation of growth and life span of bullfrog tadpole tail epidermal cells cultured in vitro. J. Exp. Zool. 1986, 230, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, F. Whole-cell and single channel K + and C1- currents in epithelial cells of frog skin. J. Gen. Physiol. 1991, 98, 131–161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiang, Y.; Gao, Q.; Su, W.; Zeng, L.; Wang, J.; Hu, Y.; Nie, W.; Ma, X.; Zhang, Y.; Lee, W.; et al. Establishment, characterization and immortalization of a fibroblast cell line from the chinese red belly toad Bombina maxima skin. Cytotechnology 2012, 64, 95–105. [Google Scholar] [CrossRef][Green Version]
- Bianchi, N.O.; Molina, J.O. DNA replication patterns in somatic chromosomes of Leptodactylus ocellatus (Amphibia, anura). Chromosoma 1967, 22, 391–400. [Google Scholar] [CrossRef]
- Handler, J.S.; Steele, B.; Sahib, M.K.; Wade, J.B.; Preston, A.S.; Lawson, N.L.; Johnson, J.P. Toad urinary bladder epithelial cells in culture: Maintenance of epithelial structure, sodium transport, and response to hormones. Proc. Natl. Acad. Sci. USA 1979, 76, 4151–4155. [Google Scholar] [CrossRef]
- Koniski, A.D.; Cohen, N. Reproducible proliferative responses of salamander (ambystoma mexicanum) lymphocytes cultured with mitogens in serum-free medium. Dev. Comp. Immunol. 1992, 16, 441–451. [Google Scholar] [CrossRef]
- Ketola-Pirie, C.; Atkinson, B.G. Cold- and heat-shock induction of new gene expression in cultured amphibian cells. J. Biochem. Cell Biol. 1983, 61, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Strand, J.; Callesen, H.; Pertoldi, C.; Purup, S. Amphibian cell lines—testing different media compositions. Unpublished material (Unpublished; manuscript in preparation).
- Rakers, S.; Umse, F.; Gebert, M. Real-time cell analysis: Sensitivity of different vertebrate cell cultures to copper sulfate measured by xCELLigence. Ecotoxicology 2014, 23, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Urcan, E.; Haertel, U.; Styllou, M.; Hickel, R.; Scherthan, H.; Reichl, F.X. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent. Mater. 2010, 26, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kustermann, S.; Manigold, T.; Ploix, C.; Skubatz, M.; Heckel, T.; Hinton, H.; Weiser, T.; Singer, T.; Suter, L.; Roth, A. A real-time impedance-based screening assay for drug-induced vascular leakage. Toxicol. Sci. 2014, 138, 333–343. [Google Scholar] [CrossRef][Green Version]
- Chalubinski, M.; Zemanek, K.; Skowron, W.; Wjodan, K.; Gorzelak, P.; Broncel, M. The effect of 7-ketocholesterol and 25-hydroxycholesterol on the integrity of the human aortic endothelial and intestinal epithelial barriers. Inflamm. Res. 2013, 62, 1015–1023. [Google Scholar] [CrossRef]
- Nad, I.B.; Au, S.H.; Wheeler, A.H. A microfluidic platform for complete mammalian cell culture. Lab Chip 2010, 10, 1493–1632. [Google Scholar]
- Sathya Sai Kumar, K.V.; Purna Sai, K.; Babu, M. Application of frog (rana tigerina daudin) skin collagen as a novel substrate in cell culture. J. Biomed. Mater. Res. 2002, 61, 197–202. [Google Scholar] [CrossRef]
- Freshney, I.R. Culture of Animal Cells: A Manual and Basic Technique and Specialized Applications, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
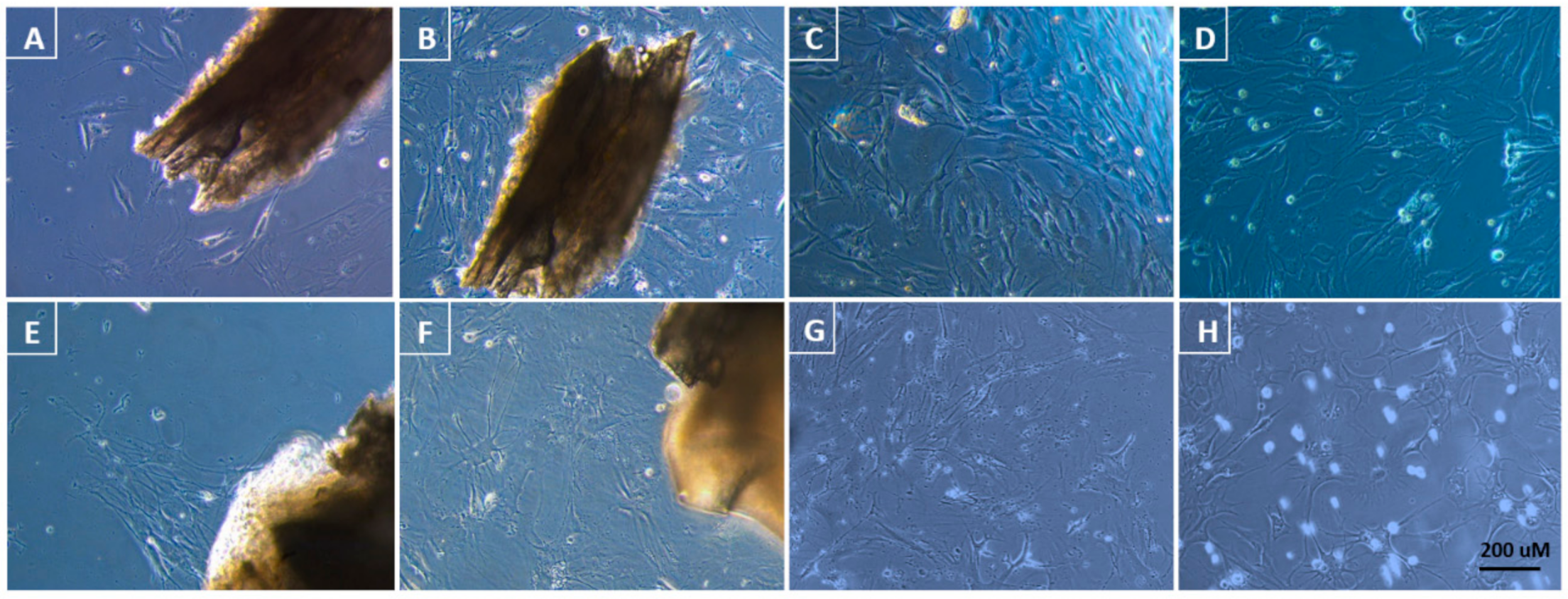
| Media | Supplements | Type of Tissue | |
|---|---|---|---|
| Medium A | 100% Cellgro Minimum Essential Medium (MEM) Alpha 1 X (Fisher Scientific) supplemented with 10% foetal bovine serum (Gibco Life Technologies, Rockville, MD, USA) and 1% penicillin-streptomycin–glutamine (29.2 mg/mL L-glutamine, 10,000 units/mL penicillin and 10,000 µg/mL streptomycin sulfate (Gibco® Life Technologies, Rockville, MD, USA) [13]. Plus 0.1% Normocin (Invivogen 500 mg) | Fresh or cryopreserved | |
| Medium B | 20 µL/mL ITS (100 µL insulin 10 mg/mL + 100 µL transferrin 5.5 mg/mL + 10 µL selenite 20 µg/mL) (Sigma-Aldrich, Inc, St.Louis, MO, USA) | Fresh or cryopreserved | |
| Medium C | 0.1 mM mercaptoethanol (Pharmacia Biotec) | Cryopreserved | |
| Medium D | 20 µL/mL ITS 0.1 mM mercaptoethanol (Pharmacia Biotec) | Cryopreserved |
| Lost to Infection | Growth Patterns | |||||
| Total no. of replicates | Unsuccessful due to fungus | Unsuccessful due to bacteria | Cell growth not observed | Culture reached 5–100 cells | Cell lines cryopreserved | |
| Fresh Tissue in Medium A/B | ||||||
| Individual 1 | 3/3 | 2/3 | 0/0 | 2/0 | 1/3 | 0/0 |
| Individual 2 | 3/3 | 1/2 | 0/1 | 0/1 | 3/2 | 1/0 |
| Individual 3 | 3/3 | 0/0 | 0/0 | 0/0 | 3/3 | 0/2 |
| Individual 4 | 3/3 | 2/2 | 0/1 | 1/1 | 1/2 | 0/0 |
| Cryopreserved Tissue in Medium A/B/C/D | ||||||
| Individual 1 | 3/3/3/3 | 1/3/1/1 | 1/0/0/0 | 3/3/3/3 | 0/0/0/0 | 0/0/0/0 |
| Individual 2 | 3/3/3/3 | 0/0/1/1 | 0/0/0/0 | 3/1/2/1 | 0/2/1/2 | 0/0/0/0 |
| Individual 3 | 3/3/3/3 | 2/2/3/1 | 0/1/2/0 | 2/3/3/1 | 1/0/0/2 | 0/0/0/1 |
| Individual 4 | 3/3/3/3 | 2/1/1/2 | 0/0/0/0 | 1/3/2/1 | 2/0/1/2 | 0/0/0/0 |
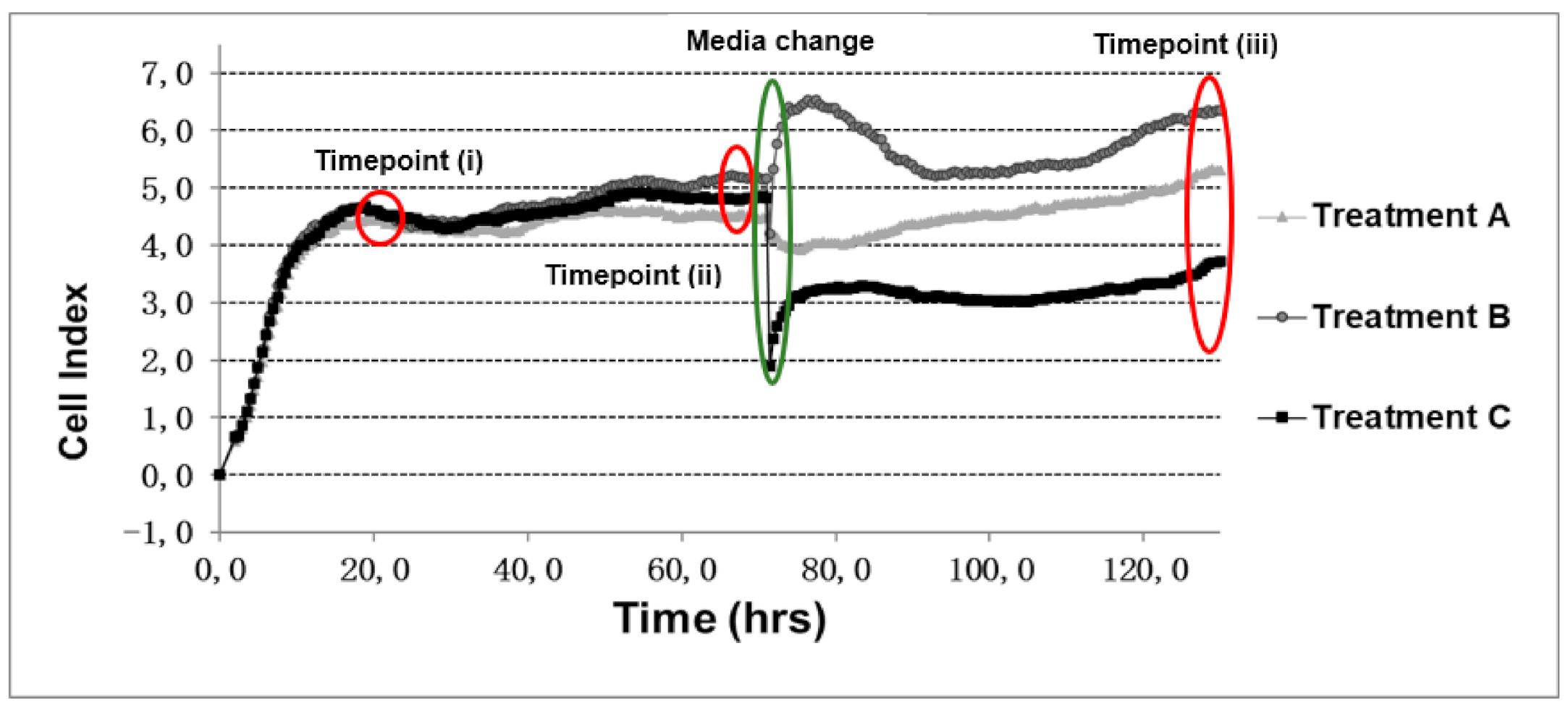
| Time Point | Cell Number | NoMC(A) (µ ± SE) | MC(B) (µ ± SE) | MCPBS(C) (µ ± SE) | One-Way ANOVA | Turkey’s Test |
|---|---|---|---|---|---|---|
| 20 h (i) | 5000 | 1.60 ± 0.12 | 1.37 ± 0.01 | 1.48 ± 0.13 | F = 2.9 = 1.36, p = 0.30 | |
| 7500 | 3.15 ± 0.13 | 3.06 ± 0.17 | 3.03 ± 0.03 | F = 2.9 = 0.23, p = 0.79 | ||
| 10,000 | 4.45 ± 0.08 | 4.60 ± 0.05 | 4.59 ± 0.05 | F = 2.9 = 1.96, p = 0.19 | ||
| 70 h (ii) | 5000 | 0.85 ± 0.14 | 0.74 ± 0.05 | 0.87 ± 0.09 | F = 2.9 = 0.47, p = 0.63 | |
| 7500 | 2.07 ± 0.23 | 2.10 ± 0.12 | 1.85 ± 0.14 | F = 2.9 = 0.60, p = 0.56 | ||
| 10,000 | 4.47 ± 0.19 | 5.13 ± 0.09 | 4.86 ± 0.17 | F = 2.9 = 4.39, p = 0.04 | (B > A) * | |
| 130 h (iii) | 5000 | 0.48 ± 0.19 | 0.22 ± 0.11 | -0.12 ± 0.04 | F = 2.9 = 5.54, p = 0.02 | (A > C) * |
| 7500 | 1.99 ± 0.47 | 1.34 ± 0.17 | 0.18 ± 0.07 | F = 2.9 = 10.06, p = 0.00 | (A > C) **, (B > C) * | |
| 10,000 | 5.26 ± 0.32 | 6.42 ± 0.23 | 3.72 ± 0.36 | F = 2.9 = 18.89, p = 0.00 | (A > C) *, (B > C) *** |
| Time Point | Cell Number | NoMC(A) (µ ± SE) | MC (B) (µ ± SE) | MCPBS (C) (µ ± SE) | One-Way ANOVA | Turkey’s Test |
|---|---|---|---|---|---|---|
| 20 h (i) | 5000 | 1.61 ± 0.08 | 1.2 ± 0.08 | 1.61 ± 0.06 | F = 2.9 = 1.88, p = 0.20 | |
| 7500 | 2.63 ± 0.13 | 2.16 ± 0.11 | 2.28 ± 0.07 | F = 2.9 = 5.26, p = 0.03 | (A > B) * | |
| 10,000 | 4.90 ± 0.15 | 5.11 ± 0.13 | 4.72 ± 0.08 | F = 2.9 = 2.47, p = 0.13 | ||
| 70 h (ii) | 5000 | 1.84 ± 0.08 | 1.79 ± 0.11 | 1.69 ± 0.05 | F = 2.9 = 0.75, p = 0.49 | |
| 7500 | 6.18 ± 0.15 | 6.08 ± 0.35 | 3.85 ± 0.10 | F = 2.9 = 4.35, p = 0.04 | (A > C) ***, (B > C) *** | |
| 10,000 | 6.45 ± 0.37 | 7.13 ± 0.13 | 6.86 ± 0.11 | F = 2.9 = 2.11, p = 0.17 | ||
| 130 h (iii) | 5000 | 2.21 ± 0.11 | 2.24 ± 0.13 | 1.27 ± 0.03 | F = 2.9 = 30.46, p = 0.00 | (A > C) ***, (B > C) *** |
| 7500 | 3.32 ± 0.12 | 2.81 ± 0.27 | 2.25 ± 0.18 | F = 2.9 = 7.11, p = 0.01 | (A > C) * | |
| 10,000 | 6.19 ± 0.15 | 6.08 ± 0.35 | 3.85 ± 0.10 | F = 2.9 = 33.72, p = 0.00 | (A > C) ***, (B > C) *** |
| Time Points | Treatment | Fresh Tissue (µ ± SE) | Cryopreserved Tissue (µ ± SE) | t-Test (p-Value) = Exact Permutation |
|---|---|---|---|---|
| 20 h (i) | NoMC (A) | 4.90 ± 0.15 | 2.94 ± 0.03 | (0.000013) = 0.0142 |
| MC (B) | 5.11 ± 0.13 | 2.77 ± 0.04 | (0.00000) = 0.0142 | |
| MCPBS (C) | 4.72 ± 0.08 | 2.74 ± 0.08 | (0.00000) = 0.0142 | |
| 70 h (ii) | NoMC (A) | 6.45 ± 0.37 | 6.57 ± 0.09 | (0.76074) = 0.8285 |
| MC (B) | 7.13 ± 0.13 | 6.55 ± 0.07 | (0.00796) = 0.0142 | |
| MCPBS (C) | 6.86 ± 0.11 | 6.59 ± 0.34 | (0.50642) = 0.5857 | |
| 130 h (iii) | NoMC (A) | 6.19 ± 0.15 | 8.61 ± 0.05 | (0.000004) = 0.0285 |
| MC (B) | 6.08 ± 0.35 | 8.25 ± 0.10 | (0.001035) = 0.0142 | |
| MCPBS (C) | 3.85 ± 0.10 | 5.98 ± 0.49 | (0.005869) = 0.0285 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strand, J.; Callesen, H.; Pertoldi, C.; Purup, S. Establishing Cell Lines from Fresh or Cryopreserved Tissue from the Great Crested Newt (Triturus cristatus): A Preliminary Protocol. Animals 2021, 11, 367. https://doi.org/10.3390/ani11020367
Strand J, Callesen H, Pertoldi C, Purup S. Establishing Cell Lines from Fresh or Cryopreserved Tissue from the Great Crested Newt (Triturus cristatus): A Preliminary Protocol. Animals. 2021; 11(2):367. https://doi.org/10.3390/ani11020367
Chicago/Turabian StyleStrand, Julie, Henrik Callesen, Cino Pertoldi, and Stig Purup. 2021. "Establishing Cell Lines from Fresh or Cryopreserved Tissue from the Great Crested Newt (Triturus cristatus): A Preliminary Protocol" Animals 11, no. 2: 367. https://doi.org/10.3390/ani11020367
APA StyleStrand, J., Callesen, H., Pertoldi, C., & Purup, S. (2021). Establishing Cell Lines from Fresh or Cryopreserved Tissue from the Great Crested Newt (Triturus cristatus): A Preliminary Protocol. Animals, 11(2), 367. https://doi.org/10.3390/ani11020367








