Overfeeding Extends the Period of Annual Cyclicity but Increases the Risk of Early Embryonic Death in Shetland Pony Mares
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
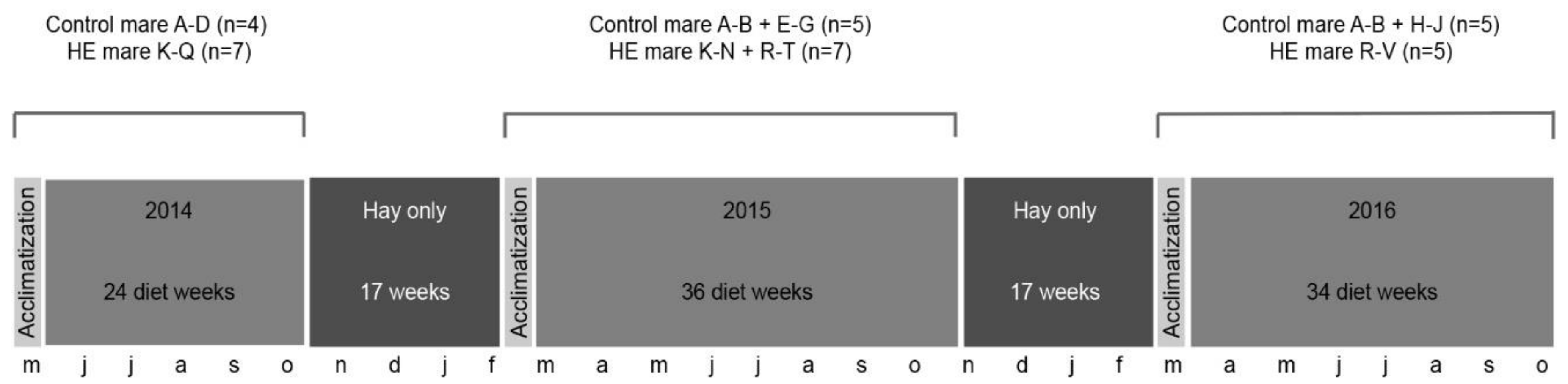
2.1. Horses and Husbandry
2.2. Diet
2.3. Measurements
2.3.1. Assessment of Obesity
2.3.2. Hemorrhagic Anovulatory Follicles
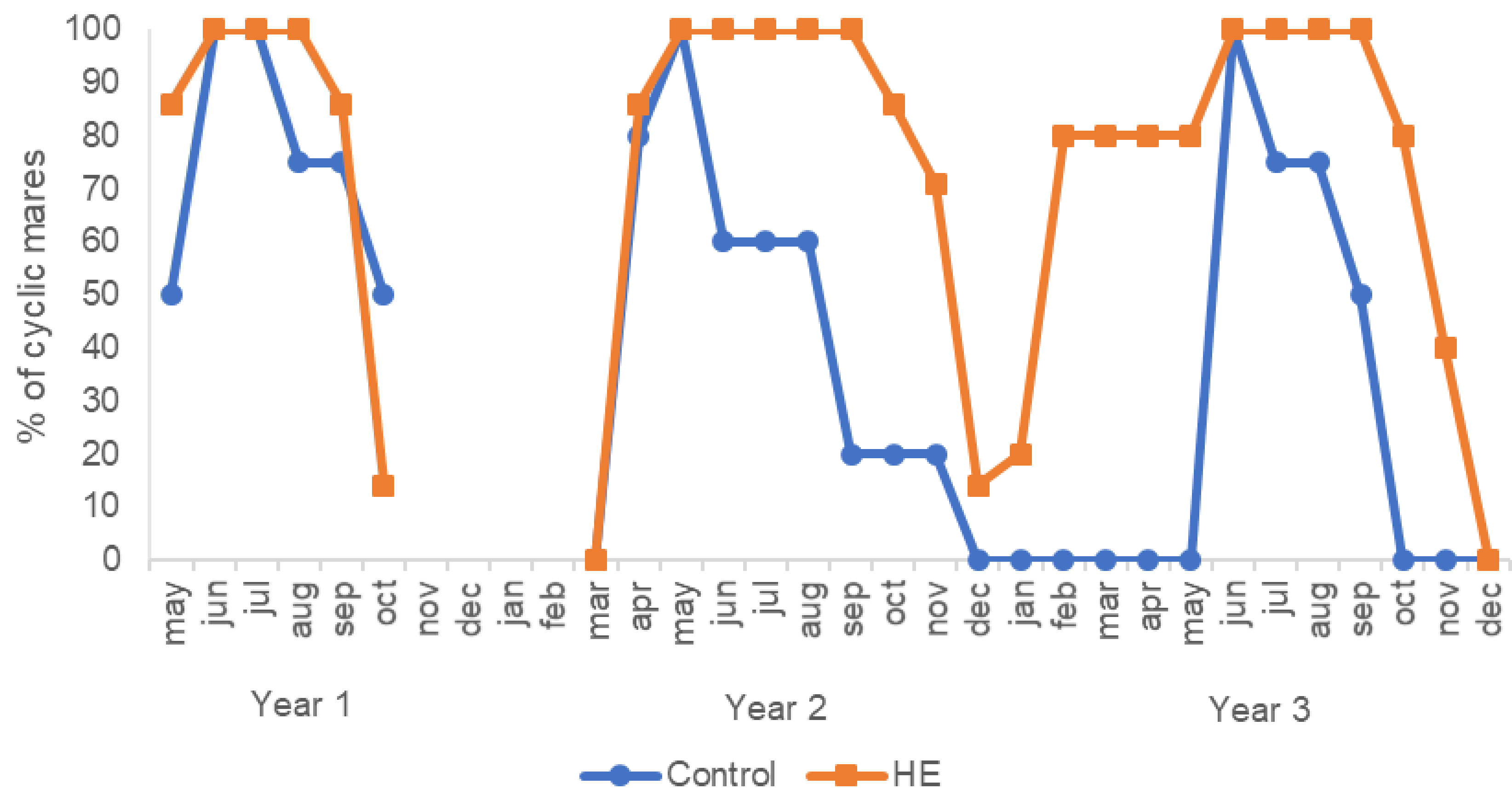
2.3.3. Duration of Annual Cyclicity
2.3.4. Embryonic Development
- -
- Control donor to control recipient mare (C-C)
- -
- HE donor to HE recipient mare (HE-HE)
- -
- Control donor to HE recipient mare (C-HE)
- -
- HE donor to control recipient mare (HE-C)
2.4. Statistical Analysis
3. Results
3.1. Obesity
3.2. Hemorrhagic Anovulatory Follicles
3.3. Duration of Annual Cyclicity
3.4. Embryonic Development
3.4.1. Embryo Recovery and Pregnancy Rates
3.4.2. Diameter and Area of the Conceptus Vesicle
3.4.3. Size and Weight of the Embryo Proper
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, R.M.; Boston, R.C.; Stefanovski, D.; Kronfeld, D.S.; Harris, P.A. Obesity and diet affect glucose dynamics and insulin sensitivity in Thoroughbred geldings. J. Anim. Sci. 2003, 81, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Treiber, K.H.; Kronfeld, D.S.; Hess, T.M.; Byrd, B.M.; Splan, R.K.; Staniar, W.B. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies. J. Am. Vet. Med. Assoc. 2006, 228, 1538–1545. [Google Scholar] [CrossRef]
- Heliczer, N. Cardiovascular findings in ponies with equine metabolic syndrome. J. Am. Vet. Med. Assoc. 2017, 250, 1027–1035. [Google Scholar] [CrossRef]
- Durham, A.E.; Frank, N.; McGowan, C.M.; Menzies-Gow, N.; Roelfsema, E.; Vervuert, I.; Feige, K.; Fey, K. ECEIM consensus statement on equine metabolic syndrome. J. Vet. Intern. Med. 2019, 33, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Vick, M.M.; Sessions, D.R.; Murphy, B.A.; Kennedy, E.L.; Reedy, S.E.; Fitzgerald, B.P. Obesity is associated with altered metabolic and reproductive activity in the mare: Effects of metformin on insulin sensitivity and reproductive cyclicity. Reprod. Fertil. Dev. 2006, 18, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Guillaume, D.; Daels, P. Seasonality in mares. Anim. Reprod. Sci. 2000, 60, 245–262. [Google Scholar] [CrossRef]
- Aurich, C. Reproductive cycles of horses. Anim. Reprod. Sci. 2011, 124, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Ortiz, J.; Camous, S.; Briant, C.; Lardic, L.; Chesneau, D.; Guillaume, D. Effects of nutritional cues on the duration of the winter anovulatory phase and on associated hormone levels in adult female Welsh pony horses (Equus caballus). Reprod. Biol. Endocrinol. 2011, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, B.P.; McManus, C.J. Photoperiodic Versus Metabolic Signals as Determinants of Seasonal Anestrus in the Mare1. Biol. Reprod. 2000, 63, 335–340. [Google Scholar] [CrossRef]
- Cuervo-Arango, J.; Newcombe, J.R. Risk factors for the development of haemorrhagic anovulatory follicles in the mare. Reprod. Domest. Anim. 2010, 45, 473–480. [Google Scholar] [CrossRef]
- McCue, P.M.; Squires, E.L. Persistent anovulatory follicles in the mare. Theriogenology 2002, 58, 541–543. [Google Scholar]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Rittenberg, V.; Seshadri, S.; Sunkara, S.K.; Sobaleva, S.; Oteng-Ntim, E.; El-Toukhy, T. Effect of body mass index on IVF treatment outcome: An updated systematic review and meta-analysis. Reprod. Biomed. Online 2011, 23, 421–439. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.; Mohammadi Roushandeh, A.; Alizadeh, Z.; Vahidinia, A.; Vahabian, M.; Hosseini, M. Effect of a high fat diet on ovary morphology, in vitro development, in vitro fertilisation rate and oocyte quality in mice. Singap. Med. J. 2015, 56, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2008, 23, 271–278. [Google Scholar] [CrossRef] [PubMed]
- D’ Fonseca, N.M.M.; Gibson, C.M.E.; Doorn, D.A.; Ruijter-Villani, M.; Stout, T.A.E.; Roelfsema, E. Effect of long-term overfeeding of a high-energy diet on glucose tolerance in Shetland pony mares. J. Vet. Intern. Med. 2020, 34, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Centraal Veevoederbureau. Het EWpa en VREp systeem. CVB documentatierapport No. 31; Centraal Veevoederbureau: Lelystad, The Netherlands, 2004. (In Dutch) [Google Scholar]
- Carter, R.A.; McCutcheon, L.J.; George, L.A.; Smith, T.L.; Frank, N.; Geor, R.J. Effects of diet-induced weight gain on insulin sensitivity and plasma hormone and lipid concentrations in horses. Am. J. Vet. Res. 2009, 70, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Arango, J.; Newcombe, J.R. Ultrasound characteristics of experimentally induced luteinized unruptured follicles (LUF) and naturally occurring hemorrhagic anovulatory follicles (HAF) in the mare. Theriogenology 2012, 77, 514–524. [Google Scholar] [CrossRef]
- Stout, T.A.E. Equine embryo transfer: Review of developing potential. Equine Vet. J. 2010, 38, 467–478. [Google Scholar] [CrossRef]
- Clark, K.E.; Squires, E.L.; McKinnon, A.O.; Seidel, G.E., Jr. Viability of stored equine embryos. J. Anim. Sci. 1987, 65, 534–542. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.; de Ruijter-Villani, M.; Stout, T.A.E. Negative uterine asynchrony retards early equine conceptus development and upregulation of placental imprinted genes. Placenta 2017, 57, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Wang, W. ExactCIdiff: An R Package for Computing Exact Confidence Intervals for the Difference of Two Proportions. R J. 2013, 5, 62–71. [Google Scholar] [CrossRef]
- Sleutjens, J.; Serra Bragança, F.M.; van Empelen, M.W.; ten Have, R.E.; de Zwaan, J.; Roelfsema, E.; Oosterlinck, M.; Back, W. Mouldable, thermoplastic, glue-on frog-supportive shoes change hoof kinetics in normal and obese Shetland ponies. Equine Vet. J. 2018, 50, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J.; Gastal, M.O.; Gastal, E.L.; Jacob, J.C.; Beg, M.A. Induction of haemorrhagic anovulatory follicles in mares. Reprod. Fertil. Dev. 2008, 20, 947–954. [Google Scholar] [CrossRef]
- Bamford, N.J. Effect of increased adiposity on insulin sensitivity and adipokine concentrations in different equine breeds adapted to cereal-rich or fat-rich meals. Vet. J. 2016, 214, 14–20. [Google Scholar] [CrossRef] [PubMed]
- De Laat, M.A. Equine hyperinsulinemia: Investigation of the enteroinsular axis during insulin dysregulation. Am. J. Physiol. Endocrinol. Metab. 2015, 310, E61–E72. [Google Scholar] [CrossRef]
- Blache, D.; Chagas, L.M.; Blackberry, M.A.; Vercoe, P.E.; Martin, G.B. Metabolic factors affecting the reproductive axis in male sheep. J. Reprod. Fertil. 2000, 120, 1–11. [Google Scholar] [CrossRef][Green Version]
- Sessions-Bresnahan, D.R.; Carnevale, E.M. The effect of equine metabolic syndrome on the ovarian follicular environment. J. Anim. Sci. 2014, 92, 1485–1494. [Google Scholar] [CrossRef]
- Sessions-Bresnahan, D.R.; Schauer, K.L.; Heuberger, A.L.; Carnevale, E.M. Effect of Obesity on the Preovulatory Follicle and Lipid Fingerprint of Equine Oocytes. Biol. Reprod. 2016, 94, 15. [Google Scholar] [CrossRef]
- Laskowski, D.; Båge, R.; Humblot, P.; Andersson, G.; Sirard, M.-A.; Sjunnesson, Y. Insulin during in vitro oocyte maturation has an impact on development, mitochondria, and cytoskeleton in bovine day 8 blastocysts. Theriogenology 2017, 101, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lange Consiglio, A.; Dell’Aquila, M.E.; Fiandanese, N.; Ambruosi, B.; Cho, Y.S.; Bosi, G.; Arrighi, S.; Lacalandra, G.M.; Cremonesi, F. Effects of leptin on in vitro maturation, fertilization and embryonic cleavage after ICSI and early developmental expression of leptin (Ob) and leptin receptor (ObR) proteins in the horse. Reprod. Biol. Endocrinol. 2009, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Kšiňanová, M.; Čikoš, Š.; Babel’Ová, J.; Šefčíková, Z.; Špirková, A.; Koppel, J.; Fabian, D. The Responses of Mouse Preimplantation Embryos to Leptin In Vitro in a Transgenerational Model for Obesity. Front. Endocrinol. 2017, 8, 233. [Google Scholar] [CrossRef] [PubMed]
| Year | Mean ± SD BCS | Mean ± SD BW (Kg) | |||
|---|---|---|---|---|---|
| Control | HE | Control | HE | ||
| 1 | Start | 4 ± 1 | 5 ± 1 | 152 ± 22 | 161 ± 28 |
| End | 3 ± 1 | 8 ± 1 | 163 ± 23 | 205 ± 36 | |
| 2 | Start | 6 ± 0 | 7 ± 1 | 176 ± 12 | 189 ± 35 |
| End | 5 ± 1 | 9 ± 0 | 166 ± 8 | 245 ± 33 | |
| 3 | Start | 5 ± 1 | 7 ± 1 | 186 ± 10 | 208 ± 13 |
| End | 5 ± 1 | 8 ± 0 | 186 ± 12 | 263 ± 16 | |
| Year | Number of Mares | Total HAFs | Total Cycles | Mean ± SD HAFs | Mean Cycles ± SD | Percentage HAFs/Cycles | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | HE | Control | HE | Control | HE | Control | HE | Control | HE | Control | HE | |
| 1 | 4 | 7 | 3 | 20 | 24 | 56 | 1 ± 1 | 3 ± 2 | 6 ± 2 | 8 ± 1 | 13% | 36% |
| 2 | 5 | 7 | 8 | 4 | 32 | 80 | 2 ± 1 | 1 ± 1 | 6 ± 2 | 11 ± 1 | 25% | 5% |
| 3 | 5 | 5 | 5 | 12 | 29 | 46 | 1 ± 1 | 2 ± 3 | 6 ± 1 | 9 ± 1 | 17% | 26% |
| Parameter | Group of Transfer | ET + 7 | ET + 14 | ET + 21 |
|---|---|---|---|---|
| Pregnancy rate at day 28 of gestation (day 21 after ET) | C-C | − | − | 100% (4/4 successful pregnancies) |
| HE-HE | − | − | 56% (5/9 successful pregnancies) | |
| C-HE | − | − | 100% (5/5 successful pregnancies) | |
| HE-C | − | − | 63% (5/8 successful pregnancies) | |
| Diameter of the conceptus (mm) | C-C | 10.9 ± 2.5 | 28.6 ± 3.1 | 32.4 ± 3.6 |
| HE-HE | 14.1 ± 1.6 | 25.4 ± 4.8 | 30.2 ± 1.7 | |
| C-HE | 13.1 ± 4.5 | 27.0 ± 3.8 | 31.1 ± 5.5 | |
| HE-C | 15.6 ± 2.7 | 29.1 ± 4.3 | 33.2 ± 5.0 | |
| Area of the vesicle (mm) | C-C | 93.7 ± 43.4 | 570.9 ± 129.7 | 735.0 ± 149.5 |
| HE-HE | 155.0 ± 36.7 | 449.7 ± 99.0 | 652.3 ± 87.1 | |
| C-HE | 146.7 ± 93.0 | 506.2 ± 152.0 | 738.8 ± 192.7 | |
| HE-C | 185.8 ± 61.1 | 609.8 ± 152.3 | 789.1 ± 186.8 | |
| Size of the embryo (mm) | C-C | − | 3.29 ± 0.53 | - |
| HE-HE | − | 3.46 ± 0.81 | - | |
| C-HE | − | 3.89 ± 0.84 | - | |
| HE-C | − | 3.60 ± 0.74 | - | |
| Crown-rump length (mm) | C-C | − | − | 9.3 ± 0.3 |
| HE-HE | − | − | 10.1± 1.5 | |
| C-HE | − | − | 9.7 ± 1.4 | |
| HE-C | − | − | 11 ± 0 | |
| Weight (mg) | C-C | − | − | 162 ± 26 |
| HE-HE | − | − | 182 ± 66 | |
| C-HE | − | − | 185 ± 63 | |
| HE-C | − | − | 170 ± 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Fonseca, N.M.M.; Gibson, C.M.E.; Hummel, I.; van Doorn, D.A.; Roelfsema, E.; Stout, T.A.E.; van den Broek, J.; de Ruijter-Villani, M. Overfeeding Extends the Period of Annual Cyclicity but Increases the Risk of Early Embryonic Death in Shetland Pony Mares. Animals 2021, 11, 361. https://doi.org/10.3390/ani11020361
D’Fonseca NMM, Gibson CME, Hummel I, van Doorn DA, Roelfsema E, Stout TAE, van den Broek J, de Ruijter-Villani M. Overfeeding Extends the Period of Annual Cyclicity but Increases the Risk of Early Embryonic Death in Shetland Pony Mares. Animals. 2021; 11(2):361. https://doi.org/10.3390/ani11020361
Chicago/Turabian StyleD’Fonseca, Nicky M. M., Charlotte M. E. Gibson, Iris Hummel, David A. van Doorn, Ellen Roelfsema, Tom A. E. Stout, Jan van den Broek, and Marta de Ruijter-Villani. 2021. "Overfeeding Extends the Period of Annual Cyclicity but Increases the Risk of Early Embryonic Death in Shetland Pony Mares" Animals 11, no. 2: 361. https://doi.org/10.3390/ani11020361
APA StyleD’Fonseca, N. M. M., Gibson, C. M. E., Hummel, I., van Doorn, D. A., Roelfsema, E., Stout, T. A. E., van den Broek, J., & de Ruijter-Villani, M. (2021). Overfeeding Extends the Period of Annual Cyclicity but Increases the Risk of Early Embryonic Death in Shetland Pony Mares. Animals, 11(2), 361. https://doi.org/10.3390/ani11020361






