Serological and Molecular Characterization of Mycobacterium avium Subsp. paratuberculosis (MAP) from Sheep, Goats, Cattle and Camels in the Eastern Province, Saudi Arabia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Samples Collection
2.3. Serological Identification of MAP in Serum Samples
2.4. Molecular Identification of MAP in Fecal Samples
2.5. Data Analysis
3. Results
3.1. Clinical and Post-Mortem Lesions
3.2. Serological Detection of MAP
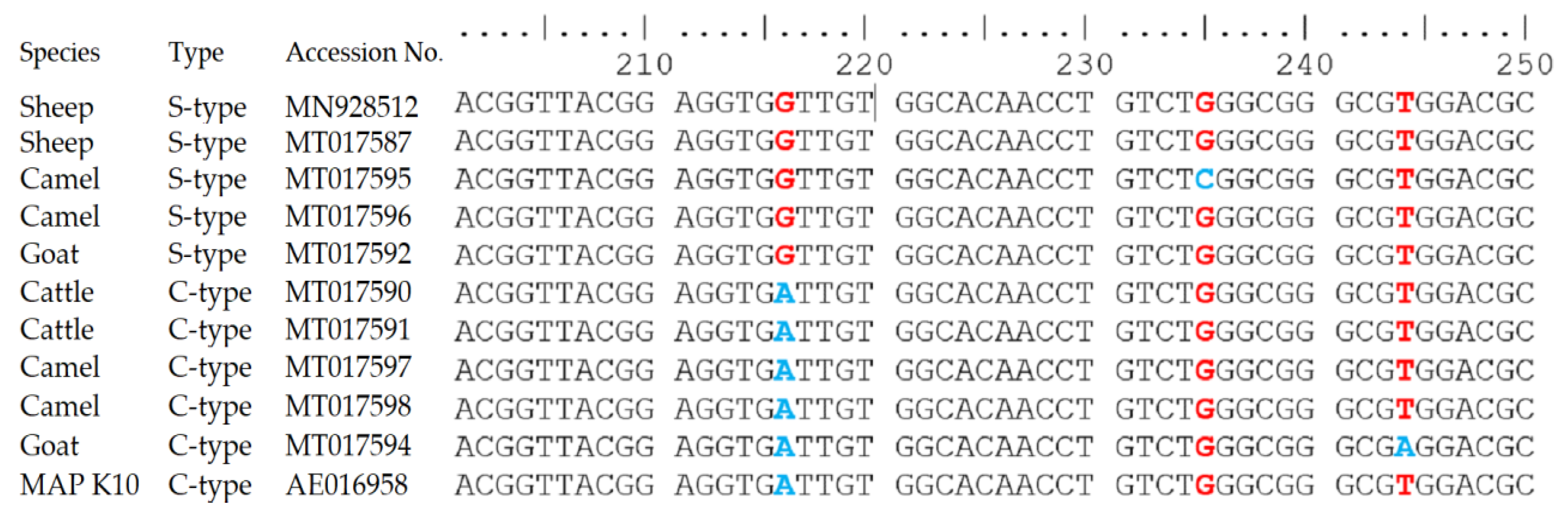
3.3. Molecular Detection of MAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittington, R.; Hope, A.; Marshall, D.; Taragel, C.; Marsh, I. Molecular epidemiology of Mycobacterium avium subsp. paratu-berculosis: IS900 restriction fragment length polymorphism and IS1311 polymorphism analyses of isolates from animals and a human in Australia. JCM. 2000, 38, 3240–3248. [Google Scholar] [CrossRef]
- Corbett, C.S.; De Jong, M.C.M.; Orsel, K.; De Buck, J.; Barkema, H.W. Quantifying transmission of Mycobacterium avium subsp. paratuberculosis among group-housed dairy calves. Vet. Res. 2019, 50, 1–14. [Google Scholar] [CrossRef]
- Harris, N.B.; Barletta, R.G. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 2001, 14, 489–512. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Schukken, Y.; Koets, A.P.; Weber, M.; Bakker, D.; Stabel, J.R.; Whitlock, R.H.; Louzoun, Y. Differences in intermittent and continuous fecal shedding patterns between natural and experimental Mycobacterium avium subspecies paratuberculosis infections in cattle. Vet. Res. 2015, 46, 1–10. [Google Scholar] [CrossRef]
- Rathnaiah, G.; Zinniel, D.K.; Bannantine, J.P.; Stabel, J.R.; Gröhn, Y.T.; Collins, M.T.; Barletta, R.G. Pathogenesis, Molecular Genetics, and Genomics of Mycobacterium avium subsp. paratuberculosis, the Etiologic Agent of Johne’s Disease. Front. Veter Sci. 2017, 4, 187. [Google Scholar] [CrossRef]
- Behr, M.A.; Kapur, V. The evidence for Mycobacterium paratuberculosis in Crohn’s disease. Curr. Opin. Gastroenterol. 2008, 24, 17–21. [Google Scholar] [CrossRef]
- Pierce, E.S. Could Mycobacterium avium subspecies paratuberculosis cause Crohn’s disease, ulcerative colitis… and colorectal cancer? Infect. Agents Cancer 2018, 13, 1–6. [Google Scholar] [CrossRef]
- Gardner, I.A.; Nielsen, S.S.; Whittington, R.; Collins, M.T.; Bakker, D.; Harris, B.; Sreevatsan, S.; Lombard, J.E.; Sweeney, R.; Smith, D.R.; et al. Consensus-based reporting standards for diagnostic test accuracy studies for paratuberculosis in ruminants. Prev. Veter Med. 2011, 101, 18–34. [Google Scholar] [CrossRef]
- Prendergast, D.M.; Pearce, R.; Yearsley, D.; Ramovic, E.; Egan, J. Evaluation of three commercial PCR kits for the direct detection of Mycobacterium avium subsp. paratuberculosis (MAP) in bovine faeces. Vet. J. 2018, 241, 52–57. [Google Scholar] [CrossRef]
- Aly, S.S.; Anderson, R.; Adaska, J.; Jiang, J.; Gardner, I. Association between Mycobacterium avium subspecies ++++ infection and milk production in two California dairies. J. Dairy Sci. 2010, 93, 1030–1040. [Google Scholar] [CrossRef]
- Clark, D., Jr.; Koziczkowski, J.; Radcliff, R.; Carlson, R.; Ellingson, J. Detection of Mycobacterium avium subspecies paratubercu-losis: Comparing fecal culture versus serum enzyme-linked immunosorbent assay and direct fecal polymerase chain reaction. J. Dairy Sci. 2008, 91, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Logar, K.; Kopinč, R.; Bandelj, P.; Starič, J.; Lapanje, A.; Ocepek, M. Evaluation of combined high-efficiency DNA extraction and real-time PCR for detection of Mycobacterium avium subsp. paratuberculosis in subclinically infected dairy cattle: Comparison with faecal culture, milk real-time PCR and milk ELISA. BMC Vet. Res. 2012, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Sting, R.; Hrubenja, M.; Mandl, J.; Seemann, G.; Salditt, A.; Waibel, S. Detection of Mycobacterium avium subsp. paratuberculosis in faeces using different procedures of pre-treatment for real-time PCR in comparison to culture. Vet. J. 2014, 199, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Stabel, J.R.; Bannantine, J.P. Development of a Nested PCR Method Targeting a Unique Multicopy Element, ISMap02, for Detection of Mycobacterium avium subsp. paratuberculosis in Fecal Samples. J. Clin. Microbiol. 2005, 43, 4744–4750. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; Gabric, D.M.; De Lisle, G.W. Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization. J. Clin. Microbiol. 1990, 28, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, K.; Hughes, V.M.; De Juan, L.; Inglis, N.F.; Wright, F.; Sharp, J.M. Molecular Characterization of Pigmented and Nonpigmented Isolates of Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 2002, 40, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, E.; Aranaz, A.; De Juan, L.; Alvarez, J.; Rodriguez-Campos, S.; Romero, B.; Bezos, J.; Stevenson, K.; Mateos, A.; Domínguez, L. Single Nucleotide Polymorphisms in the IS900 Sequence of Mycobacterium avium subsp. paratuberculosis Are Strain Type Specific. J. Clin. Microbiol. 2009, 47, 2260–2264. [Google Scholar] [CrossRef]
- Sonawane, G.G.; Tripathi, B.N. Comparison of a quantitative real-time polymerase chain reaction (qPCR) with conventional PCR, bacterial culture and ELISA for detection of Mycobacterium avium subsp. paratuberculosis infection in sheep showing pathology of Johne’s disease. SpringerPlus 2013, 2, 1–9. [Google Scholar] [CrossRef]
- De Juan, L.; Mateos, A.; Dominguez, L.; Sharp, J.; Stevenson, K. Genetic diversity of Mycobacterium avium subspecies paratu-berculosis isolates from goats detected by pulsed-field gel electrophoresis. Vet. Microbiol. 2005, 106, 249–257. [Google Scholar] [CrossRef]
- Bryant, J.; Thibault, V.C.; Smith, D.G.E.; McLuckie, J.; Heron, I.; Sevilla, I.A.; Biet, F.; Harris, S.R.; Maskell, D.J.; Bentley, S.D.; et al. Phylogenomic exploration of the relationships between strains of Mycobacterium avium subspecies paratuberculosis. BMC Genom. 2016, 17, 1–12. [Google Scholar] [CrossRef]
- Asghar, A.H.; El-rahim, I.; Mohamed, A.M.; Ahmed, O.B. Clinical and molecular investigations of Johne’s disease among small ruminants in Makkah, Saudi Arabia. Int. J. Bioassays 2014, 3445–3451. [Google Scholar]
- Al-Dubaib, M.A.; Mahmoud, O. Paratuberculosis of goats at Qassim region of Central Saudi Arabia. Bulg. J. Vet. Med. 2008, 11, 65–69. [Google Scholar]
- Shabana, I.I.; Aljohani, A.A. Sero-surveillance of Mycobacterium avium subspecies paratuberculosis infection in ruminants in Medina. J. Adv. Vet. Anim. Res. 2020, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.B.; Al-Swailem, A.M.; Al-Dubaib, M.A.; Al-Yamani, E.; Al-Naeem, A.; Shehata, M.; Hashad, M.E.; Albusadah, K.A.; Mahmoud, O.M. Pathology and molecular diagnosis of paratuberculosis of camels. Trop. Anim. Heal. Prod. 2011, 44, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; El-Deeb, W.M.; Zaghawa, A.A.; Housawi, F.M.; Alluwaimi, A.M. Investigation of Mycobacterium paratuberculosis in Arabian dromedary camels (Camelus dromedarius). Veter World 2019, 12, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; De Zoete, M.; Cavaignac, S.M. Mycobacterium avium subsp. paratuberculosis Strains from Cattle and Sheep Can Be Distinguished by a PCR Test Based on a Novel DNA Sequence Difference. J. Clin. Microbiol. 2002, 40, 4760–4762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dohmann, K.; Strommenger, B.; Stevenson, K.; De Juan, L.; Stratmann, J.; Kapur, V.; Bull, T.J.; Gerlach, G.-F. Characterization of Genetic Differences between Mycobacterium avium subsp. paratuberculosis Type I and Type II Isolates. J. Clin. Microbiol. 2003, 41, 5215–5223. [Google Scholar] [CrossRef][Green Version]
- Losinger, W.C. Economic impact of reduced milk production associated with Johne’s disease on dairy operations in the USA. J. Dairy Res. 2005, 72, 425–432. [Google Scholar] [CrossRef]
- Whitlock, R.H.; Buergelt, C. Preclinical and Clinical Manifestations of Paratuberculosis (Including Pathology). Veter Clin. N. Am. Food Anim. Pr. 1996, 12, 345–356. [Google Scholar] [CrossRef]
- Tiwari, A.; VanLeeuwen, J.A.; McKenna, S.L.; Keefe, G.P.; Barkema, H.W. Johne’s disease in Canada: Part I: Clinical symptoms, pathophysiology, diagnosis, and prevalence in dairy herds. Can. Vet. J. La Rev. Vet. Can. 2006, 47, 874–882. [Google Scholar]
- Dargatz, D.A.; Byrum, B.A.; Barber, L.K.; Sweeney, R.W.; Whitlock, R.H.; Shulaw, W.P.; Jacobson, R.H.; Stabel, J.R. Evaluation of a commercial ELISA for diagnosis of paratuberculosis in cattle. J. Am. Veter Med Assoc. 2001, 218, 1163–1166. [Google Scholar] [CrossRef]
- McKenna, S.; Keefe, G.; Barkema, H.W.; McClure, J.; VanLeeuwen, J.; Hanna, P.; Sockett, D. Cow-Level Prevalence of Paratuberculosis in Culled Dairy Cows in Atlantic Canada and Maine. J. Dairy Sci. 2004, 87, 3770–3777. [Google Scholar] [CrossRef]
- Fecteau, M.-E. Paratuberculosis in Cattle. Veter Clin. N. Am. Food Anim. Pract. 2018, 34, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Maroudam, V.; Mohana Subramanian, B.; Praveen Kumar, P.; Dhinakar Raj, G. Paratuberculosis: Diagnostic methods and their constraints. J. Vet. Sci. Technol. 2015, 6, 4172. [Google Scholar]
- Manning, E.; Collins, M. Mycobacterium avium subsp. paratuberculosis: Pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. l’OIE 2001, 20, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Attili, A.-R.; Victor, N.N.; Preziuso, S.; Luciana, P.; Anastasia, D.; Vincenzo, C. Ovine Paratuberculosis: A Seroprevalence Study in Dairy Flocks Reared in the Marche Region, Italy. Veter Med. Int. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Ocepek, M.; Pogačnik, M.; Logar, K.; Ferme, D.; Pate, M.; Krt, B. Seroprevalence of paratuberculosis in cattle in Slovenia. In Proceedings of the 10th International Colloquium on Paratuberculosis, Minneapolis, MI, USA, 9–14 August 2009; International Association for Paratuberculosis: Minneapolis, MN, USA, 2009; pp. 73–75. [Google Scholar]
- Böttcher, J.; Gangl, A. Mycobacterium avium ssp. Paratuberculosis–Combined Serological Testing and Classification of Individual Animals and Herds. J. Veter Med. Ser. B 2004, 51, 443–448. [Google Scholar] [CrossRef]
- Bauman, C.; Jones-Bitton, A.; Jansen, J.; Kelton, D.; Menzies, P.I. Evaluation of fecal culture and fecal RT-PCR to detect Mycobacterium avium ssp. paratuberculosis fecal shedding in dairy goats and dairy sheep using latent class Bayesian modeling. BMC Vet. Res. 2016, 12, 1–9. [Google Scholar] [CrossRef]
- Dreier, S.; Khol, J.L.; Stein, B.; Fuchs, K.; Gütler, S.; Baumgartner, W. Serological, Bacteriological and Molecularbiological Survey of Paratuberculosis (Johne’s Disease) in Austrian Cattle. J. Veter Med. Ser. B 2006, 53, 477–481. [Google Scholar] [CrossRef]
- Garrido, J.M.; Cortabarria, N.; A Oguiza, J.; Aduriz, G.; A Juste, R. Use of a PCR method on fecal samples for diagnosis of sheep paratuberculosis. Veter Microbiol. 2000, 77, 379–386. [Google Scholar] [CrossRef]
- Ikiz, S.; Bagcigil, A.; Ak, S.; Ozgur, N.; Lgaz, A. Paratuberculosis in cattle in Turkey detected by PCR. MED WETER 2005, 61, 881–883. [Google Scholar]
- Chiodini, R.J.; Hansen, D.; Whitlock, R.H. Council for agricultural science and technology-3 for agricultural science and technology. Int. J. Mycobacteriol. 2001, 3, 101–107. [Google Scholar]
- Pillai, S.; Jayarao, B. Application of IS900 PCR for Detection of Mycobacterium avium subsp. paratuberculosis Directly from Raw Milk. J. Dairy Sci. 2002, 85, 1052–1057. [Google Scholar] [CrossRef]
- Wells, S.J.; Collins, M.T.; Faaberg, K.S.; Wees, C.; Tavornpanich, S.; Petrini, K.R.; Collins, J.E.; Cernicchiaro, N.; Whitlock, R.H. Evaluation of a Rapid Fecal PCR Test for Detection of Mycobacterium avium subsp. paratuberculosis in Dairy Cattle. Clin. Vaccine Immunol. 2006, 13, 1125–1130. [Google Scholar] [CrossRef]
- Debroy, B.; Tripathi, B.; Sonawane, G.; Bind, R. Detection of Mycobacterium avium subspecies paratuberculosis (MAP) in alco-hol-fixed tissues of sheep by ISMav2 gene PCR and its comparison with histopathology, bacterial culture and IS900 PCR. Small Rumin. Res. 2012, 105, 329–334. [Google Scholar] [CrossRef]
- Motiwala, A.S.; Li, L.; Kapur, V.; Sreevatsan, S. Current understanding of the genetic diversity of Mycobacterium avium subsp. paratuberculosis. Microbes Infect. 2006, 8, 1406–1418. [Google Scholar] [CrossRef]
- Castellanos, E.; Aranaz, A.; Romero, B.; De Juan, L.; Alvarez, J.; Bezos, J.; Rodriguez-Campos, S.; Stevenson, K.; Mateos, A.; Domínguez, L. Polymorphisms in gyrA and gyrB Genes among Mycobacterium avium subsp. paratuberculosis Type I, II, and III Isolates. J. Clin. Microbiol. 2007, 45, 3439–3442. [Google Scholar] [CrossRef]
- Stevenson, K.; Alvarez, J.; Bakker, D.; Biet, F.; De Juan, L.; Denham, S.; Dimareli-Malli, Z.; Dohmann, K.; Gerlach, G.-F.; Heron, I.; et al. Occurrence of Mycobacterium avium subspecies paratuberculosis across host species and European countries with evidence for transmission between wildlife and domestic ruminants. BMC Microbiol. 2009, 9, 212. [Google Scholar] [CrossRef]
- Hutchings, M.R.; Stevenson, K.; Greig, A.; Davidson, R.; Marion, G.; Judge, J. Infection of non-ruminant wildlife by Mycobacterium avium subsp. paratuberculosis. In Paratuberculosis: Organism, Disease, Control; Behr, M.A., Collins, D.M., Eds.; CABI: Wallingford, UK, 2010; pp. 188–200. [Google Scholar]
- Verdugo, C.; Pleydell, E.; Price-Carter, M.; Prattley, D.; Collins, D.; De Lisle, G.; Vogue, H.; Wilson, P.; Heuer, C. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis isolated from sheep, cattle and deer on New Zealand pastoral farms. Prev. Veter Med. 2014, 117, 436–446. [Google Scholar] [CrossRef]
- Whittington, R.; Taragel, C.; Ottaway, S.; Marsh, I.; Seaman, J.; Fridriksdottir, V. Molecular epidemiological confirmation and circumstances of occurrence of sheep (S) strains of Mycobacterium avium subsp. paratuberculosis in cases of paratuberculosis in cattle in Australia and sheep and cattle in Iceland. Veter Microbiol. 2001, 79, 311–322. [Google Scholar] [CrossRef]
- Pickup, R.; Rhodes, G.; Arnott, S.; Sidi-Boumedine, K.; Bull, T.; Weightman, A.; Hurley, M.; Hermon-Taylor, J. Mycobacterium avium subsp. paratuberculosis in the catchment area and water of the River Taff in South Wales, United Kingdom, and its po-tential relationship to clustering of Crohn’s disease cases in the city of Cardiff. Appl. Environ. Microbiol. 2005, 71, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Semret, M.; Turenne, C.Y.; Behr, M.A. Insertion Sequence IS900 Revisited. J. Clin. Microbiol. 2006, 44, 1081–1083. [Google Scholar] [CrossRef] [PubMed][Green Version]
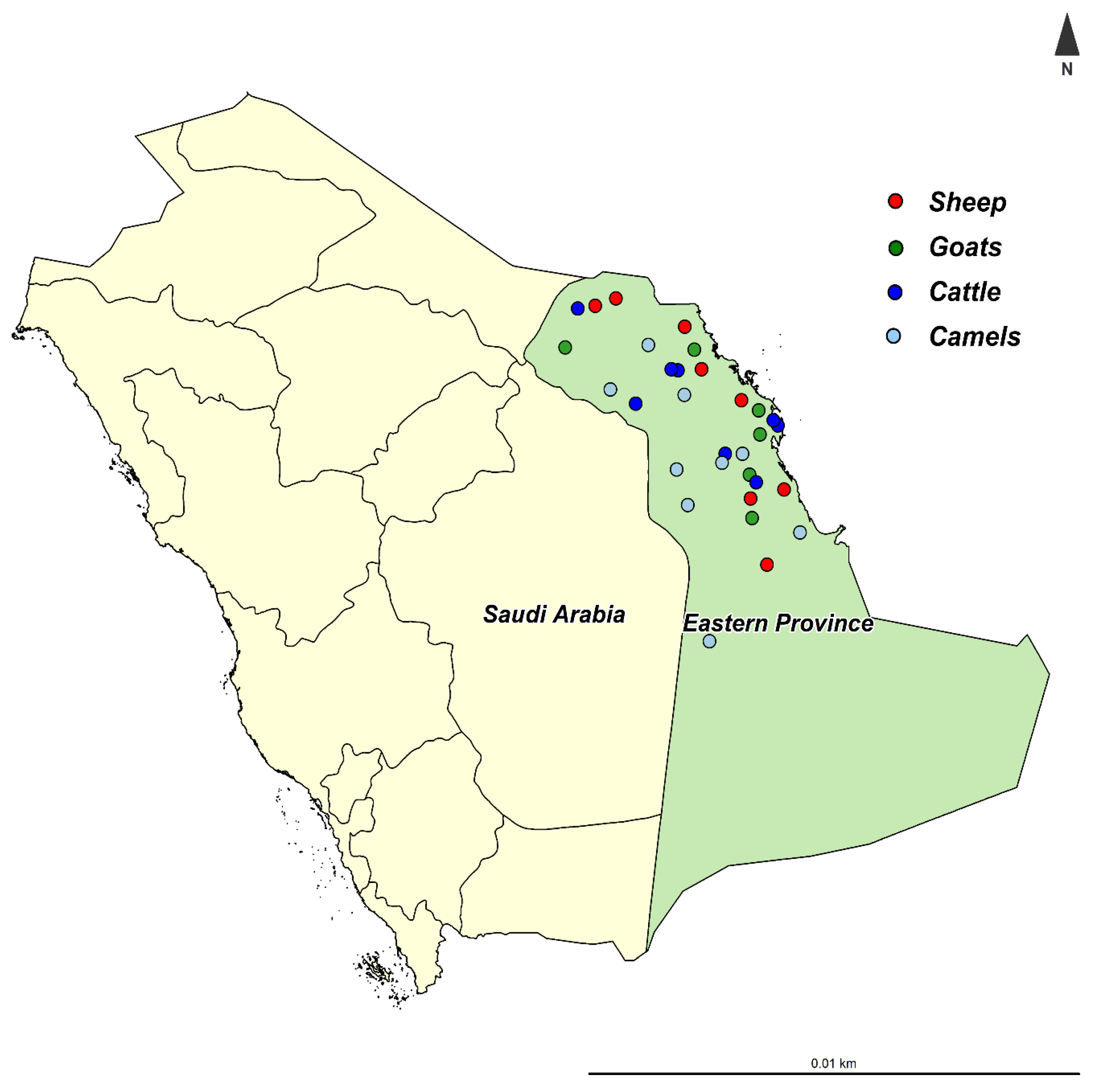


| Herds | Sheep | Goats | Cattle | Camels | ||||
|---|---|---|---|---|---|---|---|---|
| n | >2 Years | n | >2 Years | n | >2 Years | n | >2 Years | |
| 1 | 50 | 28 | 60 | 38 | 20 | 11 | 40 | 22 |
| 2 | 20 | 11 | 50 | 21 | 15 | 9 | 50 | 31 |
| 3 | 35 | 19 | 30 | 19 | 10 | 8 | 40 | 35 |
| 4 | 60 | 32 | 20 | 11 | 8 | 8 | 15 | 10 |
| 5 | 15 | 6 | 25 | 11 | 19 | 10 | 49 | 27 |
| 6 | 90 | 55 | 45 | 23 | 11 | 7 | 68 | 41 |
| 7 | 85 | 47 | - | - | 9 | 6 | 73 | 39 |
| 8 | 40 | 22 | - | - | 13 | 7 | 30 | 19 |
| 9 | - | - | - | - | - | - | 26 | 16 |
| Total | 220 | 123 | 66 | 240 | ||||
| Primer Name | Primer Sequences | Annealing (°C) | Product Size (bp) | References |
|---|---|---|---|---|
| IS900-F | 5′ CCTTTCTTGAAGGGTGTTCG 3′ | 58 | 548 | [17] |
| IS900-R | 5′ CCACCAGATCGGAACGTC 3′ | |||
| DMC1-529 F | 5′ GCTGTTGGCTGCGTCATGAAG 3′ | 60 | 310 (C-type) | [26] |
| DMC1-531 F | 5′ TCTTATCGGACTTCTTCT GGC 3′ | 162 (S-type) | ||
| DMC1-533 R | 5′ CGGATTGACCTGCGTTTCAC 3′ | |||
| pig-RDA10 P19 | 5′ TAG CGG TCC CGC AGT TTG GC 3′ | 61 | 382 | [27] |
| pig-RDA10 P20 | 5′ TCA AGC CGA ACG AGG TGG TCG 3′ | |||
| pig-RDA20 P21 | 5′ TCG TCC CGT CCC GAT GCT GT 3′ | 560 | ||
| pig-RDA20 P22 | 5′ TGA GTC CTG TCG TGC ATG CG 3′ | |||
| pig-RDA30 P23 | 5′ TGA AGA GCC CGG ACA AGG GG 3′ | 525 | ||
| pig-RDA30 P24 | 5′ TAG GTC TCA GTG GTC CAC CAG C 3′ |
| Herds | Sheep | Goats | Cattle | Camels | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Samples | No. of ELISA Positive (%) | No. of IS900 Positive (%) | No. of Samples | No. of ELISA Positive (%) | No. of IS900 Positive (%) | No. of Samples | No. of ELISA Positive (%) | No. of IS900 Positive (%) | No. of Samples | No. of ELISA Positive (%) | No. of IS900 Positive (%) | |
| 1 | 28 | 6 (21.4) | 10 (35.7) | 38 | 6 (15.8) | 11 (28.9) | 11 | 6 (54.5) | 7 (63.6) | 22 | 0 (0.0) | 2 (9.1) |
| 2 | 11 | 2 (18.2) | 4 (36.4) | 21 | 3 (14.3) | 6 (28.6) | 9 | 0 (0.0) | 2 (22.2) | 31 | 3 (9.7) | 5 (16.1) |
| 3 | 19 | 5 (26.3) | 8 (42.1) | 19 | 7 (36.8) | 5 (26.3) | 8 | 2 (25.0) | 3 (37.5) | 35 | 0 (0.0) | 0 (0.0) |
| 4 | 32 | 7 (21.9) | 9 (28.1) | 11 | 0 (0.0) | 0 (0.0) | 8 | 0 (0.0) | 0 (0.0) | 10 | 0 (0.0) | 0 (0.0) |
| 5 | 6 | 0 (0.0) | 0 (0.0) | 11 | 2 (18.2) | 4 (36.4) | 10 | 0 (0.0) | 2 (20.0) | 27 | 3 (11.1) | 2 (7.4) |
| 6 | 55 | 12 (21.8) | 16 (29.1) | 23 | 3 (13.0) | 8 (34.8) | 7 | 0 (0.0) | 0 (0.0) | 41 | 6 (14.6) | 8 (19.5) |
| 7 | 47 | 11 (23.4) | 12 (25.5) | - | - | - | 6 | 2 (33.3) | 2 (33.3) | 39 | 10 (25.6) | 6 (15.4) |
| 8 | 22 | 0 (0.0) | 0 (0.0) | - | - | - | 7 | 3 (42.9) | 4 (57.1) | 19 | 0 (0.0) | 0 (0.0) |
| 9 | - | - | - | - | - | - | - | - | - | 16 | 0 (0.0) | 1 (6.3) |
| Total | 220 | 43 (19.5) | 59 (26.8) | 123 | 21 (17.1) | 34 (27.6) | 66 | 13 (19.7) | 20 (30.3) | 240 | 22 (9.1) | 24 (10.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsohaby, I.; Fayez, M.; Alkafafy, M.; Refaat, M.; Al-Marri, T.; Alaql, F.A.; Al Amer, A.S.; Abdallah, A.; Elmoslemany, A. Serological and Molecular Characterization of Mycobacterium avium Subsp. paratuberculosis (MAP) from Sheep, Goats, Cattle and Camels in the Eastern Province, Saudi Arabia. Animals 2021, 11, 323. https://doi.org/10.3390/ani11020323
Elsohaby I, Fayez M, Alkafafy M, Refaat M, Al-Marri T, Alaql FA, Al Amer AS, Abdallah A, Elmoslemany A. Serological and Molecular Characterization of Mycobacterium avium Subsp. paratuberculosis (MAP) from Sheep, Goats, Cattle and Camels in the Eastern Province, Saudi Arabia. Animals. 2021; 11(2):323. https://doi.org/10.3390/ani11020323
Chicago/Turabian StyleElsohaby, Ibrahim, Mahmoud Fayez, Mohamed Alkafafy, Mohamed Refaat, Theeb Al-Marri, Fanan A. Alaql, Abdulaziz S. Al Amer, Abdelmonem Abdallah, and Ahmed Elmoslemany. 2021. "Serological and Molecular Characterization of Mycobacterium avium Subsp. paratuberculosis (MAP) from Sheep, Goats, Cattle and Camels in the Eastern Province, Saudi Arabia" Animals 11, no. 2: 323. https://doi.org/10.3390/ani11020323
APA StyleElsohaby, I., Fayez, M., Alkafafy, M., Refaat, M., Al-Marri, T., Alaql, F. A., Al Amer, A. S., Abdallah, A., & Elmoslemany, A. (2021). Serological and Molecular Characterization of Mycobacterium avium Subsp. paratuberculosis (MAP) from Sheep, Goats, Cattle and Camels in the Eastern Province, Saudi Arabia. Animals, 11(2), 323. https://doi.org/10.3390/ani11020323









