Hepatitis E Virus Occurrence in Pigs Slaughtered in Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Slaughterhouse and Sampling
2.2. Nucleic Acid Extractions from Fecal Samples
2.3. Nucleic Acid Extractions from Diaphragmatic Muscle Samples
2.4. Nucleic Acid Extractions from Liver Samples
2.5. Nucleic Acid Extractions from Plasma
2.6. Nucleic Acid Extraction Efficiency
2.7. HEV Real-Time RT-qPCR
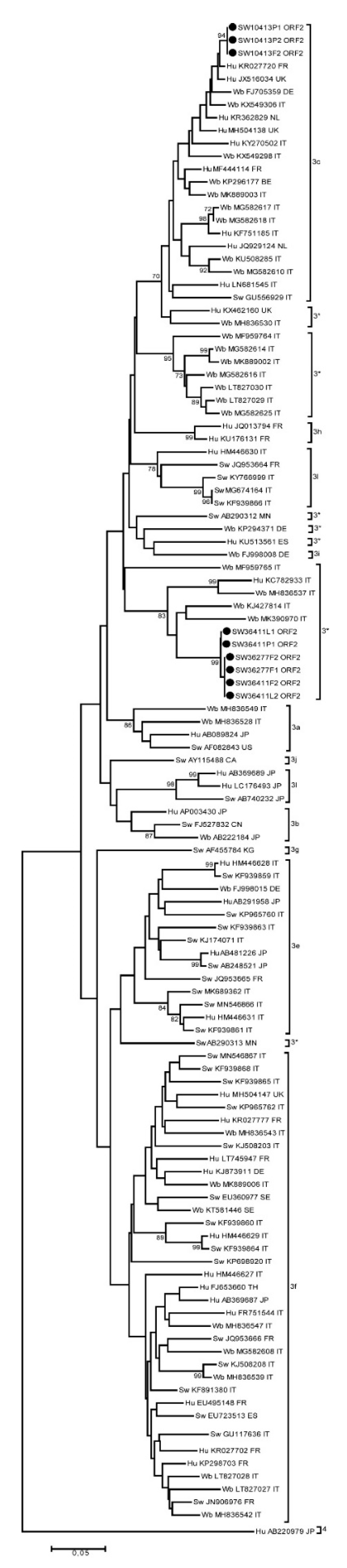
2.8. Sequencing and Subtyping
2.9. Meat Juice Preparation
2.10. Antibodies Detection
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Global Prevalence of Hepatitis E Viral Prevalence and Susceptibility: A Systematic Review. Available online: https://apps.who.int/iris/bitstream/handle/10665/70513/WHO_IVB_10.14_eng.pdf?sequence=1 (accessed on 19 January 2021).
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.-J.; Okamoto, H.; Van Der Poel, W.H.M.; Purdy, M.A.; International Committee on the Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef]
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Dalton, H.R.; Abravanel, F.; Izopet, J. Hepatitis E Virus Infection. Clin. Microbiol. Rev. 2014, 27, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ling, R.; Erker, J.C.; Zhang, H.; Li, H.; Desai, S.; Mushahwar, I.K.; Harrison, T.J. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J. Gen. Virol. 1999, 80 Pt 1, 169–177. [Google Scholar] [CrossRef]
- Van Der Poel, W.H.M.; Dalton, H.R.; Johne, R.; Pavio, N.; Bouwknegt, M.; Wu, T.; Cook, N.; Meng, X.J. Knowledge gaps and research priorities in the prevention and control of hepatitis E virus infection. Transbound. Emerg. Dis. 2018, 65 (Suppl. 1), 22–29. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Izopet, J. Transmission and Epidemiology of Hepatitis E Virus Genotype 3 and 4 Infections. Cold Spring Harb. Perspect. Med. 2018, 8, a032144. [Google Scholar] [CrossRef] [PubMed]
- Izopet, J.; Tremeaux, P.; Marion, O.; Migueres, M.; Capelli, N.; Chapuy-Regaud, S.; Mansuy, J.-M.; Abravanel, F.; Kamar, N.; Lhomme, S. Hepatitis E virus infections in Europe. J. Clin. Virol. 2019, 120, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Escamez, P.S.F.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Norrung, B.; et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017, 15, e04886. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Available online: https://ec.europa.eu/eurostat/web/products-datasets/-/tag00042 (accessed on 5 November 2020).
- Andraud, M.; Casas, M.; Pavio, N.; Rose, N. Early-Life Hepatitis E Infection in Pigs: The Importance of Maternally-Derived Antibodies. PLoS ONE 2014, 9, e105527. [Google Scholar] [CrossRef]
- Capai, L.; Maestrini, O.; Casabianca, F.; Villechenaud, N.; Masse, S.; Bosseur, F.; De Lamballerie, X.; Charrel, R.N.; Falchi, A. Drastic decline of hepatitis E virus detection in domestic pigs after the age of 6 months, Corsica, France. Transbound. Emerg. Dis. 2019, 66, 2462–2473. [Google Scholar] [CrossRef]
- Kanai, Y.; Tsujikawa, M.; Yunoki, M.; Nishiyama, S.; Ikuta, K.; Hagiwara, K. Long-term shedding of hepatitis E virus in the feces of pigs infected naturally, born to sows with and without maternal antibodies. J. Med. Virol. 2010, 82, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Salines, M.; Andraud, M.; Pellerin, M.; Bernard, C.; Grasland, B.; Pavio, N.; Rose, N. Impact of porcine circovirus type 2 (PCV2) infection on hepatitis E virus (HEV) infection and transmission under experimental conditions. Vet. Microbiol. 2019, 234, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salines, M.; Andraud, M.; Rose, N. From the epidemiology of hepatitis E virus (HEV) within the swine reservoir to public health risk mitigation strategies: A comprehensive review. Vet. Res. 2017, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Feurer, C.; Le Roux, A.; Rossel, R.; Barnaud, E.; Dumarest, M.; Garry, P.; Pavio, N. High load of hepatitis E viral RNA in pork livers but absence in pork muscle at French slaughterhouses. Int. J. Food Microbiol. 2018, 264, 25–30. [Google Scholar] [CrossRef]
- Leblanc, D.; Poitras, E.; Gagné, M.-J.; Ward, P.; Houde, A. Hepatitis E virus load in swine organs and tissues at slaughterhouse determined by real-time RT-PCR. Int. J. Food Microbiol. 2010, 139, 206–209. [Google Scholar] [CrossRef]
- Di Bartolo, I.; Ponterio, E.; Castellini, L.; Ostanello, F.; Ruggeri, F.M. Viral and antibody HEV prevalence in swine at slaughterhouse in Italy. Vet. Microbiol. 2011, 149, 330–338. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, C.; Guo, T.; Xu, Y.; Wang, X.; Huang, W.; Liu, H.; Wang, Y. Detection of Hepatitis E Virus in Raw Pork and Pig Viscera as Food in Hebei Province of China. Foodborne Pathog. Dis. 2019, 16, 325–330. [Google Scholar] [CrossRef]
- Grierson, S.; Heaney, J.; Cheney, T.; Morgan, D.; Wyllie, S.; Powell, L.; Smith, D.; Ijaz, S.; Steinbach, F.; Choudhury, B.; et al. Prevalence of Hepatitis E Virus Infection in Pigs at the Time of Slaughter, United Kingdom, 2013. Emerg. Infect. Dis. 2015, 21, 1396–1401. [Google Scholar] [CrossRef]
- Sooryanarain, H.; Heffron, C.L.; Hill, D.E.; Fredericks, J.; Rosenthal, B.M.; Werre, S.R.; Opriessnig, T.; Meng, X.-J. Hepatitis E Virus in Pigs from Slaughterhouses, United States, 2017–2019. Emerg. Infect. Dis. 2020, 26, 354–357. [Google Scholar] [CrossRef]
- Crossan, C.; Grierson, S.; Thomson, J.; Ward, A.; Nunez-Garcia, J.; Banks, M.; Scobie, L. Prevalence of hepatitis E virus in slaughter-age pigs in Scotland. Epidemiol. Infect. 2014, 143, 2237–2240. [Google Scholar] [CrossRef]
- García, N.; Hernández, M.; Gutierrez-Boada, M.; Valero, A.; Navarro, A.; Muñoz-Chimeno, M.; Fernández-Manzano, A.; Escobar, F.M.; Martínez, I.; Bárcena, C.; et al. Occurrence of Hepatitis E Virus in Pigs and Pork Cuts and Organs at the Time of Slaughter, Spain, 2017. Front. Microbiol. 2020, 10, 2990. [Google Scholar] [CrossRef] [PubMed]
- Lainšček, P.R.; Toplak, I.; Kirbiš, A. A comprehensive study of hepatitis E virus infection in pigs entering a slaughterhouse in Slovenia. Vet. Microbiol. 2017, 212, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Angeloni, G.; Salata, C.; Vonesch, N.; D’Amico, W.; Campagna, G.; Natale, A.; Zuliani, F.; Ceglie, L.; Monne, I.; et al. Hepatitis E virus infection in North Italy: High seroprevalence in swine herds and increased risk for swine workers. Epidemiol. Infect. 2017, 145, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Diez-Valcarce, M.; Vasickova, P.; Kralik, P.; Hernandez, M.; Angeloni, G.; Ostanello, F.; Bouwknegt, M.; Rodrìguez-Lázaro, D.; Pavlik, I.; et al. Hepatitis E Virus in Pork Production Chain in Czech Republic, Italy, and Spain, 2010. Emerg. Infect. Dis. 2012, 18, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Alfonsi, V.; Romanò, L.; Ciccaglione, A.R.; La Rosa, G.; Bruni, R.; Zanetti, A.; Della Libera, S.; Iaconelli, M.; Bagnarelli, P.; Capobianchi, M.R.; et al. Hepatitis E in Italy: 5 years of national epidemiological, virological and environmental surveillance, 2012 to 2016. Eurosurveillance 2018, 23, 1700517. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, C.; Spada, E.; Taliani, G.; Chionne, P.; Madonna, E.; Marcantonio, C.; Pezzotti, P.; Bruni, R.; La Rosa, G.; Pisani, G.; et al. High prevalence of anti-hepatitis E virus antibodies among blood donors in central Italy, February to March 2014. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, C.; Pezzotti, P.; Bruni, R.; Taliani, G.; Chionne, P.; Madonna, E.; Villano, U.; Pisani, G.; Equestre, M.; Dell’Orso, L.; et al. Incidence of hepatitis E virus infection among blood donors in a high endemic area of Central Italy. J. Viral Hepat. 2018, 26, 506–512. [Google Scholar] [CrossRef]
- Spada, E.; Pupella, S.; Pisani, G.; Bruni, R.; Chionne, P.; Madonna, E.; Villano, U.; Simeoni, M.; Fabi, S.; Marano, G.; et al. A nationwide retrospective study on prevalence of hepatitis E virus infection in Italian blood donors. Blood Transfus. 2018, 16, 413–421. [Google Scholar]
- ISTAT (Italian Central Institute of Statistics). Available online: http://dati-censimentoagricoltura.istat.it/Index.aspx?lang=en (accessed on 5 November 2020).
- Szabo, K.; Trojnar, E.; Anheyer-Behmenburg, H.; Binder, A.; Schotte, U.; Ellerbroek, L.; Klein, G.; Johne, R. Detection of hepatitis E virus RNA in raw sausages and liver sausages from retail in Germany using an optimized method. Int. J. Food Microbiol. 2015, 215, 149–156. [Google Scholar] [CrossRef]
- Baert, L.; Wobus, C.E.; Van Coillie, E.; Thackray, L.B.; Debevere, J.; Uyttendaele, M. Detection of Murine Norovirus 1 by Using Plaque Assay, Transfection Assay, and Real-Time Reverse Transcription-PCR before and after Heat Exposure. Appl. Environ. Microbiol. 2007, 74, 543–546. [Google Scholar] [CrossRef]
- Costafreda, M.I.; Bosch, A.; Pinto, R.M. Development, Evaluation, and Standardization of a Real-Time TaqMan Reverse Transcription-PCR Assay for Quantification of Hepatitis A Virus in Clinical and Shellfish Samples. Appl. Environ. Microbiol. 2006, 72, 3846–3855. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Garson, J.A.; Ferns, R.B.; Grant, P.R.; Ijaz, S.; Nastouli, E.; Szypulska, R.; Tedder, R.S. Minor groove binder modification of widely used TaqMan probe for hepatitis E virus reduces risk of false negative real-time PCR results. J. Virol. Methods 2012, 186, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, S.; De Santis, P.; La Rosa, G.; Di Domenico, K.; Iaconelli, M.; Micarelli, G.; Martini, E.; Bilei, S.; De Medici, D.; Suffredini, E. Quantification and genetic diversity of Hepatitis E virus in wild boar (Sus scrofa) hunted for domestic consumption in Central Italy. Food Microbiol. 2019, 82, 194–201. [Google Scholar] [CrossRef]
- Meng, X.-J.; Purcell, R.H.; Halbur, P.G.; Lehman, J.R.; Webb, D.M.; Tsareva, T.S.; Haynes, J.S.; Thacker, B.J.; Emerson, S.U. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 1997, 94, 9860–9865. [Google Scholar] [CrossRef]
- De Sabato, L.; Ostanello, F.; De Grossi, L.; Marcario, A.; Franzetti, B.; Monini, M.; Di Bartolo, I. Molecular survey of HEV infection in wild boar population in Italy. Transbound. Emerg. Dis. 2018, 65, 1749–1756. [Google Scholar] [CrossRef]
- Erker, J.C.; Desai, S.M.; Mushahwar, I.K. Rapid detection of Hepatitis E virus RNA by reverse transcription-polymerase chain reaction using universal oligonucleotide primers. J. Virol. Methods 1999, 81, 109–113. [Google Scholar] [CrossRef]
- NCBI. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 6 September 2020).
- Mulder, A.C.; Kroneman, A.; Franz, E.; Vennema, H.; Tulen, A.D.; Takkinen, J.; Hofhuis, A.; Adlhoch, C.; Members of HEVnet. HEVnet: A One Health, collaborative, interdisciplinary network and sequence data repository for enhanced hepatitis E virus molecular typing, characterisation and epidemiological investigations. Eurosurveillance 2019, 24, 1800407. [Google Scholar] [CrossRef]
- Smith, D.B.; Simmonds, P.; Izopet, J.; Oliveira-Filho, E.F.; Ulrich, R.G.; Johne, R.; Koenig, M.; Jameel, S.; Harrison, T.J.; Meng, X.-J.; et al. Proposed reference sequences for hepatitis E virus subtypes. J. Gen. Virol. 2016, 97, 537–542. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Martella, V.; Di Felice, E.; Di Francesco, C.E.; Ceci, C.; Marsilio, F. Detection of hepatitis E virus in slaughtered pigs in Italy. Arch. Virol. 2009, 155, 103–106. [Google Scholar] [CrossRef]
- Martino, C.; Rampacci, E.; Pierini, I.; Giammarioli, M.; Stefanetti, V.; Hyatt, D.R.; Ianni, A.; Di Paolo, G.; Coletti, M.; Passamonti, F. Detection of anti-HEV antibodies and RNA of HEV in pigs from a hyperendemic Italian region with high human seroprevalence. Eur. J. Public Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Martelli, F.; Inglese, N.; Pourshaban, M.; Caprioli, A.; Ostanello, F.; Ruggeri, F.M. Widespread diffusion of genotype 3 hepatitis E virus among farming swine in Northern Italy. Vet. Microbiol. 2008, 132, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, E.; Barbieri, I.; Bertasi, B.; Lombardi, G.; Giangrosso, G.; Cordioli, P.; Losio, M.N. Detection and Molecular Characterisation of Swine Hepatitis E Virus in Brescia Province, Italy. Ital. J. Food Saf. 2015, 4, 4587. [Google Scholar] [CrossRef] [PubMed]
- Milojević, L.; Velebit, B.; Teodorović, V.; Kirbiš, A.; Petrović, T.; Karabasil, N.; Dimitrijević, M. Screening and Molecular Characterization of Hepatitis E Virus in Slaughter Pigs in Serbia. Food Environ. Virol. 2019, 11, 410–419. [Google Scholar] [CrossRef]
- Lopez-Lopez, P.; Risalde, M.L.A.; Frias, M.; García-Bocanegra, I.; Brieva, T.; Caballero-Gómez, J.; Camacho, A.; Fernández-Molera, V.; Machuca, I.; Gomez-Villamandos, J.C.; et al. Risk factors associated with hepatitis E virus in pigs from different production systems. Vet. Microbiol. 2018, 224, 88–92. [Google Scholar] [CrossRef]
- Wacheck, S.; Werres, C.; Mohn, U.; Dorn, S.; Soutschek, E.; Fredriksson-Ahomaa, M.; Märtlbauer, E. Detection of IgM and IgG Against Hepatitis E Virus in Serum and Meat Juice Samples from Pigs at Slaughter in Bavaria, Germany. Foodborne Pathog. Dis. 2012, 9, 655–660. [Google Scholar] [CrossRef]
- Dzierzon, J.; Oswaldi, V.; Merle, R.; Langkabel, N.; Meemken, D. High Predictive Power of Meat Juice Serology on the Presence of Hepatitis E Virus in Slaughter Pigs. Foodborne Pathog. Dis. 2020, 17, 687–692. [Google Scholar] [CrossRef]
- Pavio, N.; Doceul, V.; Bagdassarian, E.; Johne, R. Recent knowledge on hepatitis E virus in Suidae reservoirs and transmission routes to human. Vet. Res. 2017, 48, 1–14. [Google Scholar] [CrossRef]
- Meng, X.-J.; Halbur, P.G.; Haynes, J.S.; Tsareva, T.S.; Bruna, J.D.; Royer, R.L.; Purcell, R.H.; Emerson, S.U. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch. Virol. 1998, 143, 1405–1415. [Google Scholar] [CrossRef]
- Halbur, P.G.; Kasorndorkbua, C.; Gilbert, C.; Guenette, D.; Potters, M.B.; Purcell, R.H.; Emerson, S.U.; Toth, T.E.; Meng, X.J. Comparative Pathogenesis of Infection of Pigs with Hepatitis E Viruses Recovered from a Pig and a Human. J. Clin. Microbiol. 2001, 39, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Barnaud, E.; Rogée, S.; Garry, P.; Rose, N.; Pavio, N. Thermal Inactivation of Infectious Hepatitis E Virus in Experimentally Contaminated Food. Appl. Environ. Microbiol. 2012, 78, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.M.; Di Bartolo, I.; Ponterio, E.; Angeloni, G.; Trevisiani, M.; Ostanello, F. Zoonotic transmission of hepatitis E virus in industrialized countries. New Microbiol. 2013, 36, 331–344. [Google Scholar]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van Der Poel, W.H.M.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Giordani, M.T.; Fabris, P.; Brunetti, E.; Goblirsch, S.; Romanò, L. Hepatitis E and Lymphocytic Leukemia in Man, Italy. Emerg. Infect. Dis. 2013, 19, 2054–2056. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.A.; Nardini, R.; Verin, R.; Forzan, M.; Poli, A.; Tolari, F. Serologic and molecular survey for hepatitis E virus in wild boar (Sus scrofa) in Central Italy. New Microbes New Infect. 2015, 7, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Vanek, J.; Wellington, L.; Johannessen, I.; Ramalingam, S.; Simmonds, P. Hepatitis E Virus Mixed Infection in Immunocompetent Patient. Emerg. Infect. Dis. 2013, 19, 468–470. [Google Scholar] [CrossRef]
- De Sabato, L.; Di Bartolo, I.; Lapa, D.; Capobianchi, M.R.; Garbuglia, A.R. Molecular Characterization of HEV Genotype 3 in Italy at Human/Animal Interface. Front. Microbiol. 2020, 11, 137. [Google Scholar] [CrossRef]
| Samples | Diagnostic Test Used | Total Examined Samples |
|---|---|---|
| Liver | Real-time RT-qPCR | 585 |
| Feces | Real-time RT-qPCR | 569 |
| Plasma | Real-time RT-qPCR | 91 |
| Muscle | Real-time RT-qPCR | 21 * |
| Sera | ELISA | 335 |
| Meat juice | ELISA | 74 |
| Variables | Categories | Total Pigs | No. Pigs HEV-RNA Positive | P | ||
|---|---|---|---|---|---|---|
| No. | (%) | 95%CI | ||||
| Geographic area of the slaughterhouse | Northern Italy (A) | 120 | 0 | (0.0) | [0.0–3.0] | <0.001 |
| Central Italy (B and C) | 362 | 4 | (1.1) | [0.3–2.8] | ||
| Southern Italy (D) | 103 | 17 | (16.5) | [9.9–25.1] | ||
| Geographical origin of pigs slaughtered | Northern Italy | 228 | 2 | (0.9) | [0.1–3.1] | <0.001 |
| Central Italy | 254 | 2 | (0.8) | [0.1–2.8] | ||
| Southern Italy | 91 | 14 | (15.4) | [8.7–24.5] | ||
| Other EU countries | 12 | 3 | (25.0) | [5.5–57.2] | ||
| Age class | lightweight pigs (4.5 months 1) | 87 | 10 | (11.5) | [5.6–20.1] | <0.001 |
| heavy pigs (9 months 1) | 498 | 11 | (2.2) | [1.1–3.9] | ||
| Total | 585 | 21 | (3.6) | [2.2–5.4] | ||
| Variables | Category | Total Pigs | No. Positive Pigs for Anti-HEV Antibodies | p | ||
|---|---|---|---|---|---|---|
| No. | % | 95%CI | ||||
| Geographic area of the slaughterhouse | Northern Italy (A) | 120 | 68 | (56.7) | [47.3–65.7] | <0.001 |
| Central Italy (B) | 115 | 115 | (100) | [96.8–100] | ||
| Central Italy (C) 1 | 73 | 44 | (60.3) | [48.1–71.6] | ||
| Southern Italy (D) | 101 | 87 | (86.1) | [77.8–92.2] | ||
| Geographical origin of pigs slaughtered | Northern Italy | 210 | 149 | (71.0) | [64.3–77.0] | 0.023 |
| Central Italy | 98 | 78 | (79.6) | [70.3–87.1] | (0.015) 3 | |
| Southern Italy | 91 | 78 | (85.7) | [76.8–92.2] | ||
| Other EU countries | 10 | 9 | (90.0) | [55.5–99.8] | ||
| Age class | lightweight pigs (4.5 months) 2 | 87 | 73 | (83.9) | [74.5–90.9] | 0.08 |
| heavy pigs (9 months) 2 | 322 | 241 | (74.8) | [69.3–79.5] | ||
| Total | 409 | 314 | (76.8) | [72.4–80.8] | ||
| Presence of HEV-RNA | ||||
|---|---|---|---|---|
| Anti-HEV Antibodies | Liver | Feces | Muscle | No. of Animals |
| Positive (No. 17) | + 1 | + | + | 2 |
| + | − 2 | + | 5 | |
| + | − | − | 2 | |
| − | + | − | 8 | |
| Negative (No. 3) | + | − | + | 1 |
| + | − | − | 2 | |
| Not tested for antibodies (No. 1) | − | + | − | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelli, E.; Suffredini, E.; De Santis, P.; De Medici, D.; Di Bella, S.; D’Amato, S.; Gucciardi, F.; Guercio, A.; Ostanello, F.; Perrone, V.; et al. Hepatitis E Virus Occurrence in Pigs Slaughtered in Italy. Animals 2021, 11, 277. https://doi.org/10.3390/ani11020277
Chelli E, Suffredini E, De Santis P, De Medici D, Di Bella S, D’Amato S, Gucciardi F, Guercio A, Ostanello F, Perrone V, et al. Hepatitis E Virus Occurrence in Pigs Slaughtered in Italy. Animals. 2021; 11(2):277. https://doi.org/10.3390/ani11020277
Chicago/Turabian StyleChelli, Eleonora, Elisabetta Suffredini, Paola De Santis, Dario De Medici, Santina Di Bella, Stefania D’Amato, Francesca Gucciardi, Annalisa Guercio, Fabio Ostanello, Vitantonio Perrone, and et al. 2021. "Hepatitis E Virus Occurrence in Pigs Slaughtered in Italy" Animals 11, no. 2: 277. https://doi.org/10.3390/ani11020277
APA StyleChelli, E., Suffredini, E., De Santis, P., De Medici, D., Di Bella, S., D’Amato, S., Gucciardi, F., Guercio, A., Ostanello, F., Perrone, V., Purpari, G., Scavia, G. S., Schembri, P., Varcasia, B. M., & Di Bartolo, I. (2021). Hepatitis E Virus Occurrence in Pigs Slaughtered in Italy. Animals, 11(2), 277. https://doi.org/10.3390/ani11020277








