Effect of Streptococcus uberis on Gamma Delta T Cell Phenotype in Bovine Mammary Gland
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Induction of Inflammatory Response
2.3. Flow Cytometry Analysis of Apoptosis and γδ T Lymphocytes
2.4. Statistical Analysis
3. Results
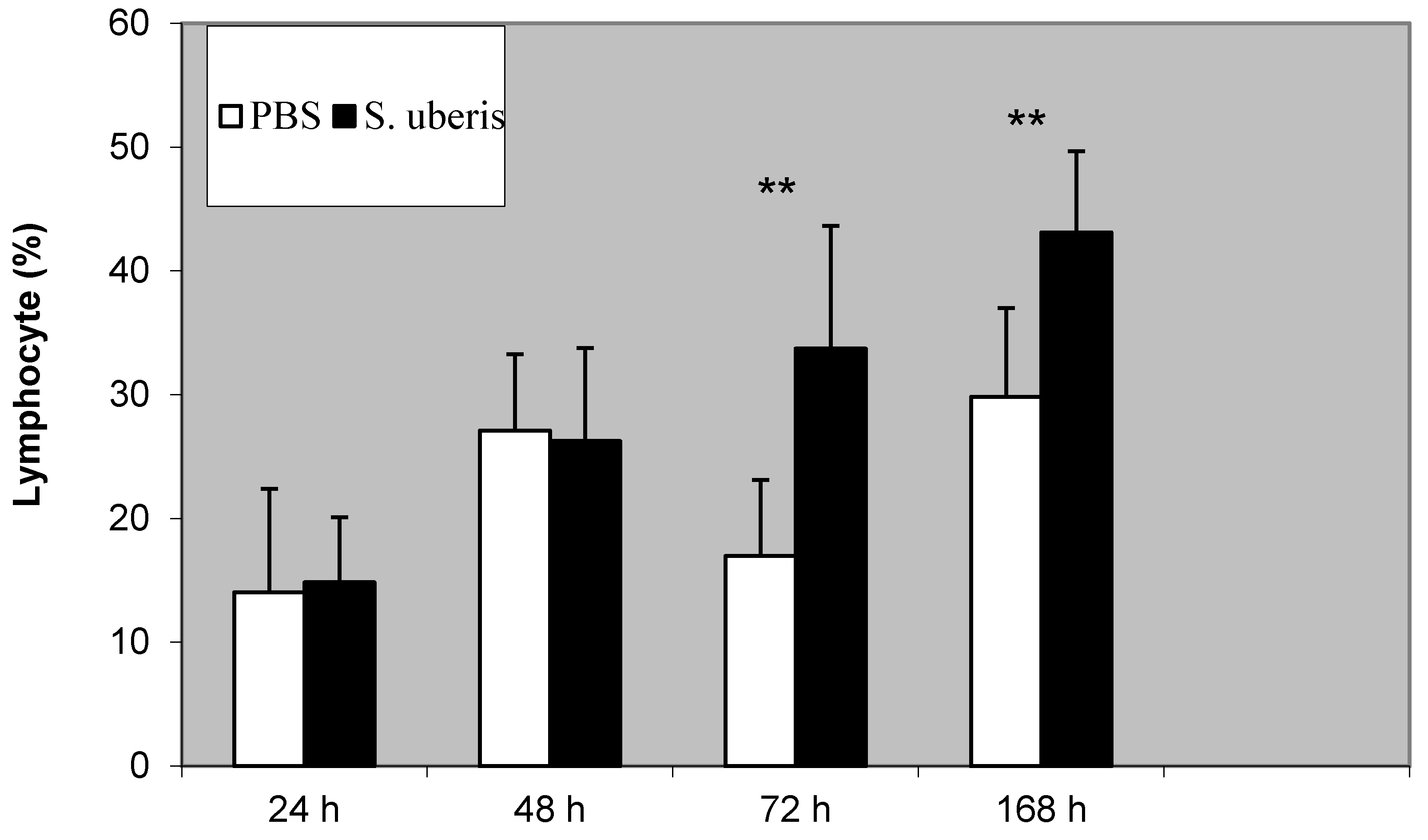
3.1. The Proportion of Lymphocytes during Inflammatory Response
3.2. The Proportion of γδ T Cells during Inflammatory Response
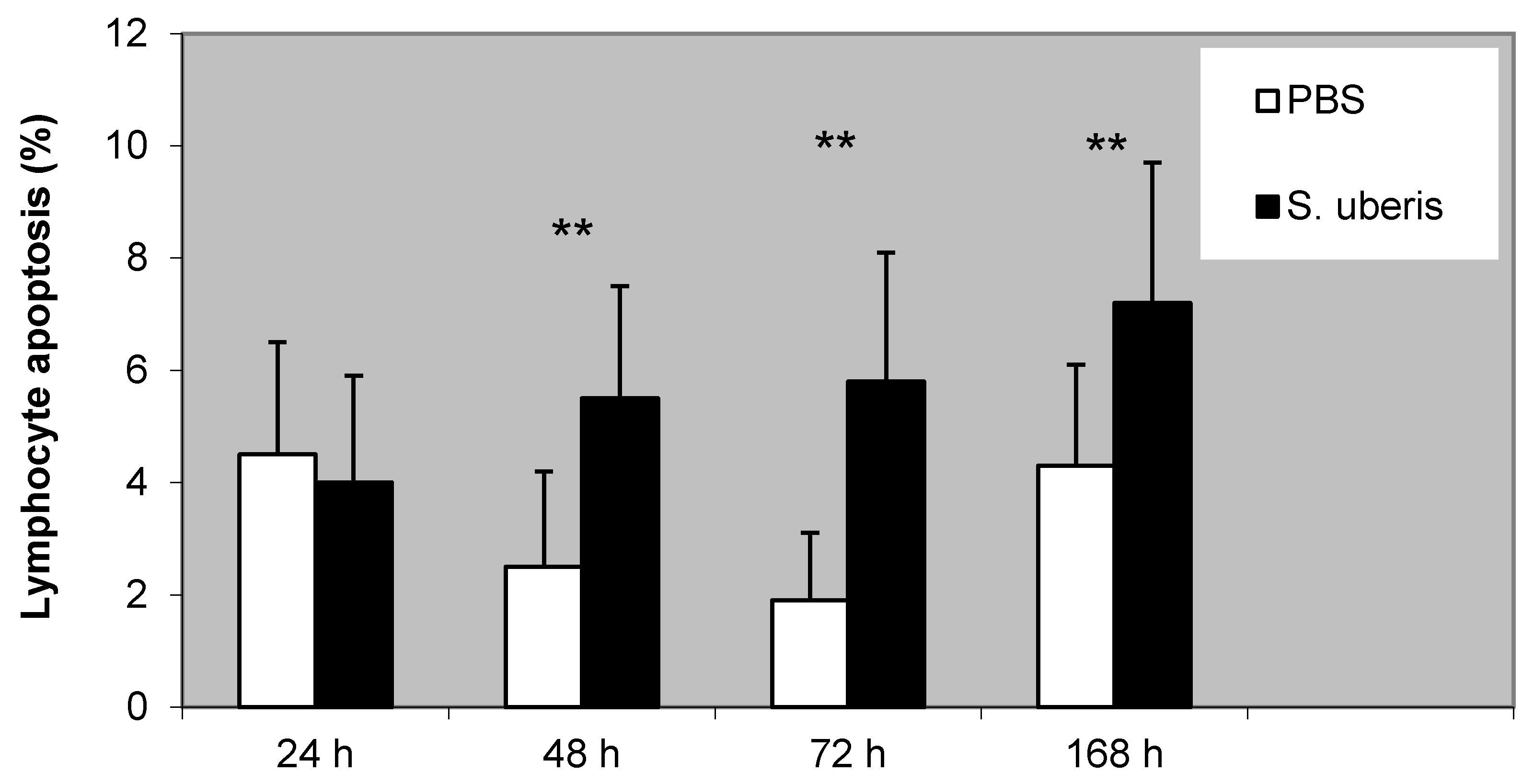
3.3. The Proportion of Lymphocyte Apoptosis during Inflammatory Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvam, B.; Almeida, R.A.; Dunlap, J.R.; Oliver, S.P. Streptococcus uberis internalizes and persists in bovine mammary epithelial cells. Microb. Pathog. 2006, 40, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.A.; Luther, D.A.; Park, H.M.; Oliver, S.P. Identification, isolation, and partial characterization of a novel Streptococcus uberis adhesion molecule (SUAM). Vet. Microbiol. 2006, 115, 183–191. [Google Scholar] [CrossRef]
- Almeida, R.A.; Dunlap, J.R.; Oliver, S.P. Binding of host factors influences internalization and intracellular trafficking of Streptococcus uberis in bovine mammary epithelial cells. Vet. Med. Int. 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, R.A.; Kerro-Dego, O.; Headrick, S.I.; Lewis, M.J.; Oliver, S.P. Role of Streptococcus uberis adhesion molecule in the pathogenesis of Streptococcus uberis mastitis. Vet. Microbiol. 2015, 179, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.A.; Kerro-Dego, O.; Prado, M.E.; Headrick, S.I.; Lewis, M.J.; Siebert, L.J.; Pighetti, G.M.; Oliver, S.P. Protective effect of anti-SUAM antibodies on Streptococcus uberis mastitis. Vet. Res. 2015, 46, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinoso, E.B. Bovine mastitis caused by Streptococcus uberis: Virulence factors and biofilm. J. Microb. Biochem. Technol. 2017, 9, 5. [Google Scholar]
- Sladek, Z.; Rysanek, D.; Ryznarova, H.; Faldyna, M. The role of neutrophil apoptosis during experimentally induced Streptococcus uberis mastitis. Vet. Med. Czech 2006, 51, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Slama, P.; Sladek, Z.; Rysanek, D.; Langrova, T. Effect of Staphylococcus aureus and Streptococcus uberis on apoptosis of bovine mammary gland lymphocytes. Res. Vet. Sci. 2009, 87, 233–238. [Google Scholar] [CrossRef]
- Slama, P.; Sladek, Z.; Rysanek, D. Lipopolysaccharide delays apoptosis of bovine lymphocytes. FEBS J. 2009, 276, 223. [Google Scholar]
- Slama, P.; Zavadilova, T.; Kratochvilova, L.; Kharkevich, K.; Uhrincat, M.; Tancin, V. Effect of peptidoglycan of Staphylococcus aureus on apoptosis of bovine mammary gland lymphocytes. J. Microbiol. Biotech. Food Sci. 2019, 9, 445–446. [Google Scholar] [CrossRef]
- Slama, P.; Kabourkova, E.; Sladek, Z.; Zavadilova, T.; Kratochvilova, L.; Kharkevich, K.; Roychoudhury, S.; Pavlik, A.; Roztocilova, A.; Uhrincat, M.; et al. Effect of Lipopolysaccharide and Muramyl Dipeptide on Apoptosis of Bovine Mammary Gland Lymphocytes. Animals 2020, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Hope, J.; Taylor, G.; Smith, A.L.; Cubillos-Zapata, C.; Charleston, B. Bovine γδ T cells are a major regulatory T cell subset. J. Immunol. 2014, 193, 208–222. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (γδ) T cells: Friend or foe in cancer development? J. Transl. Med. 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sustrova, T.; Slama, P. The effect of Staphylococcus aureus bacteria to proportion of gamma delta T-lymphocytes from bovine mammary gland. In MENDELNET 2013, Proceedings of the 20th International PhD Students Conference MENDELNET 2013, Brno, Czech Republic, 20–21 November 2013; Skarpa, P., Ryant, P., Cerkal, R., Polak, O., Kovarnik, J., Eds.; Mendel University in Brno: Brno, Czech Republic, 2013; pp. 788–792. [Google Scholar]
- Slama, P.; Sladek, Z.; Kabourkova, E.; Havlicek, Z.; Kwak, J.Y. Apoptosis of gamma delta T cells during inflammatory re-sponse of bovine mammary gland induced by Staphylococcus aureus. Eur. J. Immunol. 2016, 46 (Suppl. S1), 495. [Google Scholar]
- Telfer, J.C.; Baldwin, C.L. Bovine gamma delta T cells and the function of gamma delta T cell specific WC1 co-receptors. Cell Immunol. 2015, 296, 76–86. [Google Scholar] [CrossRef]
- Baldwin, C.L.; Telfer, J.C. The bovine model for elucidating the role of γδ T cells in controlling infectious diseases of importance to cattle and humans. Mol. Immunol. 2015, 66, 35–47. [Google Scholar] [CrossRef]
- Faldyna, M.; Leva, L.; Sladek, Z.; Rysanek, D.; Toman, M. γδ-TCR+ CD2– lymphocytes are recruited into bovine mammary gland after stimulation. Vet. Med. Czech 2006, 51, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Sladek, Z.; Rysanek, D.; Faldyna, M. Activation of phagocytes during initiation and resolution of mammary gland injury induced by lipopolysaccharide in heifers. Vet. Res. 2002, 33, 191–204. [Google Scholar] [CrossRef] [Green Version]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Davis, W.C.; Brown, W.C.; Hamilton, M.J.; Wyatt, C.R.; Orden, J.A.; Khalid, A.M.; Naessens, J. Analysis of monoclonal antibodies specific for the gamma delta TcR. Vet. Immunol. Immunopathol. 1996, 52, 275–283. [Google Scholar] [CrossRef]
- Sladek, Z.; Rysanek, D.; Ryznarova, H.; Faldyna, M. Neutrophil apoptosis during experimentally induced Staphylococcus aureus mastitis. Vet. Res. 2005, 36, 629–643. [Google Scholar] [CrossRef] [Green Version]
- Maxymiv, N.G.; Bharathan, M.; Mullarky, I.K. Bovine mammary dendritic cells: A heterogeneous population, distinct from macrophages and similar in phenotype to afferent lymph veiled cells. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, J.; Olislagers, V.; Poupot, R.; Fournie, J.J.; Goldman, M. Human γδ T Cells Induce Dendritic Cell Maturation. Clin. Immunol. 2002, 103, 296–302. [Google Scholar] [CrossRef]
- Tian, L.; Choi, S.C.; Lee, H.N.; Murakami, Y.; Qi, C.F.; Sengottuvelu, M.; Voss, O.; Krzewski, K.; Coligan, J.E. Enhanced efferocytosis by dendritic cells underlies memory T-cell expansion and susceptibility to autoimmune disease in CD300f-deficient mice. Cell Death Differ. 2016, 23, 1086–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Wu, K.; Hu, Y.; Sheng, L.; Tie, R.; Wang, B.; Huang, H. γδ T Cell and Other Immune Cells Crosstalk in Cellular Immunity. J. Immunol. Res. 2014, 2014, 960252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Cueto, P.; Magallanes-Puebla, A.; Castellanos, C.; Mancilla, R. Dendritic cells that phagocytose apoptotic macrophages loaded with mycobacterial antigens activate CD8 T cells via crosspresentation. PLoS ONE 2017, 12, e0182126. [Google Scholar] [CrossRef] [Green Version]
- Bannerman, D.D.; Paape, M.J.; Chockalingam, A. Staphylococcus aureus intramammary infection elicits increased production of transforming growth factor-α, β1, and β2. Vet. Immunol. Immunopathol. 2006, 112, 309–315. [Google Scholar] [CrossRef]
- Andreotti, C.S.; Pereyra, E.A.L.; Baravalle, C.; Renna, M.S.; Ortega, H.H.; Calvinho, L.F.; Dallard, B.E. Staphylococcus aureus chronic intramammary infection modifies protein expression of transforming growth factor beta (TGF-β) subfamily components during active involution. Res. Vet. Sci. 2014, 96, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bannermann, D.D.; Paape, M.J.; Lee, J.W.; Zhao, X.; Hoper, J.C.; Rainard, P. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin. Diagn. Lab. Immunol. 2004, 11, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, C.C.; Bashant, K.; Erikson, C.; Thwe, P.M.; Fortner, K.A.; Wang, H.; Morita, C.T.; Budd, R.C. Necroptosis of Dendritic Cells Promotes Activation of γδ T Cells. J. Innate Immun. 2016, 8, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slama, P.; Zavadilova, T.; Pavlik, A.; Horky, P.; Skalickova, S.; Skladanka, J.; Roychoudhury, S.; Baldovska, S.; Kolesarova, A.; Konecny, R.; et al. Effect of Streptococcus uberis on Gamma Delta T Cell Phenotype in Bovine Mammary Gland. Animals 2021, 11, 3594. https://doi.org/10.3390/ani11123594
Slama P, Zavadilova T, Pavlik A, Horky P, Skalickova S, Skladanka J, Roychoudhury S, Baldovska S, Kolesarova A, Konecny R, et al. Effect of Streptococcus uberis on Gamma Delta T Cell Phenotype in Bovine Mammary Gland. Animals. 2021; 11(12):3594. https://doi.org/10.3390/ani11123594
Chicago/Turabian StyleSlama, Petr, Terezie Zavadilova, Ales Pavlik, Pavel Horky, Sylvie Skalickova, Jiri Skladanka, Shubhadeep Roychoudhury, Simona Baldovska, Adriana Kolesarova, Roman Konecny, and et al. 2021. "Effect of Streptococcus uberis on Gamma Delta T Cell Phenotype in Bovine Mammary Gland" Animals 11, no. 12: 3594. https://doi.org/10.3390/ani11123594
APA StyleSlama, P., Zavadilova, T., Pavlik, A., Horky, P., Skalickova, S., Skladanka, J., Roychoudhury, S., Baldovska, S., Kolesarova, A., Konecny, R., Tancin, V., & Zouharova, M. (2021). Effect of Streptococcus uberis on Gamma Delta T Cell Phenotype in Bovine Mammary Gland. Animals, 11(12), 3594. https://doi.org/10.3390/ani11123594







