Factors Influencing Proteolysis and Protein Utilization in the Intestine of Pigs: A Review
Abstract
Simple Summary
Abstract
1. Introduction
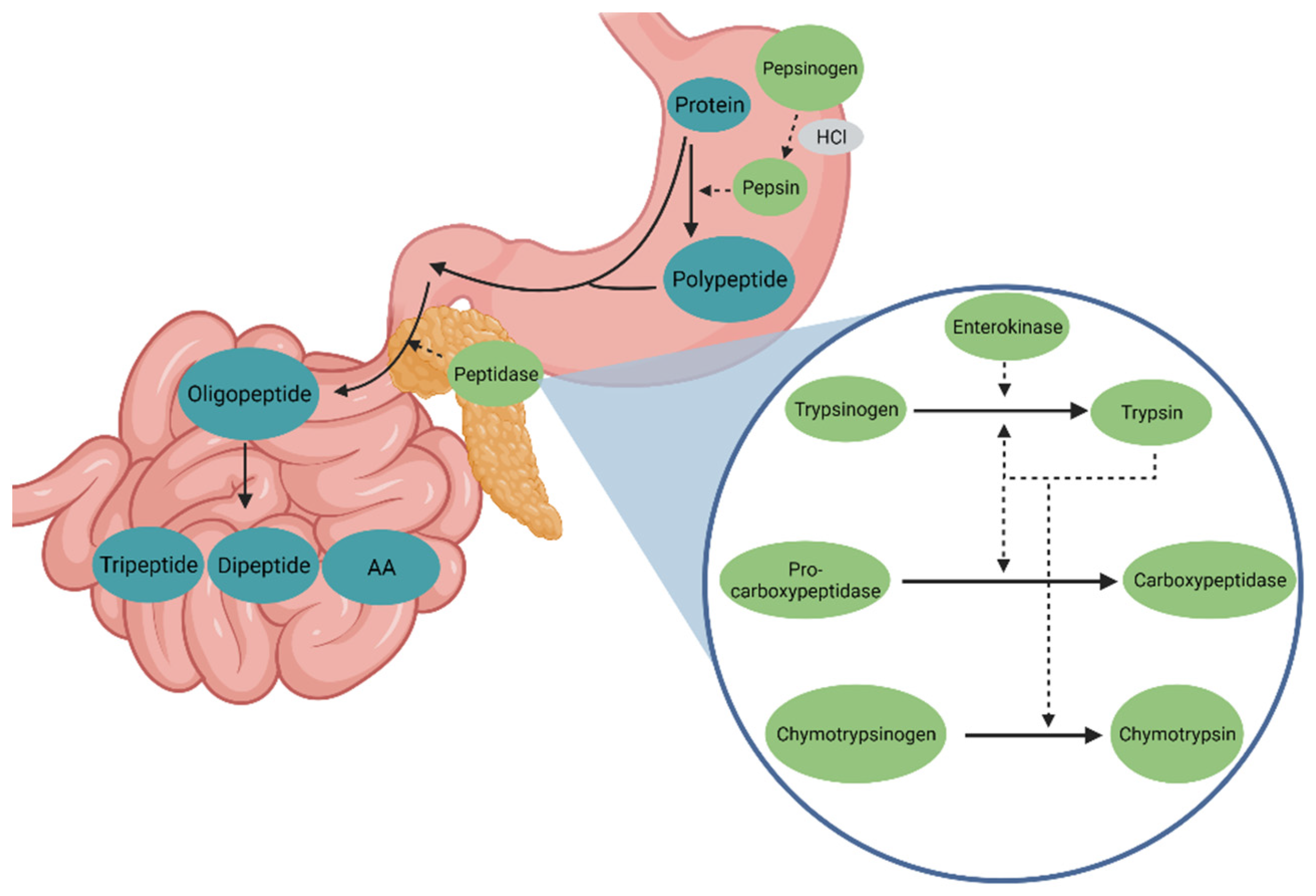
2. Proteolytic Enzymes in Pigs
2.1. Physiology and Biochemistry of Proteolytic Enzymes
2.2. Detection of Enzymatic Activities
2.3. Factors Influencing Proteolytic Enzymes and Their Effects on Proteolysis
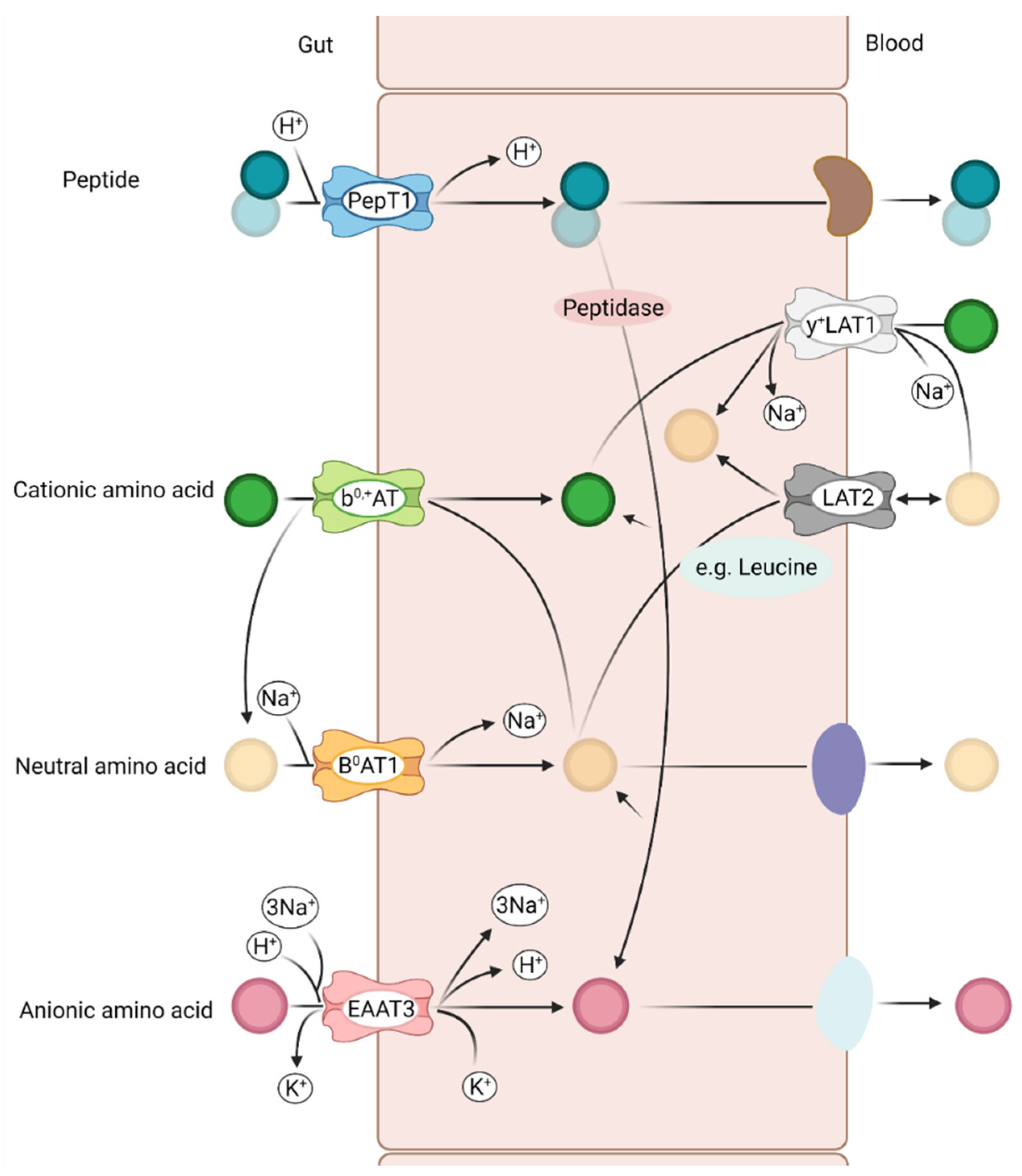
3. Amino Acid Transporter in Pig Intestine
3.1. Relevant Amino Acid Transporter in the Pig Intestine
3.2. Influence of Amino Acid Transporter Expression and Their Effect on Protein Efficiency in Pigs
4. Investigation of Proteolytic Capacities in the Host Proteome
5. Investigation of Proteolytic Capacities in the Intestinal Microbiome
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Notarnicola, B.; Tassielli, G.; Renzulli, P.A.; Castellani, V.; Sala, S. Environmental impacts of food consumption in Europe. J. Clean. Prod. 2017, 140, 753–765. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.F. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- Krul, E.S. Calculation of nitrogen-to-protein conversion factors: A review with a focus on soy protein. J. Am. Oil Chem. Soc. 2019, 96, 339–364. [Google Scholar] [CrossRef]
- Flachowsky, G.; Meyer, U.; Südekum, K.-H. Invited review: Resource inputs and land, water and carbon footprints from the production of edible protein of animal origin. Arch. Fuer Tierz. 2018, 61, 17. [Google Scholar] [CrossRef]
- Gesellschaft für Ernährungsphysiologie (GfE). Empfehlungen zur Energie-und Nährstoffversorgung von Schweinen; DLG Verlag: Frankfurt, Germany, 2006. [Google Scholar]
- Kapser, C.; Ruiz-Ascacibar, I.; Stoll, P.; Bee, G. Genetische Parameter der Proteineffizienz in Einer Schweizer Schweinepopulation. Agrar. Schweiz 2019, 10, 164–171. [Google Scholar]
- Fearnside, P.M. Soybean cultivation as a threat to the environment in Brazil. Environ. Conserv. 2001, 28, 23–38. [Google Scholar] [CrossRef]
- de Verdal, H.; Narcy, A.; Bastianelli, D.; Chapuis, H.; Même, N.; Urvoix, S.; Le Bihan-Duval, E.; Mignon-Grasteau, S. Improving the efficiency of feed utilization in poultry by selection. 2. Genetic parameters of excretion traits and correlations with anatomy of the gastro-intestinal tract and digestive efficiency. BMC Genet. 2011, 12, 71. [Google Scholar] [CrossRef]
- Ruiz-Ascacibar, I.; Stoll, P.; Kreuzer, M.; Boillat, V.; Spring, P.; Bee, G. Impact of amino acid and CP restriction from 20 to 140 kg BW on performance and dynamics in empty body protein and lipid deposition of entire male, castrated and female pigs. Animal 2017, 11, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Zervas, S.; Zijlstra, R. Effects of dietary protein and fermentable fiber on nitrogen excretion patterns and plasma urea in grower pigs. J. Anim. Sci. 2002, 80, 3247–3256. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.D.; Cromwell, G.L.; Lindemann, M.D.; Turner, L.W.; Bridges, T.C. Reducing N and P excretion by dietary manipulation in growing and finishing pigs. J. Anim. Sci. 1996, 74 (Suppl. S1), 59. [Google Scholar]
- Heda, R.; Toro, F.; Tombazzi, C.R. Physiology, Pepsin. In StatPearls (Internet); Stat Pearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Yu, D.; Zhu, W.; Hang, S. Effects of long-term dietary protein restriction on intestinal morphology, digestive enzymes, gut hormones, and colonic microbiota in pigs. Animals 2019, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.A. Trypsinogens and trypsins of various species. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1970; pp. 41–63. [Google Scholar]
- Lowe, M. The structure and function of pancreatic enzymes. In Physiology of the Gastrointestinal Tract; Johnson, L.R., Ed.; Raven Press: New York, NY, USA, 1994; pp. 1531–1542. [Google Scholar]
- Ohlsson, B.G.; WestrÖm, B.R.; Karlsson, B.W. In Vitro interaction of porcine serum and colostrum protease inhibitors with pancreatic trypsin, chymotrypsin and elastase. Biochim. Biophys. Acta 1982, 705, 357–365. [Google Scholar] [CrossRef]
- von Engelhardt, W.; Breves, G. Physiologie der Haustiere. Schweiz. Arch. Tierheilkd. 2005, 147, 507. [Google Scholar] [CrossRef]
- Herdt, T.H. Postabsorptive Nutrient Utilization. In Cunningham’s Textbook of Veterinary Physiology, 6th ed.; Klein, B.G., Ed.; W.B. Saunders: St. Louis, MO, USA, 2020; pp. 361–377. [Google Scholar]
- Shotton, D.M. Elastase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1970; pp. 113–140. [Google Scholar]
- Tomlinson, G.; Shaw, M.C.; Viswanatha, T. Chymotrypsin(s). In Methods in Enzymology; Kaplan, N.P., Colowick, N.P., Jakoby, W.B., Wilchek, M., Eds.; Academic Press: Cambridge, MA, USA, 1974; pp. 415–420. [Google Scholar]
- Adams, G. A beginner’s guide to RT-PCR, qPCR and RT-qPCR. Biochemist 2020, 42, 48–53. [Google Scholar] [CrossRef]
- He, L.; Wu, L.; Xu, Z.; Li, T.; Yao, K.; Cui, Z.; Yin, Y.; Wu, G. Low-protein diets affect ileal amino acid digestibility and gene expression of digestive enzymes in growing and finishing pigs. Amino Acids 2016, 48, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Li, F.N.; Duan, Y.H.; Guo, Q.P.; Wen, C.Y.; Wang, W.L.; Huang, X.G.; Yin, Y.L. Low-protein diet improves meat quality of growing and finishing pigs through changing lipid metabolism, fiber characteristics, and free amino acid profile of the muscle. J. Anim. Sci. 2018, 96, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhu, W.; Hang, S. Effects of low-protein diet on the intestinal morphology, digestive enzyme activity, blood urea nitrogen, and gut microbiota and metabolites in weaned pigs. Arch. Anim. Nutr. 2019, 73, 287–305. [Google Scholar] [CrossRef]
- Smyth, D.G. Techniques in enzymic hydrolysis. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1967; pp. 214–231. [Google Scholar]
- Haverback, B.J.; Dyce, B.; Bundy, H.; Edmondson, H.A. Trypsin, trypsinogen and trypsin inhibitor in human pancreatic juice: Mechanism for pancreatitis associated with hyperparathyroidism. Am. J. Med. 1960, 29, 424–433. [Google Scholar] [CrossRef]
- Rosenfelder-Kuon, P.; Klein, N.; Zegowitz, B.; Schollenberger, M.; Kühn, I.; Thuringer, L.; Seifert, J.; Rodehutscord, M. Phytate degradation cascade in pigs as affected by phytase supplementation and rapeseed cake inclusion in corn–soybean meal-based diets. J. Anim. Sci. 2020, 98, skaa053. [Google Scholar] [CrossRef]
- Green, G.M.; Levan, V.H.; Liddle, R.A. Plasma cholecystokinin and pancreatic growth during adaptation to dietary protein. Am. J. Physiol. Gastrointest. Liver Physiol. 1986, 251, G70–G74. [Google Scholar] [CrossRef]
- Fushiki, T.; Iwai, K. Two hypotheses on the feedback regulation of pancreatic enzyme secretion. FASEB J. 1989, 3, 121–126. [Google Scholar] [CrossRef]
- Adelson, J.W.; Clarizio, R.; Coutu, J.A. Pancreatic digestive enzyme secretion in the rabbit: Rapid cyclic variations in enzyme composition. Proc. Natl. Acad. Sci. USA 1995, 92, 2553–2557. [Google Scholar] [CrossRef]
- Ekelund, K.; Johansson, C. Output of bile and pancreatic enzymes after test meals with different fat content. Influence of body weight on pancreatic enzyme composition. Acta. Physiol. Scand. 1980, 110, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Buenabad, L.; Castillo, G.; Vázquez, L.; Espinoza, S.; Htoo, J.K.; Cervantes, M. Dietary levels of protein and free amino acids affect pancreatic proteases activities, amino acids transporters expression and serum amino acid concentrations in starter pigs. J. Anim. Physiol. Anim. Nutr. 2017, 101, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Ihse, I.; Lilja, P.; Lundquist, I. Trypsin as a regulator of pancreatic secretion in the rat. Scand. J. Gastroenterol. 1979, 14, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Lyman, R.L.; Olds, B.A.; Green, G.M. Chymotrypsinogen in the intestine of rats fed soybean trypsin inhibitor and its inability to suppress pancreatic enzyme secretions. J. Nutr. 1974, 104, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Murayama, H.; Tsuzuki, S.; Sugimoto, E.; Torii, K.; Fushiki, T. Long-term consumption of an amino acid diet reduces the pancreatic enzyme secretion response to a trypsin inhibitor in rats. J. Nutr. 1997, 127, 1377–1381. [Google Scholar] [CrossRef][Green Version]
- Liddle, R.A.; Green, G.M.; Conrad, C.K.; Williams, J.A. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 1986, 251, 243–248. [Google Scholar] [CrossRef]
- Guzmán-Pino, S.A.; Solà-Oriol, D.; Figueroa, J.; Pérez, J.F. Influence of the protein status of piglets on their ability to select and prefer protein sources. Physiol. Behav. 2014, 129, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Mo, W.; Cao, W.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Zhang, R.; Wang, J. Digestive abilities, amino acid transporter expression, and metabolism in the intestines of piglets fed with spermine. J. Food Biochem. 2020, 44, e13167. [Google Scholar] [CrossRef]
- Fang, T.; Liu, G.; Cao, W.; Wu, X.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Wang, J. Spermine: New insights into the intestinal development and serum antioxidant status of suckling piglets. RSC Adv. 2016, 6, 31323–31335. [Google Scholar] [CrossRef]
- Li, R.; Hou, G.; Song, Z.; Wu, C.; Zhao, J.; Sun, X.; Xiang, X.; Fan, Z.; Hou, D.-X.; He, X. Effects of different protein sources completely replacing fish meal in low-protein diet on growth performance, intestinal digestive physiology, and nitrogen digestion and metabolism in nursery pigs. Anim. Sci. J. 2019, 90, 977–989. [Google Scholar] [CrossRef]
- Yagami, K.; Takada, R. Dietary rice improves growth performance, mucosal enzyme activities and plasma urea nitrogen in weaning piglets. Anim. Sci. J. 2017, 88, 2010–2015. [Google Scholar] [CrossRef]
- Ding, H.; Cao, A.; Li, H.; Zhao, Y.; Feng, J. Effects of Eucommia ulmoides leaf extracts on growth performance, antioxidant capacity and intestinal function in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; He, W.; Yang, Z.; Fu, H.; Qiu, S.; Gao, F.; Shi, B. Effects of different emulsifiers on growth performance, nutrient digestibility, and digestive enzyme activity in weanling pigs. J. Anim. Sci. 2019, 97, 4235–4241. [Google Scholar] [CrossRef]
- Gilbert, E.; Wong, E.; Webb, K., Jr. Peptide absorption and utilization: Implications for animal nutrition and health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef]
- Nakashima, E.M.N.; Kudo, A.; Iwaihara, Y.; Tanaka, M.; Matsumoto, K.; Matsui, T. Application of 13C stable isotope labeling liquid chromatography-multiple reaction monitoring-tandem mass spectrometry method for determining intact absorption of bioactive dipeptides in rats. Anal. Biochem. 2011, 414, 109–116. [Google Scholar] [CrossRef]
- Poncet, N.; Taylor, P.M. The role of amino acid transporters in nutrition. Curr. Opin. Clin. Nutr. 2013, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gu, W.; Tang, X.; Geng, M.; Fan, M.; Li, T.; Chu, W.; Shi, C.; Huang, R.; Zhang, H.; et al. Molecular cloning, tissue distribution and ontogenetic expression of the amino acid transporter b0,+ cDNA in the small intestine of Tibetan suckling piglets. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 157–164. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Liu, Z.; Zeng, X.; Li, T.; Yin, Y. Long-term effects of lysine concentration on growth performance, intestinal microbiome, and metabolic profiles in a pig model. Food Funct. 2018, 9, 4153–4163. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Yaman, I.; Gaccioli, F.; Zeenko, V.V.; Wang, C.; Caprara, M.G.; Venema, R.C.; Komar, A.A.; Snider, M.D.; Hatzoglou, M. The hnRNA-Binding Proteins hnRNP L and PTB Are Required for Efficient Translation of the Cat-1 Arginine/Lysine Transporter mRNA during Amino Acid Starvation. Mol. Cell. Biol. 2009, 29, 2899–2912. [Google Scholar] [CrossRef]
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Phys. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, C.; Wang, Q.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Yang, H.; Yin, Y. The relationship between villous height and growth performance, small intestinal mucosal enzymes activities and nutrient transporters expression in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Nygard, A.B.; Jørgensen, C.B.; Cirera, S.; Fredholm, M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol. Biol. 2007, 8, 67. [Google Scholar] [CrossRef]
- Morales, A.; Barrera, M.A.; Araiza, A.B.; Zijlstra, R.T.; Bernal, H.; Cervantes, M. Effect of excess levels of lysine and leucine in wheat-based, amino acid-fortified diets on the mRNA expression of two selected cationic amino acid transporters in pigs. J. Anim. Physiol. Anim. Nutr. 2013, 97, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.; Grimble, G.; Rees, R. Protein digestion and amino acid and peptide absorption. Proc. Nutr. Soc. 1985, 44, 63–72. [Google Scholar] [CrossRef]
- Dave, M.H.; Schulz, N.; Zecevic, M.; Wagner, C.A.; Verrey, F. Expression of heteromeric amino acid transporters along the murine intestine. J. Physiol. 2004, 558, 597–610. [Google Scholar] [CrossRef]
- Torras-Llort, M.; Torrents, D.; Soriano-García, J.F.; Gelpí, J.L.; Estévez, R.; Ferrer, R.; Palacín, M.; Moretó, M. Sequential amino acid exchange across b0,+-like system in chicken brush border jejunum. J. Membr. Biol. 2001, 180, 213–220. [Google Scholar] [CrossRef]
- Morales, A.; Buenabad, L.; Castillo, G.; Arce, N.; Araiza, B.A.; Htoo, J.K.; Cervantes, M. Low-protein amino acid-supplemented diets for growing pigs: Effect on expression of amino acid transporters, serum concentration, performance, and carcass composition. J. Anim. Sci. 2015, 93, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Wagner, C.A.; Bröer, A.; Stehberger, P.A.; Kaltenbach, S.; Gelpí, J.L.; Martín Del Río, R.; Zorzano, A.; Palacín, M.; Lang, F.; et al. Cystinuria-specific rBAT(R365W) mutation reveals two translocation pathways in the amino acid transporter rBAT-b0,+AT. Biochem. J. 2004, 377, 665–674. [Google Scholar] [CrossRef]
- Zhang, S.; Qiao, S.; Ren, M.; Zeng, X.; Ma, X.; Wu, Z.; Thacker, P.; Wu, G. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 2013, 45, 1191–1205. [Google Scholar] [CrossRef]
- Morales, A.; García, H.; Arce, N.; Cota, M.; Zijlstra, R.T.; Araiza, B.A.; Cervantes, M. Effect of L-lysine on expression of selected genes, serum concentration of amino acids, muscle growth and performance of growing pigs. J. Anim. Physiol. Anim. Nutr. 2015, 99, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.; Rossier, G.; Spindler, B.; Meier, C.; Kühn, L.; Verrey, F. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J. 1999, 18, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.J.; Chaudhry, F.A.; Gray, A.T.; Edwards, R.H. Amino acid transport System A resembles System N in sequence but differs in mechanism. Proc. Natl. Acad. Sci. USA 2000, 97, 7715–7720. [Google Scholar] [CrossRef]
- Romeo, E.; Dave, M.H.; Bacic, D.; Ristic, Z.; Camargo, S.M.R.; Loffing, J.; Wagner, C.A.; Verrey, F. Luminal kidney and intestine SLC6 amino acid transporters of B 0AT-cluster and their tissue distribution in Mus musculus. Am. J. Physiol. Ren. Physiol. 2006, 290, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Shimada, Y.; Pan, X.; Kishimoto, K.; Sakurai, T.; Doi, R.; Onodera, H.; Katsura, T.; Imamura, M.; Inui, K.I. Expression profiles of various transporters for oligopeptides, amino acids and organic ions along the human digestive tract. Biochem. Pharmacol. 2005, 70, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.; Scislowski, P.W.D.; Brown, D.S.; Dewey, P.; Fuller, M.F. Interactions among the branched-chain amino acids and their effects on methionine utilization in growing pigs: Effects on plasma amino- and keto-acid concentrations and branched-chain keto-acid dehydrogenase activity. Br. J. Nutr. 2000, 83, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.T.; Kerr, B.J.; Easter, R.A.; Parkhurst, A.M. Difference in rates of net portal absorption between crystalline and protein-bound lysine and threonine in growing pigs fed once daily. J. Anim. Sci. 2004, 82, 1079–1090. [Google Scholar] [CrossRef]
- Vigors, S.; Sweeney, T.; O’Shea, C.J.; Kelly, A.K.; O’Doherty, J.V. Pigs that are divergent in feed efficiency, differ in intestinal enzyme and nutrient transporter gene expression, nutrient digestibility and microbial activity. Animal 2016, 10, 1848–1855. [Google Scholar] [CrossRef]
- Huang, S.; Liu, C.; Li, N.; Wu, Z.; Li, T.; Han, D.; Li, Z.; Zhao, J.; Wang, J. Membrane proteomic analysis reveals the intestinal development is deteriorated by intrauterine growth restriction in piglets. Funct. Integr. Genom. 2020, 20, 277–291. [Google Scholar] [CrossRef]
- He, Y.; Peng, X.; Liu, Y.; Wu, Q.; Zhou, Q.; Hu, L.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Effects of maternal fiber intake on intestinal morphology, bacterial profile and proteome of newborns using pig as model. Nutrients 2021, 13, 42. [Google Scholar] [CrossRef]
- Tröscher-Mußotter, J.; Tilocca, B.; Stefanski, V.; Seifert, J. Analysis of the bacterial and host proteins along and across the porcine gastrointestinal tract. Proteomes 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, Z.; Deng, D.; Cui, Y.; Qiu, Y. Effect of Dietary Protein Level on the Expression of Proteins in the Gastrointestinal Tract of Young Pigs. J. Agric. Food Chem. 2018, 66, 4364–4372. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Zhu, X.; Han, H.; Ren, W.; Chen, S.; Bin, P.; Liu, G.; Huang, X.; Fang, R.; et al. Effects of Long-Term Protein Restriction on Meat Quality, Muscle Amino Acids, and Amino Acid Transporters in Pigs. J. Agric. Food Chem. 2017, 65, 9297–9304. [Google Scholar] [CrossRef]
- Barea, R.; Nieto, R.; Aguilera, J.F. Effects of the dietary protein content and the feeding level on protein and energy metabolism in Iberian pigs growing from 50 to 100 kg body weight. Animal 2007, 1, 357–365. [Google Scholar] [CrossRef]
- Heo, J.M.; Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of postweaning diarrhea in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli. J. Anim. Sci. 2009, 87, 2833–2843. [Google Scholar] [CrossRef] [PubMed]
- Karasov, W.H.; Solberg, D.H.; Diamond, J.M. Dependence of intestinal amino acid uptake on dietary protein or amino acid levels. Am. J. Physiol. Gastrointest. Liver Physiol. 1987, 252, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, R.; Kim, H.B. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012, 13, 100–109. [Google Scholar] [CrossRef]
- De Rodas, B.; Youmans, B.P.; Danzeisen, J.L.; Tran, H.; Johnson, T.J. Microbiome profiling of commercial pigs from farrow to finish. J. Anim. Sci. 2018, 96, 1778–1794. [Google Scholar] [CrossRef]
- Looft, T.; Allen, H.K.; Cantarel, B.L.; Levine, U.Y.; Bayles, D.O.; Alt, D.P.; Henrissat, B.; Stanton, T.B. Bacteria, phages and pigs: The effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. 2014, 8, 1566–1576. [Google Scholar] [CrossRef]
- Holman, D.B.; Brunelle, B.W.; Trachsel, J.; Allen, H.K. Meta-analysis to Define a Core Microbiota in the Swine Gut. mSystems 2017, 2, e00004-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Widmer, G.; Tzipori, S. A pig model of the human gastrointestinal tract. Gut Microbes 2013, 4, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Wilmes, P.; Heintz-Buschart, A.; Bond, P.L. A decade of metaproteomics: Where we stand and what the future holds. Proteomics 2015, 15, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, B.; Tang, Y.; Liao, P.; Yao, K.; Ji, P.; Yin, Y. Extraction and identification of the chyme proteins in the digestive tract of growing pigs. Sci. China Life Sci. 2018, 61, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Gierse, L.C.; Meene, A.; Schultz, D.; Schwaiger, T.; Karte, C.; Schröder, C.; Wang, H.; Wünsche, C.; Methling, K.; Kreikemeyer, B.; et al. A multi-omics protocol for swine feces to elucidate longitudinal dynamics in microbiome structure and function. Microorganisms 2020, 8, 1887. [Google Scholar] [CrossRef]
- Kelly, J.; Daly, K.; Moran, A.W.; Ryan, S.; Bravo, D.; Shirazi-Beechey, S.P. Composition and diversity of mucosa-associated microbiota along the entire length of the pig gastrointestinal tract; dietary influences. Environ. Microbiol. 2017, 19, 1425–1438. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.; Lee, Y.K.; Xie, J.; Zhang, H. Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front. Microbiol. 2018, 9, 48. [Google Scholar] [CrossRef]
- Haange, S.B.; Oberbach, A.; Schlichting, N.; Hugenholtz, F.; Smidt, H.; Von Bergen, M.; Till, H.; Seifert, J. Metaproteome analysis and molecular genetics of rat intestinal microbiota reveals section and localization resolved species distribution and enzymatic functionalities. J. Proteome Res. 2012, 11, 5406–5417. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, Q.; Tan, B.; Guo, F.; Tang, M.; Li, T. Chronic feeding with protein-restricted diets affect ileal amino acid digestibility and the expression of nutrient-sensing, hormone secretion, gastrointestinal digestive enzyme, and nutrient transporter genes in young weaned pigs. Oncotarget 2018, 5. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Li, S.; Wu, J.; Ding, R.; Quan, J.; Zheng, E.; Yang, J.; Wu, Z. A transcriptome analysis identifies biological pathways and candidate genes for feed efficiency in DLY pigs. Genes 2019, 10, 725. [Google Scholar] [CrossRef]
- Vigors, S.; O’Doherty, J.V.; Bryan, K.; Sweeney, T. A comparative analysis of the transcriptome profiles of liver and muscle tissue in pigs divergent for feed efficiency. BMC Genom. 2019, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Horodyska, J.; Reyer, H.; Wimmers, K.; Trakooljul, N.; Lawlor, P.G.; Hamill, R.M. Transcriptome analysis of adipose tissue from pigs divergent in feed efficiency reveals alteration in gene networks related to adipose growth, lipid metabolism, extracellular matrix, and immune response. Mol. Genet. Genom. 2019, 294, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Horodyska, J.; Hamill, R.M.; Reyer, H.; Trakooljul, N.; Lawlor, P.G.; McCormack, U.M.; Wimmers, K. RNA-seq of liver from pigs divergent in feed efficiency highlights shifts in macronutrient metabolism, hepatic growth and immune response. Front. Genet. 2019, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- mayo-Caldas, Y.; Ballester, M.; Sánchez, J.P.; González-Rodríguez, O.; Revilla, M.; Reyer, H.; Wimmers, K.; Torrallardona, D.; Quintanilla, R. Integrative approach using liver and duodenum RNA-Seq data identifies candidate genes and pathways associated with feed efficiency in pigs. Sci. Rep. 2018, 8, 558. [Google Scholar] [CrossRef]
| Enzyme (Source) | Function | Product | Detection | Substrate | Wave Length |
|---|---|---|---|---|---|
| Endopeptidase | |||||
| Pepsin (stomach) | Cleavage peptides with aromatic AA | Peptide (AA) | F | Synthetic peptide substrate | Ex: 328 nm Em: 418 nm |
| Trypsin (pancreas) | Cleavage peptides with basic AA | Peptide (AA) | A | Synthetic substrate | 405 nm |
| Chymotrypsin (pancreas) | Cleavage peptides with aromatic AA and tryptophan | Peptide (AA) | F | Synthetic fluorogenic substrate | Ex: 380 nm Em: 460 nm |
| Elastase (pancreas) | Cleavage peptides with neutral AA without ring system | Peptide (AA) | F | Synthetic substrate | Ex: 380 nm Em: 500 nm |
| Exopeptidase | |||||
| Carboxypeptidase A (pancreas) | Cleavage peptides with C-terminal AA | AA, Peptide | A | N-(4-methoxyphenylazoformyl)-Phe-OH potassium salt | 350 nm |
| Carboxypeptidase B (pancreas) | Cleavage peptides with C-terminal basic AA | AA, Peptide | A | N-(4-methoxyphenylazoformyl)-Arg-OH HCl | 350 nm |
| Aminopeptidase (BBM) | Cleavage peptides with C-terminal AA | AA, Peptide | F | Fluorogenic substrate | Ex: 384 nm Em: 502 nm |
| Other Peptidase | |||||
| y-Glutamyl-transpeptidase (BBM) | Cleavage the AA glutamic acid from the tripeptide glutathione | AA, Dipeptide | A | L γ Glutamyl pNA | 418 nm |
| Dipeptidyl-peptidase (BBM) | Cleavage the AA glutamic acid from the tripeptide glutathione | Dipeptide, Peptide | F | Synthetic substrate | Ex: 360 nm Em: 460 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurz, A.; Seifert, J. Factors Influencing Proteolysis and Protein Utilization in the Intestine of Pigs: A Review. Animals 2021, 11, 3551. https://doi.org/10.3390/ani11123551
Kurz A, Seifert J. Factors Influencing Proteolysis and Protein Utilization in the Intestine of Pigs: A Review. Animals. 2021; 11(12):3551. https://doi.org/10.3390/ani11123551
Chicago/Turabian StyleKurz, Alina, and Jana Seifert. 2021. "Factors Influencing Proteolysis and Protein Utilization in the Intestine of Pigs: A Review" Animals 11, no. 12: 3551. https://doi.org/10.3390/ani11123551
APA StyleKurz, A., & Seifert, J. (2021). Factors Influencing Proteolysis and Protein Utilization in the Intestine of Pigs: A Review. Animals, 11(12), 3551. https://doi.org/10.3390/ani11123551







