Wild Felids Blood Group System
Abstract
Simple Summary
Abstract
1. Introduction
2. AB Blood System in Domestic Cats
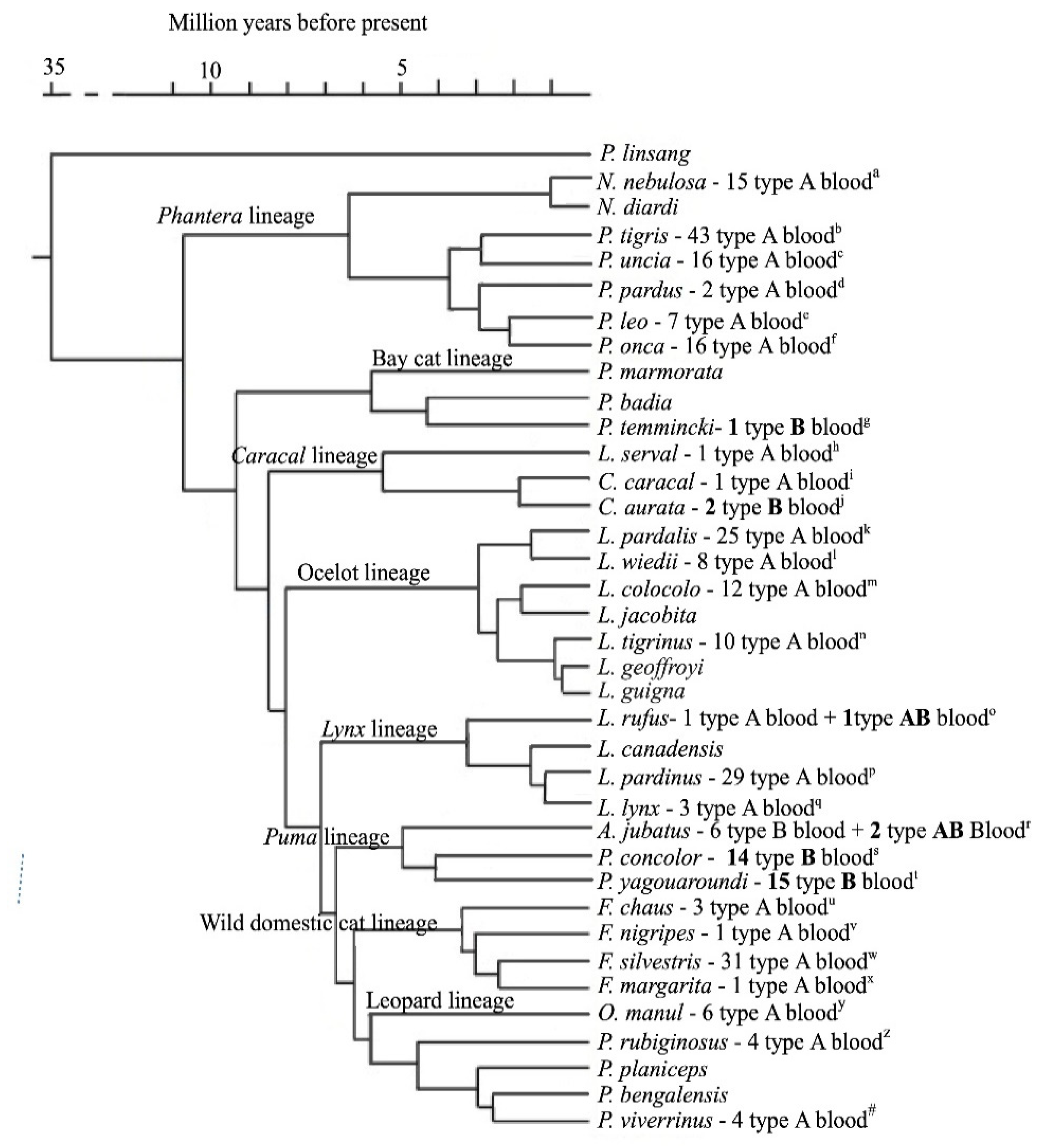
3. Wild Felids AB Blood System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Saitou, N.; Yamamoto, F. Evolution of primate ABO blood group genes and their homologous genes. Mol. Biol. Evol. 1997, 14, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.A.; Chavey, P.S.; Smith, J.E.; Rich, L. N-glycolylneuraminic acid and N-acetylneuraminic acid define feline blood group A and B antigens. Blood 1992, 79, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Kehl, A.; Truchet, L.; Langbein-Detsch, I.; Müller, E.; Giger, U. Updates on practical ABC blood compatibility testing in cats. Tierärztl. Prax. Ausg. K Kleintiere Heimtiere 2019, 47, 425–438. [Google Scholar]
- Driscoll, C.A.; Menotti-Raymond, M.; Roca, A.L.; Hupe, K.; Johnson, W.E.; Geffen, E.; Harley, E.H.; Delibes, M.; Pontier, D.; Kitchener, A.C.; et al. The near eastern origin of cat domestication. Science 2007, 317, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.C.; Moyse, J.A.; Lovstad, J.N.; Ober, C.B.; Thompson, E.E. Blood groups in the species survival Plan®, European Endangered species program, and managed in situ populations of bonobo (Pan paniscus), common chimpanzee (Pan troglodytes), gorilla (Gorilla ssp.), and orangutan (Pongo pygmaeus ssp.). Zoo Biol. 2011, 30, 427–444. [Google Scholar] [CrossRef]
- Thengchaisri, N.; Sinthusingha, C.; Arthitwong, S.; Sattasathuchana, S. Comparative serological investigation between cat and tiger blood for transfusion. J. Vet. Med. Sci. 2017, 79, 1081–1085. [Google Scholar] [CrossRef][Green Version]
- Silva, T.D.P.; Dreyer, M.O.; Back, F.P.; Lacerda, L.A.; Damasceno, A.D.; Araújo, L.B.M.; Sant’Ana, F.J.F.; Fioravanti, M.C.S. Sistema de grupos sanguíneos AB em felídeos neotropicais e compatibilidade com gatos domésticos [AB blood group system in neotropical felids and compatibility with domestic cats]. Arq. Bras. Med. Vet. Zootec. 2017, 69, 889–895. (In Portuguese) [Google Scholar] [CrossRef]
- Knottenbelt, C.M.; Day, M.J.; Cripps, P.; Mackin, A.J. Measurement of titres of naturally occurring alloantibodies against feline blood group antigens in the UK. J. Small Anim. Pract. 1999, 40, 365–370. [Google Scholar] [CrossRef]
- Auer, L.; Bell, K. Transfusion reactions in cats due to AB blood group incompatibility. Res. Vet. Sci. 1983, 35, 145–152. [Google Scholar] [CrossRef]
- Hessler, J.; Davis, L.E.; Dale, H.E. Effect of repeated transfusions of dog blood to cats. Small Anim. Clin. 1962, 2, 684–687. [Google Scholar]
- Bovens, C.; Gruffydd-Jones, T. Xenotransfusion with canine blood in the feline species: Review of the literature. J. Feline Med. Surg. 2013, 15, 62–67. [Google Scholar] [CrossRef]
- Adamantos, S.; Smith, C. Alternative transfusion methods. In Manual of Veterinary Transfusion Medicine and Blood Banking; Kenichiro, Y., Holowaychuck, M.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 296–297. [Google Scholar]
- Euler, C.C.; Raj, K.; Mizukami, K.; Murray, L.; Chen, C.; Mackin, A.; Giger, U. Xenotransfusion of anemic cats with blood compatibility issues: Pre- and post-transfusion laboratory diagnostic and crossmatching studies. Vet. Clin. Pathol. 2016, 45, 244–253. [Google Scholar] [CrossRef]
- Le Gal, A.; Thomas, E.K.; Hamm, K.R. Xenotransfusion of canine blood to cats: A review of 49 cases and their outcome. J. Small Anim. Pract. 2019, 61, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Griot-Wenk, M.; Pahlsson, P.; Chisholm-Chait, A.; Spitalnik, F.; Spitalnik, S.L.; Giger, U. Biochemical characterization of the feline AB blood group system. Anim. Genet. 1993, 24, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Ferreira, A.C.; Masso, O.; Pastor, J. High-performance liquid chromatography ganglioside pattern of the AB feline blood group. Comp. Clin. Pathol. 2011, 20, 597–605. [Google Scholar] [CrossRef]
- Bighignoli, B.; Niini, T.; Grahn, R.A.; Pedersen, N.C.; Millon, L.V.; Polli, M.; Longeri, M.; Lyons, L.A. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) mutations associated with the domestic cat AB blood group. BMC Genet. 2007, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Omi, T.; Nakazawa, S.; Udagawa, C.; Tada, N.; Ochiai, K.; Chong, Y.H.; Kato, Y.; Mitsui, H.; Gin, A.; Oda, H.; et al. Molecular characterization of the cytidine Monophosphate-N-Acetylneuraminic acid hydroxylase (CMAH) gene associated with the feline AB blood group system. PLoS ONE 2016, 11, e0165000. [Google Scholar]
- Auer, L.; Bell, K. The AB blood group system of cats. Anim. Blood Groups Biochem. Genet. 1981, 12, 287–297. [Google Scholar] [CrossRef]
- Giger, U.; Bucheler, J.; Patterson, D.F. Frequency and inheritance of A and B blood types in feline breeds of the United States. J. Hered. 1991, 82, 15–20. [Google Scholar] [CrossRef]
- Giger, U.; Bucheler, J.; Diserens, D.; Hale, A.; Griot-Wenk, M. Geographical variation of the feline blood type frequencies in the United States. Feline Pract. 1991, 19, 21–26. [Google Scholar]
- Hubler, M.; Arnold, S.; Casal, M.; Fairburn, A.; Nussbaumer, M.; Rüsch, P. The blood group distribution in domestic cats in Switzerland. Schweiz. Arch.r Tierheilkd. 1993, 135, 231–235. (In German) [Google Scholar]
- Jensen, A.L.; Olesen, A.B.; Arnbjerg, J. Distribution of feline blood types detected in the Copenhagen area of Denmark. Acta Vet. Scand. 1994, 35, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Knottenbelt, C.M.; Addie, D.D.; Day, M.J.; Mackin, A.J. Determination of the prevalence of feline blood types in the UK. J. Small Anim. Pract. 1999, 40, 115–118. [Google Scholar] [CrossRef]
- Mylonakis, M.E.; Koutinas, A.F.; Saridomichelakis, M.; Leontidis, L.; Papadogiannakis, E.; Plevraki, K. Determination of the prevalence of blood types in the non-pedigree feline population in Greece. Vet. Rec. 2001, 149, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Bagdi, N.; Magdus, M.; Leidinger, E.; Leidinger, J.; Vörös, K. Frequencies of feline blood types in Hungary. Acta Vet. Hung. 2001, 49, 369–375. [Google Scholar] [CrossRef]
- Silvestre-Ferreira, A.C.; Pastor, J.; Almeida, O.; Montoya, A. Frequencies of feline blood types in northern Portugal. Vet. Clin. Pathol. 2004, 33, 240–243. [Google Scholar] [CrossRef]
- Silvestre-Ferreira, A.C.; Pastor, J.; Sousa, A.P.; Pires, M.J.; Morales, M.; Abreu, Z.; Montoya, J.A. Blood types in the non-pedigree cat population of Gran Canaria. Vet. Rec. 2004, 155, 778–779. [Google Scholar]
- Ruiz de Gopegui, R.; Velasquez, M.; Espada, Y. Survey of feline blood types in the Barcelona area of Spain. Vet. Rec. 2004, 154, 794–795. [Google Scholar] [CrossRef]
- Malik, R.; Griffin, D.L.; White, J.D.; Rozmanec, M.; Tisdall, P.L.C.; Fosters, S.F.; Bell, K.; Nicholas, F.W. The prevalence of feline A/B blood types in the Sydney region. Aust. Vet. J. 2005, 83, 38–44. [Google Scholar] [CrossRef]
- Arikan, S.; Gurkan, M.; Ozaytekim, E.; Dodurka, T.; Giger, U. Frequencies of blood types A, B, and AB in non-pedigree domestic cats in Turkey. J. Small Anim. Pract. 2006, 47, 10–13. [Google Scholar] [CrossRef]
- Forcada, Y.; Guitian, J.; Gibson, G. Frequencies of feline blood types at a referral hospital in the south east of England. J. Small Anim. Pract. 2007, 48, 570–573. [Google Scholar] [CrossRef]
- Medeiros, M.A.S.; Soares, A.M.; Alviano, D.S.; Ejzemberg, R.; da Silva, M.H.; Almosny, N.R. Frequencies of feline blood types in the Rio de Janeiro area of Brazil. Vet. Clin. Pathol. 2008, 37, 272–276. [Google Scholar] [CrossRef]
- Arikan, S.; Guzel, M.; Ozturk, A.S.; Simsek, O. Frequencies of blood type A, B and AB in cats from the mediterranean sea coast of the Turkey. Rev. Med. Vet. 2010, 161, 322–325. [Google Scholar]
- Juvet, F.; Brennan, S.; Mooney, C.T. Assessment of feline blood for transfusion purposes in the Dublin area of Ireland. Vet. Rec. 2011, 168, 352. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Ferreira, M.; Gomes, J.F.; Leitāo, N.; Costa, M.; Serra, P.; Duarte Correia, J.H.; Pomba, C.F. Frequency of blood type A, B, and AB in 515 domestic shorthair cats from the Lisbon area. Vet. Clin. Pathol. 2011, 40, 185–187. [Google Scholar] [CrossRef]
- Zheng, L.; Zhong, Y.; Shi, Z.; Giger, U. Frequencies of blood types A, B, and AB in non-pedigree domestic cats in Beijing. Vet. Clin. Pathol. 2011, 40, 513–517. [Google Scholar] [CrossRef]
- Spada, E.; Miglio, A.; Proverbio, D.; Antognoni, M.T.; Bagnagatti De Giorgi, G.; Ferro, E.; Mangili, V. Signalment and blood types in cats being evaluated as blood donors at two Italian university blood banks. Vet. Med. Int. 2014, 2014, 704836. [Google Scholar] [CrossRef] [PubMed]
- Fosset, F.T.; Blais, M.C. Prevalence of feline blood groups in the Montreal area of Quebec, Canada. Can. Vet. J. 2014, 55, 1225–1228. [Google Scholar] [PubMed]
- Karadjole, T.; Kovačević, I.; Samardžija, M.; Babić, T.; Kreszinger, M.; Radišić, B.; Harapin, I.; Bedrica, L. Blood groups in cats in the city of Zagreb. Vet. Arh. 2016, 86, 209–216. [Google Scholar]
- Cattin, R. Distribution of blood types in a sample of 245 New Zealand non-purebred cats. N. Z. Vet. J. 2016, 64, 154–157. [Google Scholar] [CrossRef]
- Barrot, A.C.; Buttin, R.; Linsart, A.; Bachy, V.; Guidetti, M.; Blais, M.C. Frequency of feline blood types in non-pedigree cats in France. Rev. Med. Vet. 2017, 168, 235–240. [Google Scholar]
- Vieira, S.M.; Ferreira, R.R.F.; de Matos, A.J.; Cardoso, I.M.; Graça, R.M.C.; Soares, A.R.; Blasi-Brugué, C.; Sánchez, I.M.; Gopegui, R.R. Distribution of feline AB blood types: A review of frequencies and its implications in the Iberian Peninsula. J. Feline Med. Surg. Open Rep. 2017, 3, 2055116917727693. [Google Scholar] [CrossRef]
- Sorgatto, S.; Brito De Oliveira, B.; Cristina, K.; Godoy, S.; Antunes, T.R.; Almeida Lacerda, L.; De Souza, A.I. Frequência dos tipos sanguíneos de gatos domésticos mestiços no município de Campo Grande, Mato Grosso do Sul, Brasil. Med. Vet. 2017, 11, 172–178. [Google Scholar]
- Nectoux, A.; Guidetti, M.; Barthélemy, A.; Pouzot-Nevoret, C.; Hoareau, G.L.; Goy-Thollot, I. Assessment of risks of feline mismatched transfusion and neonatal isoerythrolysis in the Lyon (France) area. J. Feline Med. Surg. Open Rep. 2019, 5, 205511691986317. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Perego, R.; Baggiani, L.; Salatino, E.; Priolo, V.; Mangano, C.; Pennisi, M.G.; Proverbio, D. Prevalence of blood types and alloantibodies of the AB blood group system in non-pedigree cats from Northern (Lombardy) and Southern (Sicily) Italy. Animals 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- McDermott, F.M.; Maloney, S.; McMillan, C.; Snead, E. The prevalence of blood groups in Domestic cats in the Saskatoon and Calgary areas of Saskatchewan and Alberta, Canada. Front. Vet. Sci. 2020, 7, 160. [Google Scholar] [CrossRef]
- Arikan, S.; Duru, S.Y.; Gurkan, M.; Agaoglu, Z.T.; Giger, U. Blood type A and B frequencies in Turkish Van and Angora cats in Turkey. J. Vet. Med. A 2003, 50, 303–306. [Google Scholar] [CrossRef]
- Proverbio, D.; Spada, E.; Perego, R.; Della Pepa, A.; Bagnagatti De Giorgi, G.; Baggiani, L. Assessment of blood types of Ragdoll cats for transfusion purposes. Vet. Clin. Pathol. 2013, 42, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Antognoni, M.T.; Proverbio, D.; Ferro, E.; Mangili, V.; Miglio, A. Haematological and biochemical reference intervals in adult Maine Coon cat blood donors. J. Feline Med. Surg. 2015, 17, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, N.M.; Blais, M.-C.; Harris, K.; Oakley, D.A.; Aronson, L.R.; Giger, U. A newly recognized blood group in domestic shorthair cats: The Mik red cell antigen. J. Vet. Intern. Med. 2007, 21, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bücheler, J.; Giger, U. Alloantibodies against A and B blood types in cats. Vet. Immunol. Immunopathol. 1993, 38, 283–295. [Google Scholar] [CrossRef]
- Arikan, S.; Akkan, H.A. Titres of naturally occurring alloantibodies against feline blood group antigens in Turkish Van cats. J. Small Anim. Pract. 2004, 45, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, M.; Arikan, S.; Ozaytekin, E.; Dodurka, T. Titres of alloantibodies against A and B blood types in non-pedigree domestic cats in Turkey: Assessing the transfusion reaction risk. J. Feline Med. Surg. 2005, 7, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Giger, U.; Bucheler, J. Transfusion of type-A and type-B blood to cats. J. Am. Vet. Med. Assoc. 1991, 198, 411–418. [Google Scholar]
- Giger, U.; Akol, K.G. Acute hemolytic transfusion reaction in an Abyssinian cat with blood type B. J. Vet. Intern. Med. 1990, 4, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.; Maglaras, C.H.; Giger, U. Acute hemolytic reaction due to A-B mismatched transfusion in a cat with transient AB blood type. J. Vet. Emerg. Crit. Care 2020, 30, 325–330. [Google Scholar] [CrossRef]
- Cain, G.R.; Suzuki, Y. Presumptive neonatal isoerythrolysis in cats. J. Am. Vet. Med. Assoc. 1985, 187, 46–48. [Google Scholar]
- Griot-Wenk, M.; Callan, M.; Casal, L.; Chisholm-Chait, A.; Spitalnik, S.; Patterson, D.; Giger, U. Blood type AB in the feline AB blood group system. Am. J. Vet. Res. 1996, 57, 1438–1442. [Google Scholar]
- Silvestre-Ferreira, A.C.; Pastor, J. Feline neonatal isoerythrolysis and the importance of feline blood types. Vet. Med. Int. 2010, 2010, 753726. [Google Scholar] [CrossRef]
- Griot-Wenk, M.E.; Giger, U. The AB blood group system in wild felids. Anim. Genet. 1999, 30, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Werdelin, L.; Yamaguchi, N.; Johnson, W.E.; O’Brien, S.J. Phylogeny and evolution of cats (Felidae). In Biology and Conservation of Wild Felids; Macdonald, D.W., Loveridge, A.J., Eds.; Oxford University: Oxford, UK, 2010; pp. 59–82. [Google Scholar]
- Silvestre-Ferreira, A.C.; Marco, I.; Daussa, B.; Piñol, C.; Lavin, S.; Pastor, J. Blood group system in a captive population of European wildcats (Felis silvestris). Vet. Rec. 2006, 159, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Ferreira, A.C.; Bach-Raich, E.; Mesalles, M.; Vargas, A.; Martinez, F.; Cuenca, R.; Pastor, J. Blood group system in the iberian lynx (Lynx pardinus). In Proceedings of the 12th International Society of Animal Biochemistry Congress, Istanbul, Turkey, 22–26 May 2006. [Google Scholar]
- Bisca, J.M. Prevalência dos Tipos Sanguíneos A, B e AB em Felinos Selvagens Neotropicais Nativos do Brasil. Master’s Thesis, Universidade Estadual Paulista, São Paulo, Brasil, 2017. [Google Scholar]
- Gavazza, A.; Rossi, G.; Antognoni, M.T.; Cerquetella, M.; Miglio, A.; Mangiaterra, S. Feline blood groups: A systematic review of phylogenetic and geographical origin. Animals 2021, 11, 3339. [Google Scholar] [CrossRef]
- Binvel, M.; Arsenault, J.; Depré, B.; Blais, M.C. Identification of 5 novel feline erythrocyte antigens based on the presence of naturally occurring alloantibodies. J. Vet. Intern. Med. 2021, 35, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sogues, L.; Blois, S.L.; Manzanilla, E.G.; Abrams-Ogg, A.O.; Cosentino, P. Exploration of risk factors for non-survival and for transfusion-associated complications in cats receiving red cell transfusions: 450 cases (2009 to 2017). J. Small Anim. Pract. 2020, 61, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Melo, E.S.; Santos-Filho, M. Efeitos da BR-070 na Província Serrana de Cáceres, Mato Grosso, sobre a comunidade de vertebrados silvestres. Rev. Bras. Zoociências 2007, 9, 185–192. (In Portuguese) [Google Scholar]
- Singleton, C.L.; Oosterhuis, J.E.; Seibold, K.; Lamberski, N. Successful treatment of a southern Pacific rattlesnake (Crotalus viridis helleri) bite in a caracal (Caracal caracal). J. Zoo Wildl. Med. 2009, 40, 378–381. [Google Scholar] [CrossRef]
- Haefner, M.; Burke, T.J.; Kitchell, B.E.; Lamont, L.A.; Schaeffer, D.J.; Behr, M.; Messick, J.B. Identification of Haemobartonella felis (Mycoplasma haemofelis) in captive nondomestic cats. J. Zoo Wildl. Med. 2003, 34, 139–143. [Google Scholar] [PubMed]
- Yabsley, M.J.; Murphy, S.M.; Mark, W. Cunningham molecular detection and characterization of Cytauxzoon felis and a Babesia species in cougars from Florida. J. Wildl. Dis. 2006, 42, 366–374. [Google Scholar] [CrossRef]
- Harvey, J.W.; Dunbar, M.R.; Norton, T.M.; Yabsley, M.J. Laboratory findings in acute Cytauxzoon felis infection in cougars (Puma concolor couguar) in Florida. J. Zoo Wildl. Med. 2007, 38, 285–291. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Marr, H.S.; Warren, C.; Acton, A.E.; Mucker, E.M.; Humphreys, J.G.; Tucker, M.D. Cytauxzoon felis infections are present in bobcats (Lynx rufus) in a region where cytauxzoonosis is not recognized in domestic cats. Vet. Parasitol. 2008, 153, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Berentsen, A.; Shock, B.C.; Teixiera, M.; Dunbar, M.R.; Becker, M.S.; Yabsley, M.J. Prevalence and diversity of Babesia, Hepatozoon, Ehrlichia, and Bartonella in wild and domestic carnivores from Zambia, Africa. Parasitol. Res. 2014, 113, 911–918. [Google Scholar] [CrossRef]
- Zaeemi, M.; Razmi, G.R.; Khoshnegah, J. The first detection of Cytauxzoon felis in a wild cat (Felis silvestris) in Iran. Comp. Clin. Pathol. 2015, 24, 181–184. [Google Scholar] [CrossRef]
- Sacristán, I.; Acuña, F.; Aguilar, E.; García, S.; López, M.J.; Cevidanes, A.; Cabello, J.; Hidalgo-Hermoso, E.; Johnson, W.E.; Poulin, E.; et al. Assessing cross-species transmission of hemoplasmas at the wild-domestic felid interface in Chile using genetic and landscape variables analysis. Sci. Rep. 2019, 9, 16816. [Google Scholar] [CrossRef]
- Feldman, B.F. In-house canine and feline blood typing. J. Am. Anim. Hosp. Assoc. 1999, 35, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Giger, U. Blood typing and crossmatching to ensure compatible transfusions. In Kirk’s Current Veterinary Therapy. XIII Small Animal Practice; Bonagura, J.D., Ed.; WB Saunders: Philadelphia, PA, USA, 2000; pp. 396–399. [Google Scholar]
- Knottenbelt, C.M. The feline AB blood group system and its importance in transfusion medicine. J. Feline Med. Surg. 2002, 4, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Marenzoni, M.L.; Antognoni, M.T.; Baldelli, F.; Miglio, A.; Stefanetti, V.; Desario, C.; Di Summa, A.; Buonavoglia, C.; Decaro, N. Detection of parvovirus and herpesvirus DNA in the blood of feline and canine blood donors. Vet. Microbiol. 2018, 224, 66–69. [Google Scholar] [CrossRef]
- Reine, N.J. Infection and blood transfusion: A guide to donor screening. Clin. Tech. Small Anim. Pract. 2004, 19, 68–74. [Google Scholar] [CrossRef]
- Satake, M.; Hoshi, Y.; Taira, R.; Momose, S.Y.; Hino, S.; Tadokoro, K. Symptomatic parvovirus B19 infection caused by blood component transfusion. Transfusion. 2011, 51, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Hartmann, K.; Addie, D.D.; Lutz, H.; Gruffydd-Jones, T.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Horzinek, M.C.; Hosie, M.J.; et al. Blood transfusion in cats: ABCD guidelines for minimising risks of infectious iatrogenic complications. J. Feline Med. Surg. 2015, 17, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, V.; Miglio, A.; Cappelli, K.; Capomaccio, S.; Sgariglia, E.; Marenzoni, M.L.; Antognoni, M.T.; Coletti, M.; Mangili, V.; Passamonti, F. Detection of bacterial contamination and DNA quantification in stored blood units in 2 veterinary hospital blood banks. Vet. Clin. Pathol. 2016, 45, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, K.J.; Birkenheuer, A.; Blais, M.C.; Callan, M.B.; Kohn, B.; Lappin, M.R.; Sykes, J. Update on canine and feline blood donor screening for blood-borne pathogens. J. Vet. Intern. Med. 2016, 30, 15–35. [Google Scholar] [CrossRef] [PubMed]
| Lineage | Nº Animals | Blood Type | Typing Method | Alloantibodies | Country | ||
|---|---|---|---|---|---|---|---|
| A | B | AB | - | ||||
| Ocelot | 15 | 15 | Tube hemagglutination | Not detected | USA/Dubai [61] | ||
| Caracal | 8 | 6 | 2 | ||||
| Asian leopard | 4 | 4 | |||||
| Puma | 23 | 21 | 2 | ||||
| Lynx | 5 | 4 | 1 | ||||
| Bay cat | 1 | 1 | |||||
| Wild domestic cats | 17 | 17 | |||||
| Panthera | 58 | 58 | |||||
| Felis silvestris | 25 | 25 | Tube hemagglutination | Detected | Spain [63] | ||
| Lynx pardinus | 29 | 29 | Tube hemagglutination | Detected | Spain [64] | ||
| Lynx pardinus | 111 | 111 | Immunochromatographic | Not determined | Spain * | ||
| Panthera tigris tigris | 30 | 30 | Slide agglutination test | Not determined | Thailand [6] | ||
| Ocelot | 15 | 15 | Tube hemagglutination | Not determined | Brazil ** [7] | ||
| Puma | 8 | 8 | |||||
| Ocelot | 25 | 25 | Tube hemagglutination and card *** | Not determined | Brazil **** [65] | ||
| Puma | 6 | 6 | |||||
| Panthera | 11 | 11 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestre-Ferreira, A.; Pastor, J. Wild Felids Blood Group System. Animals 2021, 11, 3533. https://doi.org/10.3390/ani11123533
Silvestre-Ferreira A, Pastor J. Wild Felids Blood Group System. Animals. 2021; 11(12):3533. https://doi.org/10.3390/ani11123533
Chicago/Turabian StyleSilvestre-Ferreira, Ana, and Josep Pastor. 2021. "Wild Felids Blood Group System" Animals 11, no. 12: 3533. https://doi.org/10.3390/ani11123533
APA StyleSilvestre-Ferreira, A., & Pastor, J. (2021). Wild Felids Blood Group System. Animals, 11(12), 3533. https://doi.org/10.3390/ani11123533





