Effects of Positive Reinforcement Training and Novel Object Exposure on Salivary Cortisol Levels under Consideration of Individual Variation in Captive African Elephants (Loxodonta africana)
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Glucocorticoid Levels as an Indicator of Stress
1.2. Individual Variation of Glucocorticoid Levels
1.3. The Management of Stress in Zoo Animals
1.4. Rationale of the Present Study
2. Materials and Methods
2.1. Animals and Management
2.2. Treatments
2.2.1. Positive Reinforcement Training
2.2.2. Novel Object Exposure
2.3. Saliva Sample Collection for Cortisol Analysis
2.3.1. Social Context
2.3.2. Sampling Schedule
2.3.3. Experimental Set-Up
2.4. Cortisol Analysis
2.5. Statistical Analysis
2.5.1. Effect of the Treatment on SACort
2.5.2. Effect of Individual Parameters on SACort
3. Results
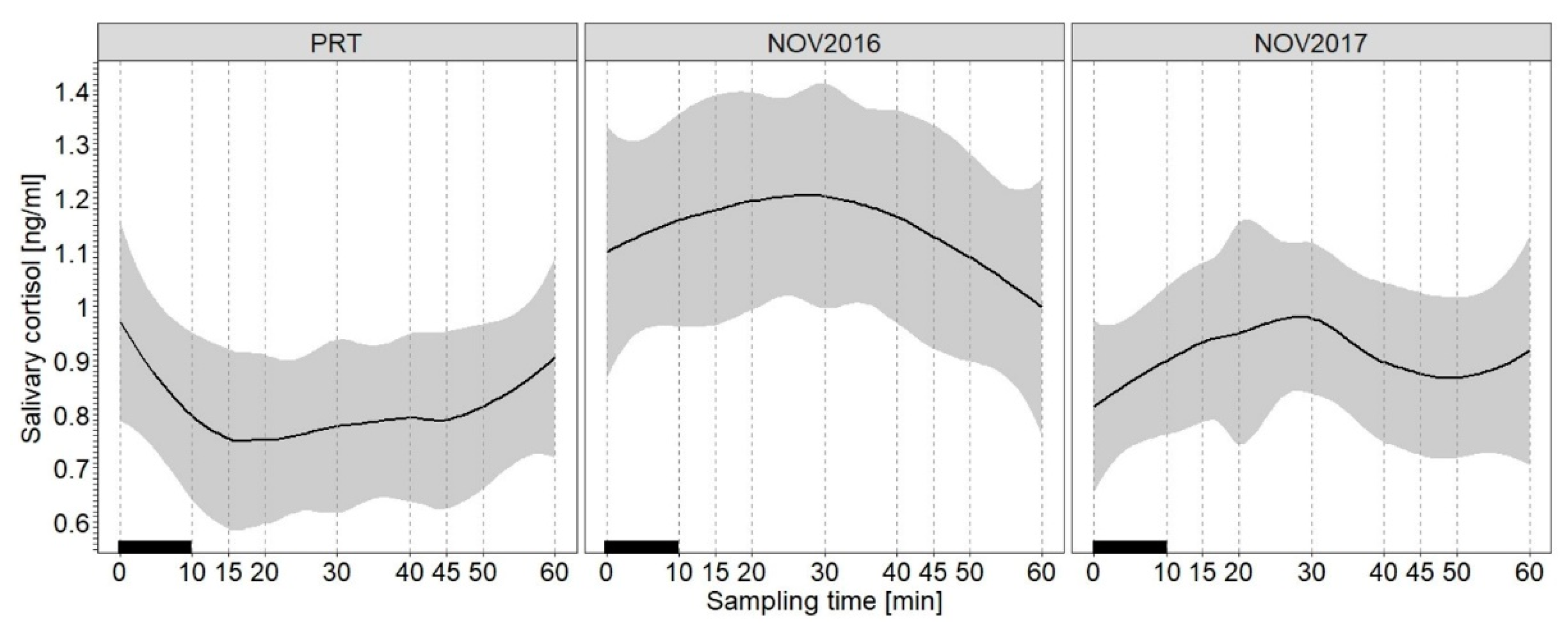
3.1. Effect of the Treatment on SACort
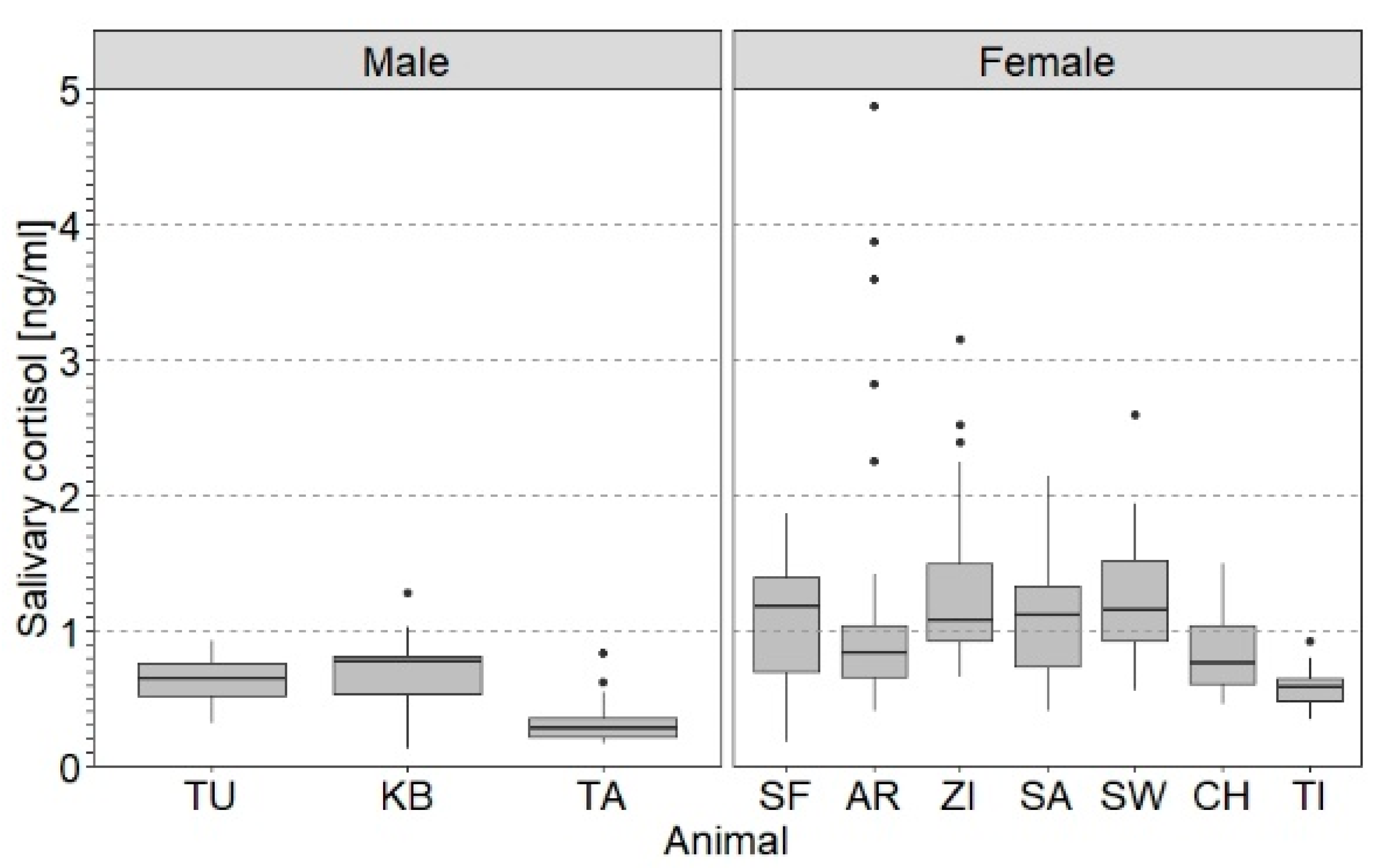
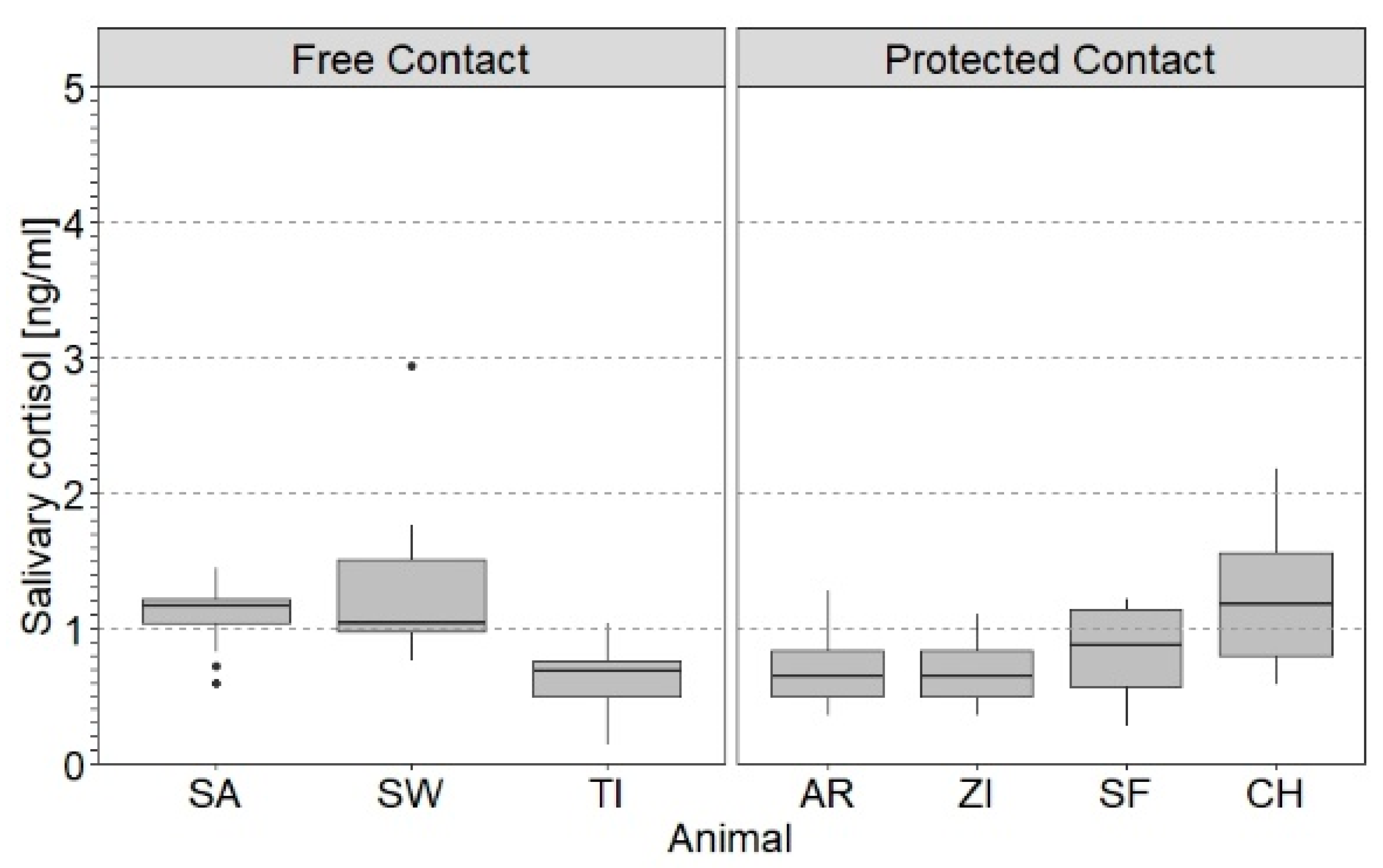
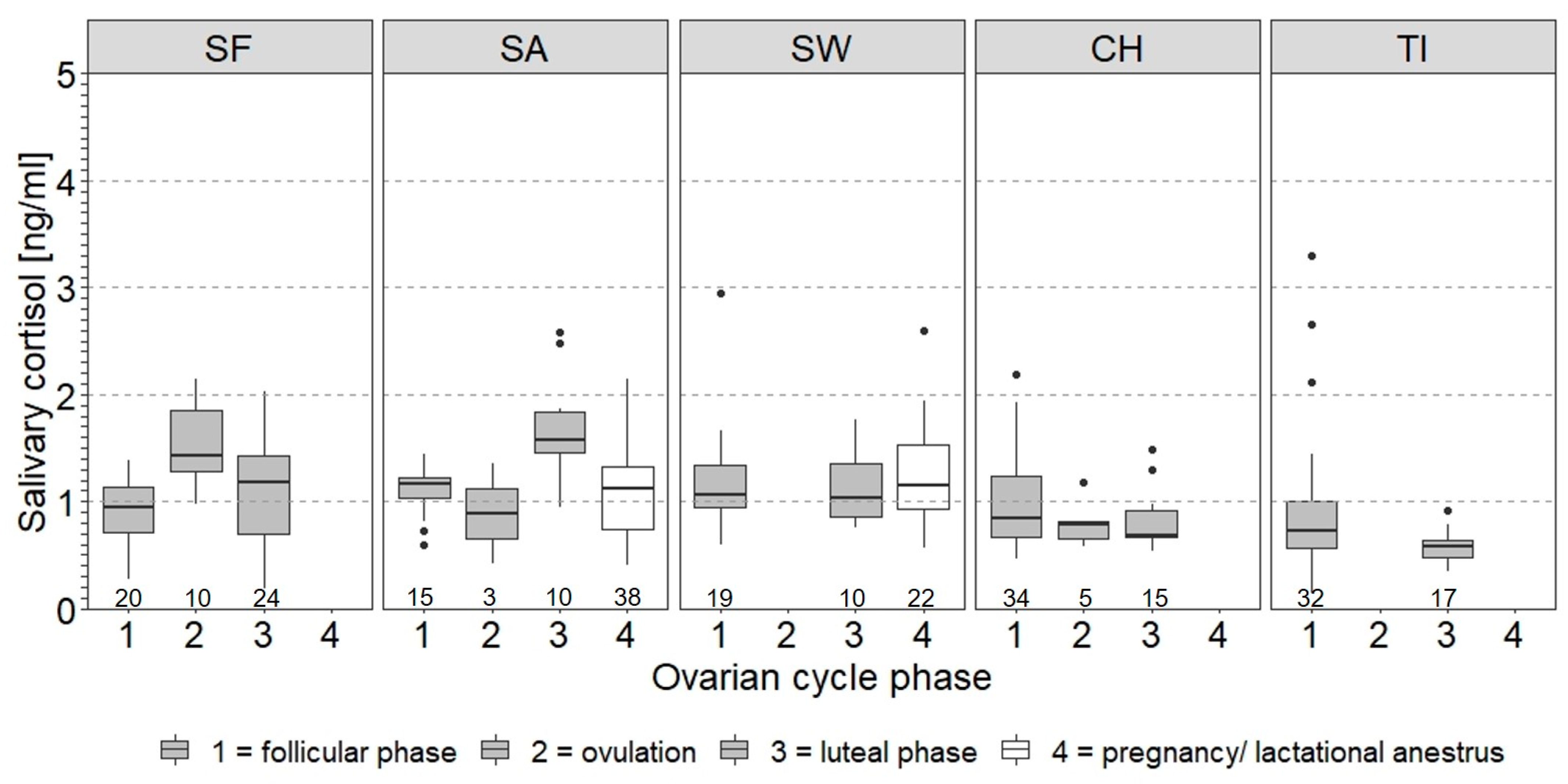
3.2. Effect of Individual Parameters on SACort
4. Discussion
4.1. Effect of the Treatment on SACort
4.2. Effect of Individual Parameters on SACort in Relation to PRT and NOV
4.3. Stress Management through Positive Reinforcement Training and Enrichment
4.4. Methodological Considerations and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilbrook, A.J.; Clarke, I.J. Neuroendocrine mechanisms of innate states of attenuated responsiveness of the hypothalamo-pituitary adrenal axis to stress. Front. Neuroendocrinol. 2006, 27, 285–307. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare, Reprinted; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 1–21. ISBN 978-0-85199-359-1. [Google Scholar]
- Wu, G.; Feder, A.; Cohen, H.; Kim, J.J.; Calderon, S.; Charney, D.S.; Mathé, A.A. Understanding resilience. Front. Behav. Neurosci. 2013, 7, 10. [Google Scholar] [CrossRef]
- Amaya, V.; Paterson, M.B.A.; Descovich, K.; Phillips, C.J.C. Effects of olfactory and auditory enrichment on heart rate variability in shelter dogs. Animals 2020, 10, 1385. [Google Scholar] [CrossRef]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Boonstra, R.; Hik, D.; Singleton, G.R.; Tinnikov, A. The impact of predator-induced stress on the snowshoe hare cycle. Ecol. Monogr. 1998, 68, 371–394. [Google Scholar] [CrossRef]
- Ralph, C.R.; Tilbrook, A.J. The usefulness of measuring glucocorticoids for assessing animal welfare. J. Anim. Sci. 2016, 94, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Möstl, E. Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann. N. Y. Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef]
- Brown, J.L.; Kersey, D.C.; Freeman, E.W.; Wagener, T. Assessment of diurnal urinary cortisol excretion in Asian and African elephants using different endocrine methods. Zoo Biol. 2010, 29, 274–283. [Google Scholar] [CrossRef]
- Smith, T.; French, J. Psychosocial stress and urinary cortisol excretion in marmoset monkeys (Callithrix kuhli). Physiol. Behav. 1997, 62, 225–232. [Google Scholar] [CrossRef]
- Sousa, M.; Ziegler, T.E. Diurnal variation on the excretion patterns of fecal steroids in common marmoset (Callithrix jacchus) females. Am. J. Primatol. 1998, 46, 105–117. [Google Scholar] [CrossRef]
- Heistermann, M. Non-invasive monitoring of endocrine status in laboratory primates: Methods, guidelines and applications. Adv. Sci. Res. 2010, 5, 1–9. [Google Scholar] [CrossRef][Green Version]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Desportes, G.; Buholzer, L.; Anderson-Hansen, K.; Blanchet, M.-A.; Acquarone, M.; Shephard, G.; Brando, S.; Vossen, A.; Siebert, U. Decrease stress; train your animals: The effect of handling methods on cortisol levels in harbour porpoises (Phocoena phocoena) under human care. Aquat. Mamm. 2007, 33, 286–292. [Google Scholar] [CrossRef]
- Grandin, T. Habituating antelope and bison to cooperate with veterinary procedures. J. Appl. Anim. Welf. Sci. 2000, 3, 253–261. [Google Scholar] [CrossRef]
- Lambeth, S.P.; Hau, J.; Perlman, J.E.; Martino, M.; Schapiro, S.J. Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodytes). Am. J. Primatol. 2006, 68, 245–256. [Google Scholar] [CrossRef]
- Foley, C.A.H.; Papageorge, S.; Wasser, S.K. Noninvasive stress and reproductive measures of social and ecological pressures in free-ranging African elephants. Conserv. Biol. 2001, 15, 1134–1142. [Google Scholar] [CrossRef]
- Hunninck, L.; Ringstad, I.H.; Jackson, C.R.; May, R.; Fossøy, F.; Uiseb, K.; Killian, W.; Røskaft, E. Being stressed outside the park—conservation of African elephants (Loxodonta africana) in Namibia. Conserv. Physiol. 2017, 5, cox067. [Google Scholar] [CrossRef]
- Viljoen, J.J.; Ganswindt, A.; du Toit, J.T.; Langbauer, W.R. Translocation stress and faecal glucocorticoid metabolite levels in free-ranging African savanna elephants. S. Afr. J. Wildl. Res. 2008, 38, 146–152. [Google Scholar] [CrossRef]
- Boyle, S.A.; Roberts, B.; Pope, B.M.; Blake, M.R.; Leavelle, S.E.; Marshall, J.J.; Smith, A.; Hadicke, A.; Falcone, J.F.; Knott, K.; et al. Assessment of flooring renovations on African elephant (Loxodonta africana) behavior and glucocorticoid response. PLoS ONE 2015, 10, e0141009. [Google Scholar] [CrossRef]
- Carlstead, K.; Paris, S.; Brown, J.L. Good keeper-elephant relationships in North American zoos are mutually beneficial to welfare. Appl. Anim. Behav. Sci. 2019, 211, 103–111. [Google Scholar] [CrossRef]
- Proctor, C.M.; Brown, J.L. A preliminary analysis of the influence of handling method on adrenal activity in zoo African and Asian elephants. J. Zoo Aquar. Res. 2015, 3, 1–5. [Google Scholar] [CrossRef]
- White, B.C.; Taylor, S.R.; Gyimesi, Z.S.; Rieskamp, C.L.; Sarros, W.; Burton, S.D., II. Serum cortisol concentrations associated with artificial insemination events in an African elephant (Loxodonta africana). J. Zoo Aquar. Res. 2019, 7, 134–137. [Google Scholar] [CrossRef]
- Williams, E.; Chadwick, C.; Yon, L.; Asher, L. A review of current indicators of welfare in captive elephants (Loxodonta africana and Elephas maximus). Anim. Welf. 2018, 27, 235–249. [Google Scholar] [CrossRef]
- Brown, J.L.; Carlstead, K.; Bray, J.D.; Dickey, D.; Farin, C.; Ange-van Heugten, K. Individual and environmental risk factors associated with fecal glucocorticoid metabolite concentrations in zoo-housed Asian and African elephants. PLoS ONE 2019, 14, e0217326. [Google Scholar] [CrossRef] [PubMed]
- Burks, K.D.; Mellen, J.D.; Miller, G.W.; Lehnhardt, J.; Weiss, A.; Figueredo, A.J.; Maple, T.L. Comparison of two introduction methods for African elephants (Loxodonta africana). Zoo Biol. 2004, 23, 109–126. [Google Scholar] [CrossRef]
- Casares, M.; Silván, G.; Carbonell, M.D.; Gerique, C.; Martinez-Fernandez, L.; Cáceres, S.; Illera, J.C. Circadian rhythm of salivary cortisol secretion in female zoo-kept African elephants (Loxodonta africana). Zoo Biol. 2016, 35, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Grand, A.P.; Kuhar, C.W.; Leighty, K.A.; Bettinger, T.L.; Laudenslager, M.L. Using personality ratings and cortisol to characterize individual differences in African Elephants (Loxodonta africana). Appl. Anim. Behav. Sci. 2012, 142, 69–75. [Google Scholar] [CrossRef]
- Hambrecht, S.; Oerke, A.-K.; Heistermann, M.; Dierkes, P.W. Diurnal variation of salivary cortisol in captive African elephants (Loxodonta africana) under routine management conditions and in relation to a translocation event. Zoo Biol. 2020, 39, 186–196. [Google Scholar] [CrossRef]
- Kelling, A.S. An Examination of Salivary Cortisol Concentrations and Behavior in Three Captive African Elephants (Loxodonta africana) at Zoo Atlanta. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2008. [Google Scholar]
- Cook, N.J. Minimally invasive sampling media and the measurement of corticosteroids as biomarkers of stress in animals. Can. J. Anim. Sci. 2012, 92, 227–259. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.; Janssen, N.S.; Mol, J.A. The use of saliva cortisol, urinary cortisol, and catecholamine measurements for a noninvasive assessment of stress responses in dogs. Horm. Behav. 1996, 30, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Hiramatsu, R.; Iwaoka, T.; Shimada, T.; Miura, F.; Sato, T. Use of saliva for monitoring unbound free cortisol levels in serum. Clin. Chim. Acta 1981, 110, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol. In Encyclopedia of Stress, 1st ed.; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 379–383. [Google Scholar]
- Hernandez, C.E.; Thierfelder, T.; Svennersten-Sjaunja, K.; Berg, C.; Orihuela, A.; Lidfors, L. Time lag between peak concentrations of plasma and salivary cortisol following a stressful procedure in dairy cattle. Acta Vet. Scand. 2014, 56, 61. [Google Scholar] [CrossRef]
- Heintz, M.R.; Santymire, R.M.; Parr, L.A.; Lonsdorf, E.V. Validation of a cortisol enzyme immunoassay and characterization of salivary cortisol circadian rhythm in chimpanzees (Pan troglodytes). Am. J. Primatol. 2011, 73, 903–908. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.H.; van Hooff, J.A.R.A.M.; de Vries, H.W.; Mol, J.A. Behavioural, saliva cortisol and heart rate responses to different types of stimuli in dogs. Appl. Anim. Behav. Sci. 1998, 58, 365–381. [Google Scholar] [CrossRef]
- Peeters, M.; Sulon, J.; Beckers, J.-F.; Ledoux, D.; Vandenheede, M. Comparison between blood serum and salivary cortisol concentrations in horses using an adrenocorticotropic hormone challenge. Equine Vet. J. 2011, 43, 487–493. [Google Scholar] [CrossRef]
- Peeters, M.; Closson, C.; Beckers, J.-F.; Vandenheede, M. Rider and horse salivary cortisol levels during competition and impact on performance. J. Equine Vet. Sci. 2013, 33, 155–160. [Google Scholar] [CrossRef]
- Cockrem, J.F. Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef]
- Anestis, S.F.; Bribiescas, R.G.; Hasselschwert, D.L. Age, rank, and personality effects on the cortisol sedation stress response in young chimpanzees. Physiol. Behav. 2006, 89, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Guimont, F.S.; Wynne-Edwards, K.E. Individual variation in cortisol responses to acute “on-back” restraint in an outbred hamster. Horm. Behav. 2006, 50, 252–260. [Google Scholar] [CrossRef]
- Turner, A.I.; Rivalland, E.T.A.; Clarke, I.J.; Tilbrook, A.J. Stressor specificity of sex differences in hypothalamo-pituitary-adrenal axis activity: Cortisol responses to exercise, endotoxin, wetting, and isolation/restraint stress in gonadectomized male and female sheep. Endocrinology 2010, 151, 4324–4331. [Google Scholar] [CrossRef]
- Carere, C.; Caramaschi, D.; Fawcett, T.W. Covariation between personalities and individual differences in coping with stress: Converging evidence and hypotheses. Curr. Zool. 2010, 56, 728–740. [Google Scholar] [CrossRef]
- Creel, S.; Dantzer, B.; Goymann, W.; Rubenstein, D.R. The ecology of stress: Effects of the social environment. Funct. Ecol. 2013, 27, 66–80. [Google Scholar] [CrossRef]
- van Jaarsveld, A.S.; Skinner, J.D. Adrenocortical responsiveness to immobilization stress in spotted hyenas (Crocuta crocuta). Comp. Biochem. Physiol. 1992, 103, 73–79. [Google Scholar] [CrossRef]
- Schmitt, T.L.; St. Aubin, D.J.; Schäfer, A.M.; Dunn, J.L. Baseline, diurnal variations, and stress-induced changes of stress hormones in three captive beluga, Delphinapterus leucas. Mar. Mamm. Sci. 2010, 26, 635–647. [Google Scholar] [CrossRef]
- Vincent, I.C.; Michell, A.R. Comparison of cortisol concentrations in saliva and plasma of dogs. Res. Vet. Sci. 1992, 53, 342–345. [Google Scholar] [CrossRef]
- Dembiec, D.P.; Snider, R.J.; Zanella, A.J. The effects of transport stress on tiger physiology and behavior. Zoo Biol. 2004, 23, 335–346. [Google Scholar] [CrossRef]
- Rooney, N.J.; Gaines, S.A.; Bradshaw, J.W.S. Behavioural and glucocorticoid responses of dogs (Canis familiaris) to kennelling: Investigating mitigation of stress by prior habituation. Physiol. Behav. 2007, 92, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Grissom, N.; Bhatnagar, S. Habituation to repeated stress: Get used to it. Neurobiol. Learn. Mem. 2009, 92, 215–224. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; van der Spek, R.; Lei, J.; Endert, E.; Buijs, R.M.; Fliers, E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol. Cell. Endocrinol. 2012, 349, 20–29. [Google Scholar] [CrossRef]
- Fanson, K.V.; Lynch, M.; Vogelnest, L.; Miller, G.; Keeley, T. Response to long-distance relocation in Asian elephants (Elephas maximus): Monitoring adrenocortical activity via serum, urine, and feces. Eur. J. Wildl. Res. 2013, 59, 655–664. [Google Scholar] [CrossRef]
- Glaeser, S.S.; Edwards, K.L.; Wielebnowski, N.; Brown, J.L. Effects of physiological changes and social life events on adrenal glucocorticoid activity in female zoo-housed Asian elephants (Elephas maximus). PLoS ONE 2020, 15, e0241910. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Somerville, M.; Riddle, H.S.; Keele, M.; Duer, C.K.; Freeman, E.W. Comparative endocrinology of testicular, adrenal and thyroid function in captive Asian and African elephant bulls. Gen. Comp. Endocrinol. 2007, 151, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Bechert, U.; Hixon, S.; Schmitt, D. Diurnal variation in serum concentrations of cortisol in captive African (Loxodonta africana) and Asian (Elephas maximus) elephants. Zoo Biol. 2021, 40, 458–471. [Google Scholar] [CrossRef]
- Menargues, A.; Urios, V.; Limiñana, R.; Mauri, M. Circadian rhythm of salivary cortisol in Asian elephants (Elephas maximus): A factor to consider during welfare assessment. J. Appl. Anim. Welf. Sci. 2012, 15, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Plangsangmas, T.; Brown, J.L.; Thitaram, C.; Silva-Fletcher, A.; Edwards, K.L.; Punyapornwithaya, V.; Towiboon, P.; Somgird, C. Circadian Rhythm of Salivary Immunoglobulin A and Associations with Cortisol as A Stress Biomarker in Captive Asian Elephants (Elephas maximus). Animals 2020, 10, 157. [Google Scholar] [CrossRef]
- Brown, J.L.; Walker, S.L.; Moeller, T. Comparative endocrinology of cycling and non-cycling Asian (Elephas maximus) and African (Loxodonta africana) elephants. Gen. Comp. Endocrinol. 2004, 136, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Bechert, U.S.; Swanson, L.; Wasser, S.K.; Hess, D.L.; Stormshak, F. Serum prolactin concentrations in the captive female African elephant (Loxodonta africana): Potential effects of season and steroid hormone interactions. Gen. Comp. Endocrinol. 1999, 114, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ganswindt, A.; Palme, R.; Heistermann, M.; Borragan, S.; Hodges, J. Non-invasive assessment of adrenocortical function in the male African elephant (Loxodonta africana) and its relation to musth. Gen. Comp. Endocrinol. 2003, 134, 156–166. [Google Scholar] [CrossRef]
- Fanson, K.V.; Keeley, T.; Fanson, B.G. Cyclic changes in cortisol across the estrous cycle in parous and nulliparous Asian elephants. Endocr. Connect. 2014, 3, 57–66. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Felippe, E.C.G.; Chelini, M.O.M. Serum cortisol and progestin concentrations in pregnant and non-pregnant Asian elephants (Elephas maximus). Res. Vet. Sci. 2008, 84, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Beehner, J.C.; Bergman, T.J.; Cheney, D.L.; Seyfarth, R.M.; Whitten, P.L. The effect of new alpha males on female stress in free-ranging baboons. Anim. Behav. 2005, 69, 1211–1221. [Google Scholar] [CrossRef]
- Macbeth, B.J.; Cattet, M.R.L.; Obbard, M.E.; Middel, K.; Janz, D.M. Evaluation of hair cortisol concentration as a biomarker of long-term stress in free-ranging polar bears. Wildl. Soc. Bull. 2012, 36, 747–758. [Google Scholar] [CrossRef]
- Tecot, S.R.; Irwin, M.T.; Raharison, J. Faecal glucocorticoid metabolite profiles in diademed sifakas increase during seasonal fruit scarcity with interactive effects of age/sex class and habitat degradation. Conserv. Physiol. 2019, 7, coz001. [Google Scholar] [CrossRef]
- Wielebnowski, N. Stress and distress: Evaluating their impact for the well-being of zoo animals. J. Am. Vet. Med. Assoc. 2003, 223, 973–977. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Alleviating stress in zoo animals with environmental enrichment. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare, Reprinted; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 337–354. ISBN 978-0-85199-359-1. [Google Scholar]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Carlstead, K.; Brown, J.L.; Seidensticker, J. Behavioral and adrenocortical responses to environmental changes in leopard cats (Felis bengalensis). Zoo Biol. 1993, 12, 321–331. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Effects of environmental enrichment on reproduction. Zoo Biol. 1994, 13, 447–458. [Google Scholar] [CrossRef]
- Lyons, D.M.; Parker, K.J.; Schatzberg, A.F. Animal models of early life stress: Implications for understanding resilience. Dev. Psychol. 2010, 52, 616–624. [Google Scholar] [CrossRef]
- Jones, B.R.; Waddington, D. Modification of fear in domestic chicks, Gallus gallus domesticus, via regular handling and early environmental enrichment. Anim. Behav. 1992, 43, 1021–1033. [Google Scholar] [CrossRef]
- Meehan, C.L.; Mench, J.A. Environmental enrichment affects the fear and exploratory responses to novelty of young Amazon parrots. Appl. Anim. Behav. Sci. 2002, 79, 75–88. [Google Scholar] [CrossRef]
- Oesterwind, S.; Nürnberg, G.; Puppe, B.; Langbein, J. Impact of structural and cognitive enrichment on the learning performance, behavior and physiology of dwarf goats (Capra aegagrus hircus). Appl. Anim. Behav. Sci. 2016, 177, 34–41. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; White, A.M.; Zhou, X.; Zhang, G.; Lindburg, D.G. How do giant pandas (Ailuropoda melanoleuca) respond to varying properties of enrichments? A comparison of behavioral profiles among five enrichment items. J. Comp. Psychol. 2005, 119, 325–334. [Google Scholar] [CrossRef]
- Shepherdson, D.J. Tracing the path of environmental enrichment in zoos. In Second Nature: Environmental Enrichment for Captive Animals; Shepherdson, D.J., Mellen, J.D., Hutchins, M., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1998; pp. 1–12. ISBN 978-1-56098-397-2. [Google Scholar]
- Cannon, T.H.; Heistermann, M.; Hankison, S.J.; Hockings, K.J.; McLennan, M.R. Tailored Enrichment Strategies and Stereotypic Behavior in Captive Individually Housed Macaques (Macaca spp.). J. Appl. Anim. Welf. Sci. 2016, 19, 171–182. [Google Scholar] [CrossRef]
- Gronqvist, G.; Kingston-Jones, M.; May, A.; Lehmann, J. The effects of three types of environmental enrichment on the behaviour of captive Javan gibbons (Hylobates moloch). Appl. Anim. Behav. Sci. 2013, 147, 214–223. [Google Scholar] [CrossRef]
- Vick, S.-J.; Anderson, J.R.; Young, R. Maracas for Macaca? Evaluation of three potential enrichment objects in two species of zoo-housed macaques. Zoo Biol. 2000, 19, 181–191. [Google Scholar] [CrossRef]
- Sach, F.; Tatchley, C.; Needham, N.; Pullen, K. Guidelines for the Management of Elephants within BIAZA Zoos; British and Irish Association of Zoos and Aquariums (BIAZA): London, UK, 2019. [Google Scholar]
- Greenberg, R.; Mettke-Hofmann, C. Ecological aspects of neophobia and neophilia in birds. Curr. Ornithol. 2001, 16, 119–178. [Google Scholar] [CrossRef]
- Greggor, A.L.; Thornton, A.; Clayton, N.S. Neophobia is not only avoidance: Improving neophobia tests by combining cognition and ecology. Curr. Opin. Behav. Sci. 2015, 6, 82–89. [Google Scholar] [CrossRef]
- van Reenen, C.G.; Hopster, H.; van der Werf, J.T.N.; Engel, B.; Buist, W.G.; Jones, R.B.; Blokhuis, H.J.; Korte, S.M. The benzodiazepine brotizolam reduces fear in calves exposed to a novel object test. Physiol. Behav. 2009, 96, 307–314. [Google Scholar] [CrossRef] [PubMed]
- van Reenen, C.G.; O’Connell, N.E.; van der Werf, J.T.N.; Korte, S.M.; Hopster, H.; Jones, R.B.; Blokhuis, H.J. Responses of calves to acute stress: Individual consistency and relations between behavioral and physiological measures. Physiol. Behav. 2005, 85, 557–570. [Google Scholar] [CrossRef]
- Apfelbeck, B.; Raess, M. Behavioural and hormonal effects of social isolation and neophobia in a gregarious bird species, the European starling (Sturnus vulgaris). Horm. Behav. 2008, 54, 435–441. [Google Scholar] [CrossRef]
- Baugh, A.T.; Witonsky, K.R.; Davidson, S.C.; Hyder, L.; Hau, M.; van Oers, K. Novelty induces behavioural and glucocorticoid responses in a songbird artificially selected for divergent personalities. Anim. Behav. 2017, 130, 221–231. [Google Scholar] [CrossRef]
- Richard, S.; Wacrenier-Ceré, N.; Hazard, D.; Saint-Dizier, H.; Arnould, C.; Faure, J.M. Behavioural and endocrine fear responses in Japanese quail upon presentation of a novel object in the home cage. Behav. Process. 2008, 77, 313–319. [Google Scholar] [CrossRef]
- EAZA Elephant Taxon Advisory Group; Kölpin, T.; Pluhackova, J. EAZA Best Practice Guidelines for Elephants. 2020. Available online: https://www.eaza.net/assets/Uploads/CCC/BPG-2020/Elephant-TAG-BPG-2020.pdf (accessed on 1 November 2020).
- Laule, G.; Desmond, T. Positive reinforcement training as an enrichment strategy. In Second Nature: Environmental Enrichment for Captive Animals; Shepherdson, D.J., Mellen, J.D., Hutchins, M., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1998; pp. 302–313. ISBN 978-1-56098-397-2. [Google Scholar]
- Ward, S.J.; Melfi, V. The implications of husbandry training on zoo animal response rates. Appl. Anim. Behav. Sci. 2013, 147, 179–185. [Google Scholar] [CrossRef]
- Behringer, V.; Stevens, J.M.G.; Hohmann, G.; Möstl, E.; Selzer, D.; Deschner, T. Testing the effect of medical positive reinforcement training on salivary cortisol levels in bonobos and orangutans. PLoS ONE 2014, 9, e108664. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.K.; Heffernan, S.; Thomson, P.C.; McGreevy, P.D. Effect of positive reinforcement training on physiological and behavioural stress responses in the hamadryas baboon (Papio hamadryas). Anim. Welf. 2008, 17, 125–138. [Google Scholar]
- Veasey, J. Concepts in the care and welfare of captive elephants. Int. Zoo Yearb. 2006, 40, 63–79. [Google Scholar] [CrossRef]
- Olson, D. Elephant Husbandry Resource Guide. International Elephant Foundation. Available online: https://elephantconservation.org/resources/elephant-care-resources/ (accessed on 20 March 2021).
- Lee, P.C.; Moss, C.J. African elephant play, competence and social complexity. Anim. Behav. Cogn. 2014, 1, 144–156. [Google Scholar] [CrossRef]
- Heistermann, M.; Trohorsch, B.; Hodges, J.K. Assessment of ovarian function in the African elephant (Loxodonta africana) by measurement of 5α-reduced progesterone metabolites in serum and urine. Zoo Biol. 1997, 16, 273–284. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Hevia, M.L.; Escribano, D.; Lamy, E.; Tecles, F.; Cerón, J.J. Effect of food contamination and collection material in the measurement of biomarkers in saliva of horses. Res. Vet. Sci. 2020, 129, 90–95. [Google Scholar] [CrossRef]
- Dreschel, N.A.; Granger, D.A. Methods of collection for salivary cortisol measurement in dogs. Horm. Behav. 2009, 55, 163–168. [Google Scholar] [CrossRef]
- Hohmann, G.; Mundry, R.; Deschner, T. The relationship between socio-sexual behavior and salivary cortisol in bonobos: Tests of the tension regulation hypothesis. Am. J. Primatol. 2009, 71, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Stöwe, M.; Bugnyar, T.; Heinrich, B.; Kotrschal, K. Effects of group size on approach to novel objects in ravens (Corvus corax). Ethology 2006, 112, 1079–1088. [Google Scholar] [CrossRef]
- Palme, R.; Möstl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Z. Saugetierkd. Mamm. Biol. 1997, 62, 192–197. [Google Scholar]
- Behringer, V.; Clauss, W.; Hachenburger, K.; Kuchar, A.; Möstl, E.; Selzer, D. Effect of giving birth on the cortisol level in a bonobo groups’ (Pan paniscus) saliva. Primates 2009, 50, 190–193. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Powell, M.J.D. The BOBYQA Algorithm for Bound Constrained Optimization without Derivatives: Technical Report DAMTP 2009/NA06; University of Cambride: Cambridge, UK, 2009; Available online: http://www.cityu.edu.hk/rcms/publications/preprint26.pdf (accessed on 20 March 2021).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Buchanan-Smith, H.M.; Badihi, I. The psychology of control: Effects of control over supplementary light on welfare of marmosets. Appl. Anim. Behav. Sci. 2012, 137, 166–174. [Google Scholar] [CrossRef]
- Hanson, J.D.; Larson, M.E.; Snowdon, C.T. The effects of control over high intensity noise on plasma cortisol levels in rhesus monkeys. Behav. Biol. 1976, 16, 333–340. [Google Scholar] [CrossRef]
- Clegg, I.L.; Rödel, H.G.; Boivin, X.; Delfour, F. Looking forward to interacting with their caretakers: Dolphins’ anticipatory behaviour indicates motivation to participate in specific events. Appl. Anim. Behav. Sci. 2018, 202, 85–93. [Google Scholar] [CrossRef]
- Hasenjager, M.J.; Bergl, R.A. Environmental conditions associated with repetitive behavior in a group of African elephants. Zoo Biol. 2015, 34, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Wittemyer, G.; Daballen, D.; Rasmussen, H.; Kahindi, O.; Douglas-Hamilton, I. Demographic status of elephants in the Samburu and Buffalo Springs National Reserves, Kenya. Afr. J. Ecol. 2005, 43, 44–47. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Stead, S.K.; Meltzer, D.G.A.; Palme, R. The measurement of glucocorticoid concentrations in the serum and faeces of captive African elephants (Loxodonta africana) after ACTH stimulation. J. S. Afr. Vet. Assoc. 2000, 71, 192–196. [Google Scholar] [CrossRef]
- Wilson, M.L.; Bloomsmith, M.A.; Maple, T.L. Stereotypic swaying and serum cortisol concentrations in three captive African elephants (Loxodonta africana). Anim. Welf. 2004, 13, 39–43. [Google Scholar]
- Kirschbaum, C.; Wüst, S.; Hellhammer, D. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med. 1992, 54, 648–657. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Huddart, F.J. The effect of training first-lactation heifers to the milking parlor on the behavioral reactivity to humans and the physiological and behavioral responses to milking and productivity. J. Dairy Sci. 2012, 95, 6983–6993. [Google Scholar] [CrossRef]
- Proctor, C.M.; Freeman, E.W.; Brown, J.L. Influence of dominance status on adrenal activity and ovarian cyclicity status in captive African elephants. Zoo Biol. 2010, 29, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.L.; Perdue, B.M.; Bloomsmith, M.A.; Maple, T.L. Rates of reinforcement and measures of compliance in free and protected contact elephant management systems. Zoo Biol. 2015, 34, 431–437. [Google Scholar] [CrossRef]
- Owen, M.A.; Swaisgood, R.R.; Czekala, N.M.; Lindburg, D.G. Enclosure choice and well-being in giant pandas: Is it all about control? Zoo Biol. 2005, 24, 475–481. [Google Scholar] [CrossRef]
- Bassett, L.; Buchanan-Smith, H.M. Effects of predictability on the welfare of captive animals. Appl. Anim. Behav. Sci. 2007, 102, 223–245. [Google Scholar] [CrossRef]
- de Boer, S.F.; de Beun, R.; Slangen, J.L.; van der Gugten, J. Dynamics of plasma catecholamine and corticosterone concentrations during reinforced and extinguished operant behavior in rats. Physiol. Behav. 1990, 47, 691–698. [Google Scholar] [CrossRef]
- Bishop, J.; Hosey, G.; Plowman, A. Handbook of Zoo Research, Guidelines for Conducting Research in Zoos; British and Irish Association of Zoos and Aquariums (BIAZA): London, UK, 2013; Available online: https://biaza.org.uk/research-resources (accessed on 27 April 2020).
- Martin, P.; Bateson, P.P.G. Measuring Behaviour: An introductory Guide, 3rd ed.; Cambridge University Press: New York, NY, USA, 2007; ISBN 9780521535632. [Google Scholar]
- Wielebnowski, N.C.; Fletchall, N.; Carlstead, K.; Busso, J.M.; Brown, J.L. Noninvasive assessment of adrenal activity associated with husbandry and behavioral factors in the North American clouded leopard population. Zoo Biol. 2002, 21, 77–98. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Charmandari, E.; Kino, T.; Chrousos, G.P. Stress-related and circadian secretion and target tissue actions of glucocorticoids: Impact on health. Front. Endocrinol. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Pirke, K.M.; Hellhammer, D.H. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef]
- Tiefenbacher, S.; Lee, B.; Meyer, J.S.; Spealman, R.D. Noninvasive technique for the repeated sampling of salivary free cortisol in awake, unrestrained squirrel monkeys. Am. J. Primatol. 2003, 60, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Gröschl, M.; Wagner, R.; Rauh, M.; Dörr, H.G. Stability of salivary steroids: The influences of storage, food and dental care. Steroids 2001, 66, 737–741. [Google Scholar] [CrossRef]
- Majchrzak, Y.N.; Mastromonaco, G.F.; Korver, W.; Burness, G. Use of salivary cortisol to evaluate the influence of rides in dromedary camels. Gen. Comp. Endocrinol. 2015, 211, 123–130. [Google Scholar] [CrossRef]

| Zoo | Animal-ID | Sex † | Age Class ‡ | Handling Method § | Social Status ¶ | Reproductive Status of Females # | Ovarian Cycle Phase # |
|---|---|---|---|---|---|---|---|
| Kronberg | AR | F | 3 | PC | High | 2016, 2017: acyclic | -- |
| ZI | F | 3 | PC | Low | 2016, 2017: acyclic | -- | |
| TA | M | 1 | PC | Lowest * | -- | -- | |
| Wuppertal | SA | F | 2 | FC | High | 2016: lactational anestrus, cyclic 2017: pregnant | 2016: ovulation, luteal 2017: -- |
| SW | F | 2 | FC | Low | 2016: cyclic 2017: pregnant | 2016: luteal, follicular 2017: -- | |
| TI | F | 1 | FC | Low | 2016, 2017: cyclic | 2016: follicular 2017: luteal, follicular | |
| TU | M | 2 | PC | Highest * | -- | -- | |
| Erfurt | SF | F | 3 | PC | High | 2016, 2017: cyclic | 2016: follicular, ovulation, luteal 2017: ovulation, luteal |
| CH | F | 1 | PC | Low | 2016, 2017: cyclic | 2016: follicular, ovulation, luteal 2017: follicular | |
| KB | M | 1 | PC | In between * | -- | -- |
| Fixed Effects | LMM Name | |||||
|---|---|---|---|---|---|---|
| All Animals/PRT and NOV | All Animals/PRT | All Animals/NOV | Females/PRT | Females/NOV | ||
| Experimental | Situation | Yes | No | No | No | No |
| Study period † | Yes | No | Yes | No | Yes | |
| Sampling time | Yes | Yes | Yes | Yes | Yes | |
| Individual | Age class | Yes | Yes | Yes | Yes | Yes |
| Sex | Yes | Yes | Yes | No | No | |
| Zoo | Yes | Yes | Yes | Yes | Yes | |
| Social status ‡ | No | No | No | Yes | Yes | |
| Handling method ‡ | No | No | No | Yes | Yes | |
| All Animals/PRT | |||||
| Fixed Effect | Estimate | SE | df | t | p |
| Intercept | −0.76 | 0.28 | 6.00 | −2.72 | 0.035 |
| Sampling time | −5.36 × 10−4 | 5.28 × 10−4 | 135.30 | −1.02 | 0.312 |
| Sampling time sq | 7.33 × 10−5 | 3.00 × 10−5 | 135.30 | 2.44 | 0.016 |
| Age class | 0.06 | 0.08 | 6.00 | 0.81 | 0.449 |
| Sex | 0.12 | 0.14 | 6.02 | 0.86 | 0.422 |
| Zoo | 0.14 | 0.08 | 5.96 | 1.79 | 0.125 |
| All Animals/NOV | |||||
| Fixed effect | Estimate | SE | df | t | p |
| Intercept | −0.53 | 0.13 | 6.77 | −4.17 | 0.005 |
| Sampling time | 1.23 × 10−4 | 4.19 × 10−4 | 373.70 | 0.29 | 0.39 |
| Sampling time sq | −3.76 × 10−5 | 2.23 × 10−5 | 360.40 | −1.69 | 0.05 |
| Study period | −0.11 | 0.02 | 388.00 | −6.34 | <0.001 |
| Age class | 0.11 | 0.04 | 6.11 | 3.10 | 0.02 |
| Sex | 0.20 | 0.06 | 6.18 | 3.27 | 0.02 |
| Zoo | 0.05 | 0.03 | 5.88 | 1.37 | 0.22 |
| Females/PRT | |||||
| Fixed Effect | Estimate | SE | df | t | p |
| Intercept | −0.27 | 0.64 | 1.99 | −0.42 | 0.713 |
| Sampling time | −7.72 × 10−4 | 6.29 × 10−4 | 93.13 | −1.23 | 0.223 |
| Sampling time sq | 1.20 × 10−4 | 3.57 × 10−5 | 93.11 | 3.37 | 0.001 |
| Age class | 0.03 | 0.15 | 1.99 | 0.22 | 0.848 |
| Handling | −0.09 | 0.18 | 1.99 | −0.48 | 0.681 |
| Social status | 0.02 | 0.19 | 1.97 | 0.12 | 0.918 |
| Zoo | 0.09 | 0.12 | 1.98 | 0.78 | 0.518 |
| Females/NOV | |||||
| Fixed effect | Estimate | SE | df | t | p |
| Intercept | −0.06 | 0.24 | 1.99 | −0.27 | 0.814 |
| Sampling time | 9.37 × 10−4 | 5.34 × 10−4 | 261.10 | 1.75 | 0.040 |
| Sampling time sq | −6.27 × 10−5 | 2.85 × 10−5 | 250.20 | −2.20 | 0.015 |
| Study period | −0.12 | 0.02 | 273.90 | −5.17 | <0.001 |
| Age class | 0.11 | 0.06 | 1.88 | 1.99 | 0.194 |
| Handling | −0.04 | 0.07 | 1.89 | −0.57 | 0.629 |
| Social status | 0.04 | 0.07 | 1.82 | 0.64 | 0.594 |
| Zoo | 0.01 | 0.04 | 1.82 | 0.24 | 0.838 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hambrecht, S.; Oerke, A.-K.; Heistermann, M.; Hartig, J.; Dierkes, P.W. Effects of Positive Reinforcement Training and Novel Object Exposure on Salivary Cortisol Levels under Consideration of Individual Variation in Captive African Elephants (Loxodonta africana). Animals 2021, 11, 3525. https://doi.org/10.3390/ani11123525
Hambrecht S, Oerke A-K, Heistermann M, Hartig J, Dierkes PW. Effects of Positive Reinforcement Training and Novel Object Exposure on Salivary Cortisol Levels under Consideration of Individual Variation in Captive African Elephants (Loxodonta africana). Animals. 2021; 11(12):3525. https://doi.org/10.3390/ani11123525
Chicago/Turabian StyleHambrecht, Susan, Ann-Kathrin Oerke, Michael Heistermann, Johannes Hartig, and Paul W. Dierkes. 2021. "Effects of Positive Reinforcement Training and Novel Object Exposure on Salivary Cortisol Levels under Consideration of Individual Variation in Captive African Elephants (Loxodonta africana)" Animals 11, no. 12: 3525. https://doi.org/10.3390/ani11123525
APA StyleHambrecht, S., Oerke, A.-K., Heistermann, M., Hartig, J., & Dierkes, P. W. (2021). Effects of Positive Reinforcement Training and Novel Object Exposure on Salivary Cortisol Levels under Consideration of Individual Variation in Captive African Elephants (Loxodonta africana). Animals, 11(12), 3525. https://doi.org/10.3390/ani11123525






