Evaluating Alternatives to Locomotion Scoring for Lameness Detection in Pasture-Based Dairy Cows in New Zealand: Infra-Red Thermography
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Farm Location
2.2. Locomotion Scoring
2.3. Infrared Thermography
2.4. Statistical Data Analyses
3. Results
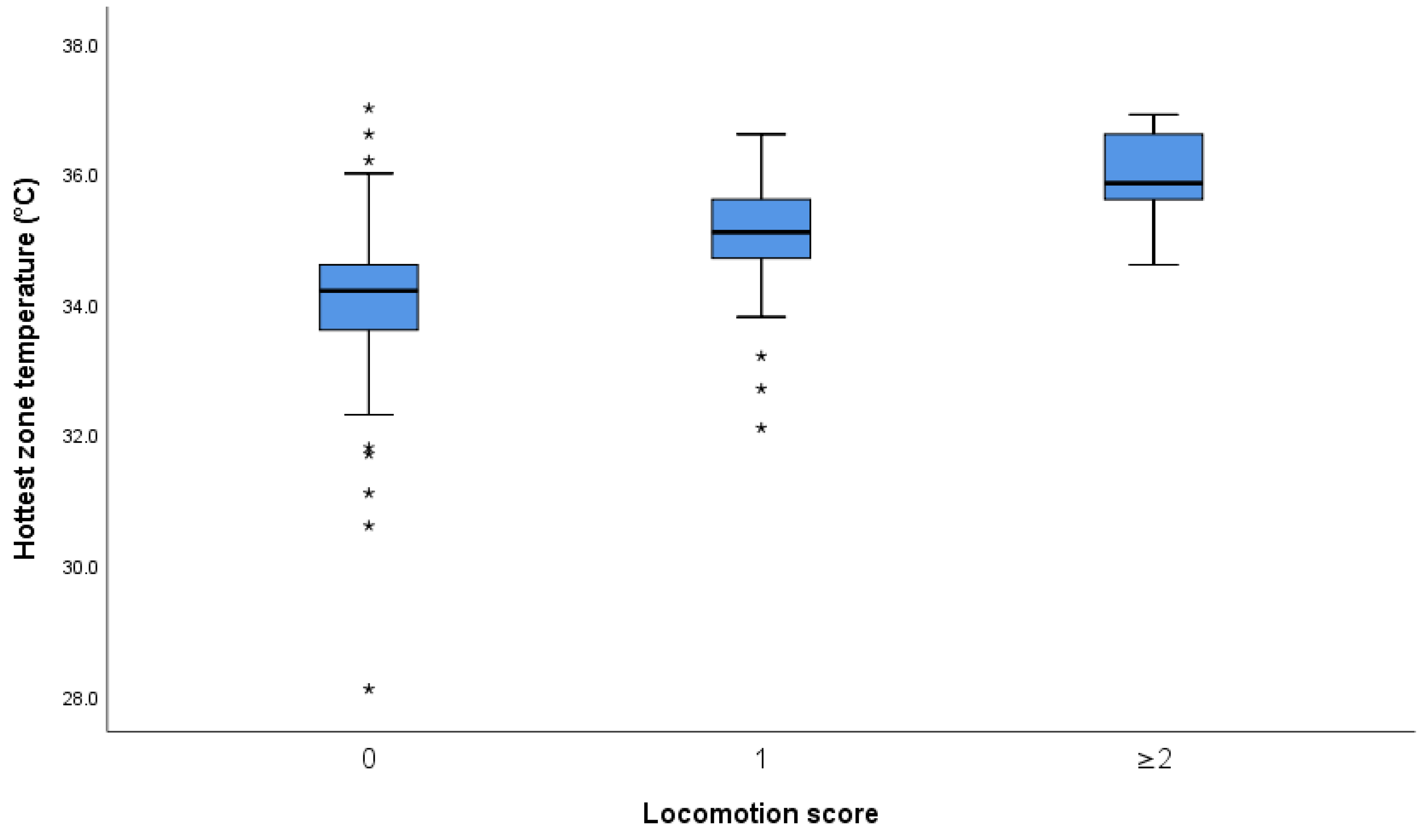
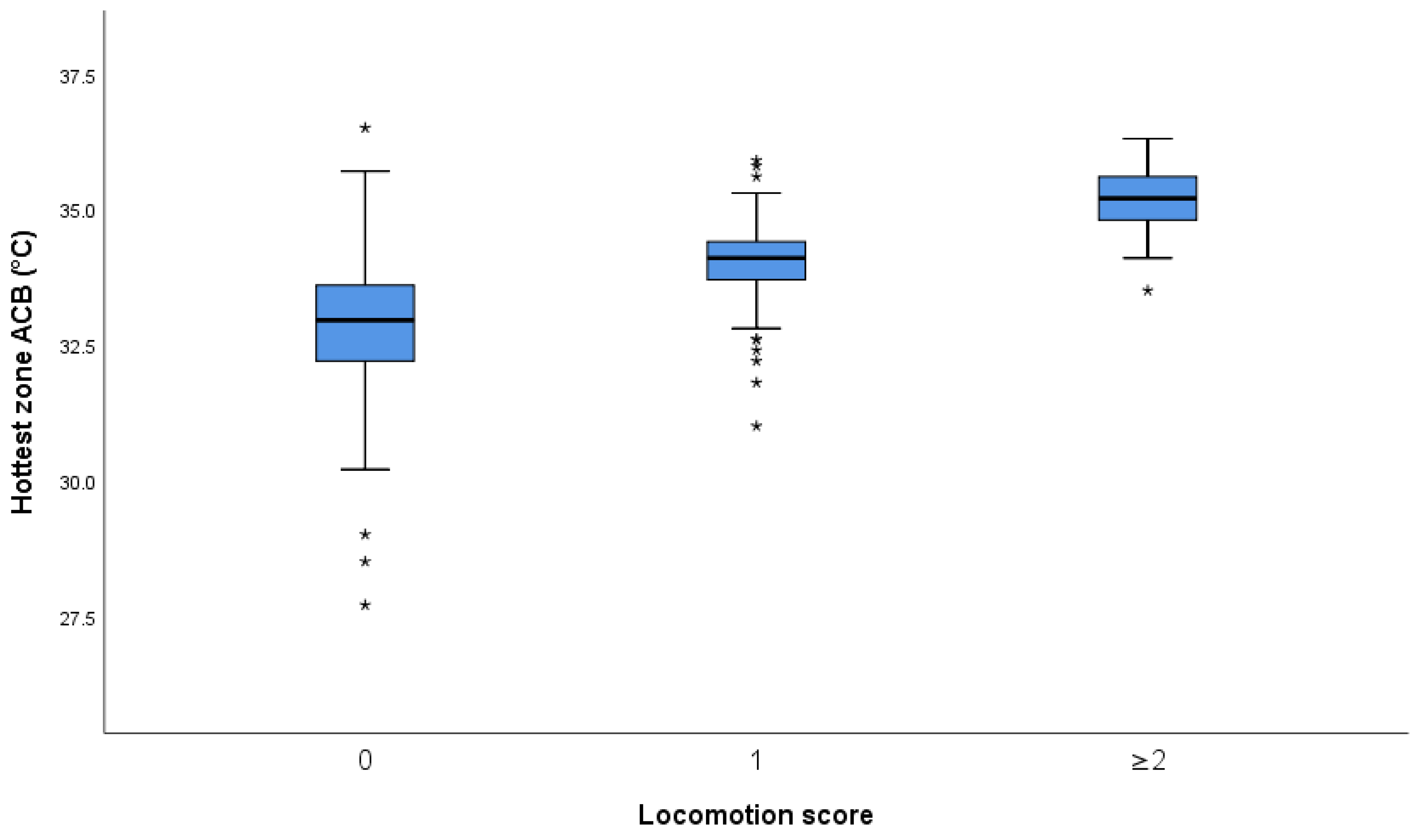
3.1. Effect of Foot and Foot Zone on Skin Temperature
3.1.1. Infrared Thermography versus Locomotion Scoring
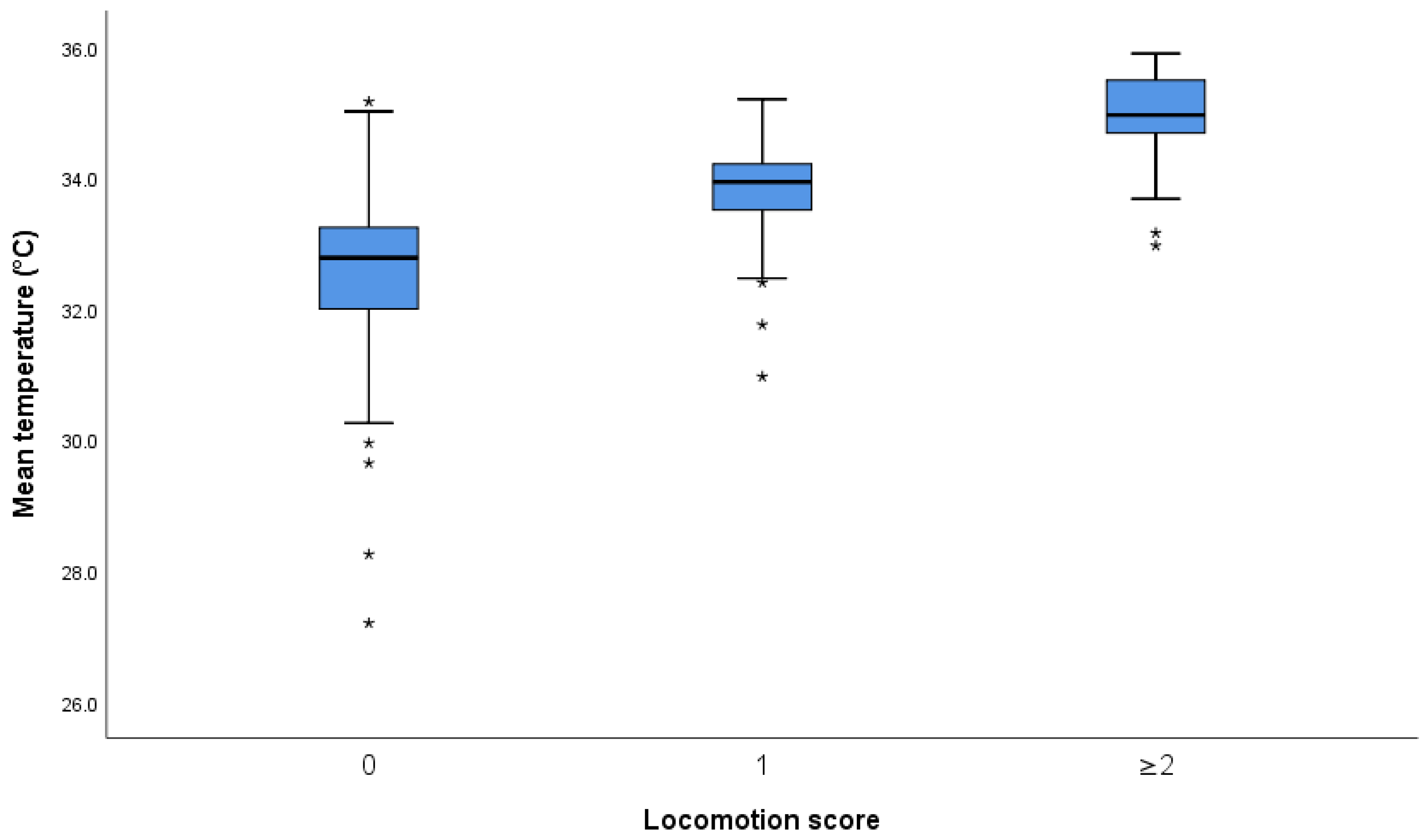
3.1.2. Association of Foot Temperatures and Locomotion Scores
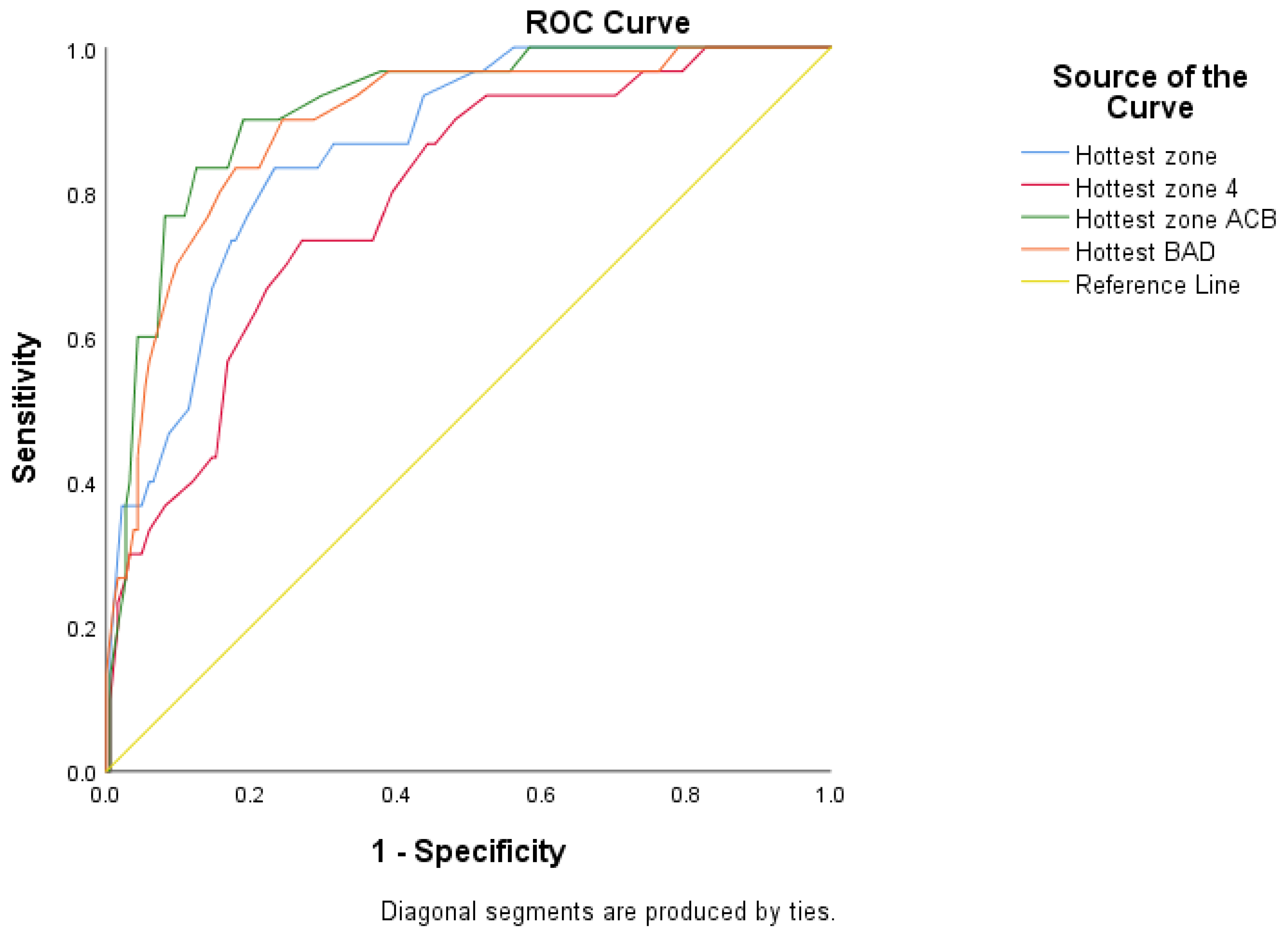
3.1.3. A Receiver Operating Characteristic (ROC) Analysis
4. Discussion
4.1. Feasibility of Infrared Thermography as a Method on New Zealand Dairy Farms
4.2. Skin Foot Temperature and Effect of Claw and Zone
4.3. Infrared Thermography as a Predictor of Locomotion Score
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model Parameter | 95% Confidence Interval | ||
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| Hottest zone (intercept) | 35.957 | 35.716 | 36.197 |
| Locomotion score * | 0.958 | 0.793 | 1.157 |
| Hottest zone 4 (intercept) | 35.590 | 35.280 | 35.900 |
| Locomotion score * | 1.218 | 1.008 | 1.471 |
| Hottest ACB (intercept) | 35.153 | 34.915 | 35.392 |
| Locomotion score * | 1.087 | 0.900 | 1.314 |
| Hottest BAD (intercept) | 35.657 | 35.385 | 35.929 |
| Locomotion score * | 1.126 | 0.932 | 1.361 |
| Temperature Measure 1 | Optimal Threshold (°C) | AUC (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | PPV (95% CI) * | NPV (95% CI) * |
|---|---|---|---|---|---|---|
| Hottest zone | 35.7 | 0.861 (0.799–0.923) | 82.2 (75.87–87.39) | 73.3 (54.11–87.72) | 40.1 (31.46–49.39) | 95.0 (91.24–97.17) |
| Hottest zone 4 | 35.2 | 0.790 (0.707–0.873) | 72.4 (65.40–78.73) | 73.3 (54.11–87.72) | 30.2 (23.96–37.31) | 94.4 (90.15–96.82) |
| Hottest zone ACB | 35.1 | 0.914 (0.865–0.963) | 95.7 (91.66–98.11) | 60.0 (40.60–77.34) | 69.3 (51.92–82.53) | 93.6 (90.45–95.80) |
| Hottest zone BAD | 35.1 | 0.893 (0.832–0.954) | 82.2 (75.87–87.39) | 83.3 (65.28–94.36) | 43.2 (34.93–51.86) | 96.8 (93.13–98.54) |
Appendix B


References
- Whay, H.R.; Shearer, J. The impact of lameness on welfare of the dairy cow. Vet. Clin. Food Anim. Pract. 2017, 33, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Laven, R.; Lawrence, K.; Weston, J.; Dowson, K.; Stafford, K. Assessment of the duration of the pain response associated with lameness in dairy cows, and the influence of treatment. N. Z. Vet. J. 2008, 56, 210–217. [Google Scholar] [CrossRef]
- Winckler, C.; Willen, S. The reliability and repeatability of a lameness scoring system for use as an indicator of welfare in dairy cattle. Acta Agric. Scand. Sect. A-Anim. Sci. 2001, 51, 103–107. [Google Scholar] [CrossRef]
- Thomas, H.; Remnant, J.; Bollard, N.; Burrows, A.; Whay, H.; Bell, N.; Mason, C.; Huxley, J. Recovery of chronically lame dairy cows following treatment for claw horn lesions: A randomised controlled trial. Vet. Rec. 2016, 178, 116. [Google Scholar] [CrossRef] [PubMed]
- Green, L.E.; Hedges, V.J.; Schukken, Y.H.; Blowey, R.W.; Packington, A.J. The impact of clinical lameness on the milk yield of dairy cows. J. Dairy Sci. 2002, 85, 2250–2256. [Google Scholar] [CrossRef] [Green Version]
- Warnick, L.D.; Janssen, D.; Guard, C.L.; Gröhn, Y.T. The effect of lameness on milk production in dairy cows. J. Dairy Sci. 2001, 84, 1988–1997. [Google Scholar] [CrossRef]
- Archer, C.S.; Green, M.J.; Huxley, J.N. Association between milk yield and serial locomotion score assessments in UK dairy cows. J. Dairy Sci. 2010, 93, 4045–4053. [Google Scholar] [CrossRef] [Green Version]
- Somers, J.R.; Huxley, J.; Lorenz, I.; Doherty, M.L.; O’Grady, L. The effect of Lameness before and during the breeding season on fertility in 10 pasture-based Irish dairy herds. Ir. Vet. J. 2015, 68, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alawneh, I.J.; Laven, R.A.; Stevenson, M.A. The effect of lameness on the fertility of dairy cattle in a seasonally breeding pasture-based system. J. Dairy Sci. 2011, 94, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, D.J.; Hostetler, D.E.; Kaneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Booth, C.; Warnick, L.; Gröhn, Y.; Maizon, D.; Guard, C.; Janssen, D. Effect of lameness on culling in dairy cows. J. Dairy Sci. 2004, 87, 4115–4122. [Google Scholar] [CrossRef] [Green Version]
- Huxley, J.N. Impact of lameness and claw lesions in cows on health and production. Livest. Sci. 2013, 156, 64–70. [Google Scholar] [CrossRef]
- Machado, V.; Caixeta, L.; McArt, J.; Bicalho, R. The effect of claw horn disruption lesions and body condition score at dry-off on survivability, reproductive performance, and milk production in the subsequent lactation. J. Dairy Sci. 2010, 93, 4071–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sogstad, Å.; Østerås, O.; Fjeldaas, T.; Nafstad, O. Bovine claw and limb disorders related to culling and carcass characteristics. Livest. Sci. 2007, 106, 87–95. [Google Scholar] [CrossRef]
- Ghotoorlar, S.M.; Ghamsari, S.M.; Nowrouzian, I.; Ghotoorlar, S.M.; Ghidary, S.S. Lameness scoring system for dairy cows using force plates and artificial intelligence. Vet. Rec. 2012, 170, 126. [Google Scholar] [CrossRef]
- Pedersen, S.; Wilson, J. Early detection and prompt effective treatment of lameness in dairy cattle. Livestock 2021, 26, 115–121. [Google Scholar] [CrossRef]
- Newsome, R.; Green, M.J.; Bell, N.J.; Chagunda, M.G.G.; Mason, C.S.; Rutland, C.S.; Sturrock, C.J.; Whay, H.R.; Huxley, J.N. Linking bone development on the caudal aspect of the distal phalanx with lameness during life. J. Dairy Sci. 2016, 99, 4512–4525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlageter-Tello, A.A.; Bokkers, E.A.; Koerkamp, P.W.G.; Van Hertem, T.; Viazzi, S.; Romanini, C.E.; Halachmi, I.; Bahr, C.; Berckmans, D.; Lokhorst, K. Manual and automatic locomotion scoring systems in dairy cows: A review. Prev. Vet. Med. 2014, 116, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Manson, F.J.; Leaver, J.D. The influence of concentrate amount on locomotion and clinical lameness in dairy cattle. Anim. Sci. 1988, 47, 185–190. [Google Scholar] [CrossRef]
- Groehn, J.; Kaneene, J.; Foster, D. Risk factors associated with lameness in lactating dairy cattle in Michigan. Prev. Vet. Med. 1992, 14, 77–85. [Google Scholar] [CrossRef]
- Flower, F.C.; Weary, D.M. Effect of hoof pathologies on subjective assessments of dairy cow gait. J. Dairy Sci. 2006, 89, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Alawneh, J.I.; RLaven, A.; Stevenson, M.A. Interval between detection of lameness by locomotion scoring and treatment for lameness: A survival analysis. Vet. J. 2012, 193, 622–625. [Google Scholar] [CrossRef]
- Fabian, J.; Laven, R.A.; Whay, H.R. The prevalence of lameness on New Zealand dairy farms: A comparison of farmer estimate and locomotion scoring. Vet. J. 2014, 201, 31–38. [Google Scholar] [CrossRef]
- Van Nuffel, A.; Zwertvaegher, I.; Van Weyenberg, S.; Pluym, L.; Pastell, M.; Thorup, V.M.; Sonck, B.; Saeys, W. Lameness detection in dairy cows: Part 1. How to distinguish between non-lame and lame cows based on differences in locomotion or behavior. Animals 2015, 5, 838–860. [Google Scholar] [CrossRef] [Green Version]
- Schlageter-Tello, A.A.; Bokkers, E.A.; Koerkamp, P.W.G.; Van Hertem, T.; Viazzi, S.; Romanini, C.E.; Halachmi, I.; Bahr, C.; Berckmans, D.; Lokhorst, K. Relation between observed locomotion traits and locomotion score in dairy cows. J. Dairy Sci. 2015, 98, 8623–8633. [Google Scholar] [CrossRef] [Green Version]
- Schlageter-Tello, A.A.; Bokkers, E.A.; Koerkamp, P.W.G.; Van Hertem, T.; Viazzi, S.; Romanini, C.E.; Halachmi, I.; Bahr, C.; Berckmans, D.; Lokhorst, K. Comparison of locomotion scoring for dairy cows by experienced and inexperienced raters using live or video observation methods. Anim. Welf. 2015, 24, 69–79. [Google Scholar] [CrossRef]
- Ranjbar, S.; Rabiee, A.R.; Gunn, A.; House, J.K. Identifying risk factors associated with lameness in pasture-based dairy herds. J. Dairy Sci. 2016, 99, 7495–7505. [Google Scholar] [CrossRef] [Green Version]
- Eddy, A.L.; van Hoogmoed, L.M.; Snyder, J.R. The role of thermography in the management of equine lameness. Vet. J. 2001, 162, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsaaod, M.; Schaefer, A.; Büscher, W.; Steiner, A. The role of infrared thermography as a non-invasive tool for the detection of lameness in cattle. Sensors 2015, 15, 14513–14525. [Google Scholar] [CrossRef] [Green Version]
- Colak, A.; Polat, B.; Okumus, Z.; Kaya, M.; Yanmaz, L.E.; Hayirli, A. Early detection of mastitis using infrared thermography in dairy cows. J. Dairy Sci. 2008, 91, 4244–4248. [Google Scholar] [CrossRef] [Green Version]
- Berry, R.; Kennedy, A.D.; Scott, S.L.; Kyle, B.L.; Schaefer, A.L. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: Potential for mastitis detection. Can. J. Anim. Sci. 2003, 83, 687–693. [Google Scholar] [CrossRef]
- Alsaaod, M.; Büscher, W. Detection of hoof lesions using digital infrared thermography in dairy cows. J. Dairy Sci. 2012, 95, 735–742. [Google Scholar] [CrossRef]
- Gloster, J.; Ebert, K.; Gubbins, S.; Bashiruddin, J.; Paton, D.J. Normal variation in thermal radiated temperature in cattle: Implications for foot-and-mouth disease detection. BMC Vet. Res. 2011, 7, 73. [Google Scholar] [CrossRef] [Green Version]
- Nikkhah, A.; Plaizier, J.C.; Einarson, M.S.; Berry, R.J.; Scott, S.L.; Kennedy, A.D. Infrared thermography and visual examination of hooves of dairy cows in two stages of lactation. J. Dairy Sci. 2005, 88, 2749–2753. [Google Scholar] [CrossRef] [Green Version]
- Bobić, T.; Mijić, P.; Gantner, V.; Glavaš, H.; Gregić, M. The effects of parity and stage of lactation on hoof temperature of dairy cows using a thermovision camera. J. Cent. Eur. Agric. 2018, 19, 777–783. [Google Scholar] [CrossRef]
- Wood, S.; Lin, Y.; Knowles, T.G.; Main, D.C.J. Infrared thermometry for lesion monitoring in cattle lameness. Vet. Rec. 2015, 176, 308. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, G.R. An Evaluation of Two Low-Cost Thermal Imaging Cameras for Wildlife Damage Management Objectives. In Proceedings of the Vertebrate Pest Conference; University of California: Los Angeles, CA, USA, 2018. [Google Scholar]
- Montanholi, Y. Using infrared radiation to detect local inflammation in cattle. Vet. Rec. 2015, 176, 306–307. [Google Scholar] [CrossRef]
- Landgraf, T.; Zipser, S.; Stewart, M.; Dowling, S.; Schaefer, A. Modelling and correction of influences on surface temperature measurements using infrared thermography for animal health and welfare assessments. In Proceedings of the The 12th International Conference on Quantitative Infrared Thermography, Bordeaux, France, 7–11 July 2014. [Google Scholar]
- Main, D.C.; Stokes, J.E.; Reader, J.D.; Whay, H.R. Detecting hoof lesions in dairy cattle using a hand-held thermometer. Vet. Rec. J. Br. Vet. Assoc. 2012, 171, 504. [Google Scholar] [CrossRef]
- Lawrence, K.E.; Laven, R.A.; Chesterton, R.N. Further investigation of lameness in cows at pasture: An analysis of the lesions found in, and some possible risk factors associated with, lame New Zealand dairy cattle requiring veterinary treatment. J. Dairy Sci. 2011, 94, 2794–2805. [Google Scholar] [CrossRef] [PubMed]
- Mülling, C.K.; Green, L.; Barker, Z.; Scaife, J.; Amory, J.; Speijers, M. Risk factors associated with foot lameness in dairy cattle and a suggested approach for lameness reduction. In World Buiatrics Congress; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Rodríguez, A.R.; Olivares, F.J.; Descouvieres, P.T.; Werner, M.P.; Tadich, N.A.; Bustamante, H.A. Thermographic assessment of hoof temperature in dairy cows with different mobility scores. Livest. Sci. 2016, 184, 92–96. [Google Scholar] [CrossRef]
- Harris-Bridge, G.; Young, L.; Handel, I.; Farish, M.; Mason, C.; Mitchell, M.A.; Haskell, M.J. The use of infrared thermography for detecting digital dermatitis in dairy cattle: What is the best measure of temperature and foot location to use? Vet. J. 2018, 237, 26–33. [Google Scholar] [CrossRef]
- DairyNZ. Lameness Scoring Poster. Available online: https://www.dairynz.co.nz/publications/animal/lameness-scoring-poster/ (accessed on 9 January 2018).
- DairyNZ. Lameness Scoring. Available online: https://www.dairynz.co.nz/animal/cow-health/lameness/lameness-scoring/ (accessed on 9 January 2018).
- AHDB. AHDB Dairy UK Mobility with AHDB Dairy. Available online: https://ahdb.org.uk/knowledge-library/mobility-scoring-for-dairy-cows (accessed on 9 January 2018).
- Stokes, J.E.; Leach, K.A.; Main, D.C.J.; Whay, H.R. An investigation into the use of infrared thermography (IRT) as a rapid diagnostic tool for foot lesions in dairy cattle. Vet. J. 2012, 193, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Whay, H.; Bell, M.; Main, D. Validation of lame limb identification through thermal imaging. In Proceedings of the 13th International Symposium and 5th Conference on Lameness in Ruminants, Maribor, Slovenia, 11–15 February 2004. [Google Scholar]
- Abdi, H. Bonferroni and Šidák corrections for multiple comparisons. Encycl. Meas. Stat. 2007, 3, 103–107. [Google Scholar]
- Astivia, O.L.O.; Zumbo, B.D. Heteroskedasticity in Multiple Regression Analysis: What it is, How to Detect it and How to Solve it with Applications in R and SPSS. Pract. Assess. Res. Eval. 2019, 24, 1. [Google Scholar]
- Lin, Y.C.; Mullan, S.; Main, D.C. Optimising lameness detection in dairy cattle by using handheld infrared thermometers. Vet. Med. Sci. 2018, 4, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Murray, R.; Downham, D.; Clarkson, M.; Faull, W.; Hughes, J.; Manson, F.; Merritt, J.; Russell, W.; Sutherst, J.; Ward, W. Epidemiology of lamenessin dairy cattle: Description and analysis of foot lesions. Vet. Rec. 1996, 138, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Chesterton, R.; Lawrence, K.; Laven, R. A descriptive analysis of the foot lesions identified during veterinary treatment for lameness on dairy farms in north Taranaki. N. Z. Vet. J. 2008, 56, 130–138. [Google Scholar] [CrossRef]
- Fabbri, G.; Fiore, E.; Piccione, G.; Giudice, E.; Gianesella, M.; Morgante, M.; Armato, L.; Bonato, O.; Giambelluca, S.; Arfuso, F. Detection of digital and interdigital dermatitis in Holstein Friesian dairy cows by means of infrared thermography. Large Anim. Rev. 2020, 26, 113–116. [Google Scholar]
- Gianesella, M.; Arfuso, F.; Fiore, E.; Giambelluca, S.; Giudice, E.; Armato, L.; Piccione, G. Infrared thermography as a rapid and non-invasive diagnostic tool to detect inflammatory foot diseases in dairy cows. Pol. J. Vet. Sci. 2018, 21, 299–305. [Google Scholar] [PubMed]
- Rainwater-Lovett, K.; Pacheco, J.M.; Packer, C.; Rodriguez, L.L. Detection of foot-and-mouth disease virus infected cattle using infrared thermography. Vet. J. 2009, 180, 317–324. [Google Scholar] [CrossRef]
- Wilhelm, K.; Wilhelm, J.; Fürll, M. Use of thermography to monitor sole haemorrhages and temperature distribution over the claws of dairy cattle. Vet. Rec. 2015, 176, 146. [Google Scholar] [CrossRef] [PubMed]
- Le Fevre, A.; Logue, D.; Offer, J.; McKendrick, I.; Gettinby, G. Correlations of measurements of subclinical claw horn lesions in dairy cattle. Vet. Rec. 2001, 148, 135–138. [Google Scholar] [CrossRef]
- Orman, A.; Endres, M.I. Use of thermal imaging for identification of foot lesions in dairy cattle. Acta Agric. Scand. Sect. A Anim. Sci. 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Alsaaod, M.; Syring, C.; Dietrich, J.; Doherr, M.G.; Gujan, T.; Steiner, A. A field trial of infrared thermography as a non-invasive diagnostic tool for early detection of digital dermatitis in dairy cows. Vet. J. 2014, 199, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.K.; van Amstel, S.R.; Brodersen, B.W. Clinical Diagnosis of Foot and Leg Lameness in Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 535–556. [Google Scholar] [CrossRef]
- Flower, F.C.; Weary, D.M. Gait assessment in dairy cattle. Animal 2009, 3, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, K. Lamneness and associated pain in cattle-challenging traditional perceptions. Practice 2002, 24, 212. [Google Scholar] [CrossRef]
- Ebrahimie, E.; Mohammadi-Dehcheshmeh, M.; Laven, R.; Petrovski, K.R. Rule Discovery in Milk Content towards Mastitis Diagnosis: Dealing with Farm Heterogeneity over Multiple Years through Classification Based on Associations. Animals 2021, 11, 1638. [Google Scholar] [CrossRef]
- O’Leary, N.; Byrne, D.; O’Connor, A.; Shalloo, L. Invited review: Cattle lameness detection with accelerometers. J. Dairy Sci. 2020, 103, 3895–3911. [Google Scholar] [CrossRef] [Green Version]
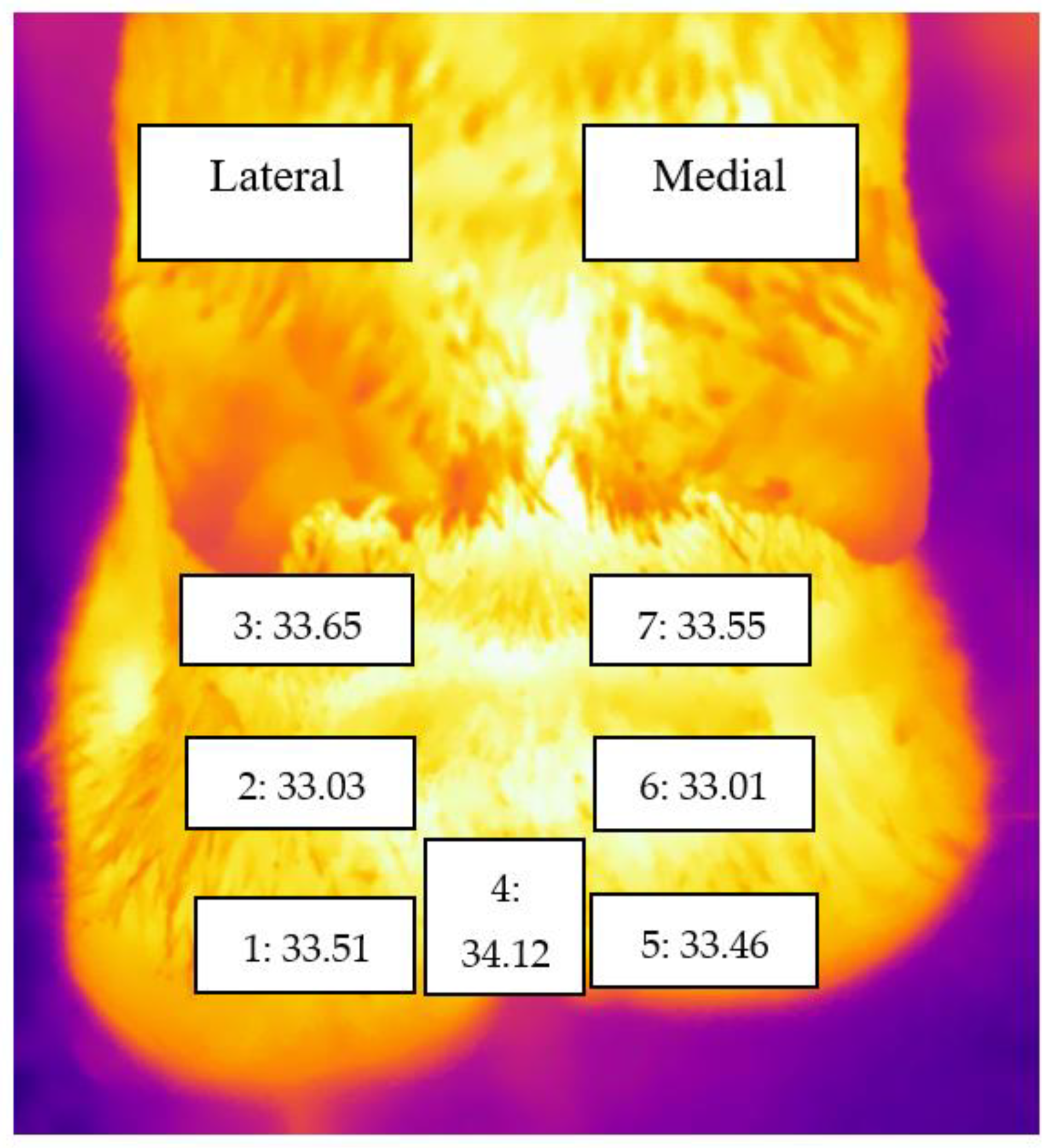


| Score | Clinical Term | Evaluation Criteria |
|---|---|---|
| 0 | Sound | The cow walks confidently, even weight-bearing and tracks up. |
| 1 | Imperfect locomotion | The cow walks unevenly, does not track up, with a mildly arched back when walking. |
| 2 | Lame | An arched back, the favoured limb moves faster than the lame leg, feet placed unevenly, head bobs up and down when walking. |
| 3 | Severely lame | Walks very slow, reluctant to bear weight, arched back, and head bobs obvious. |
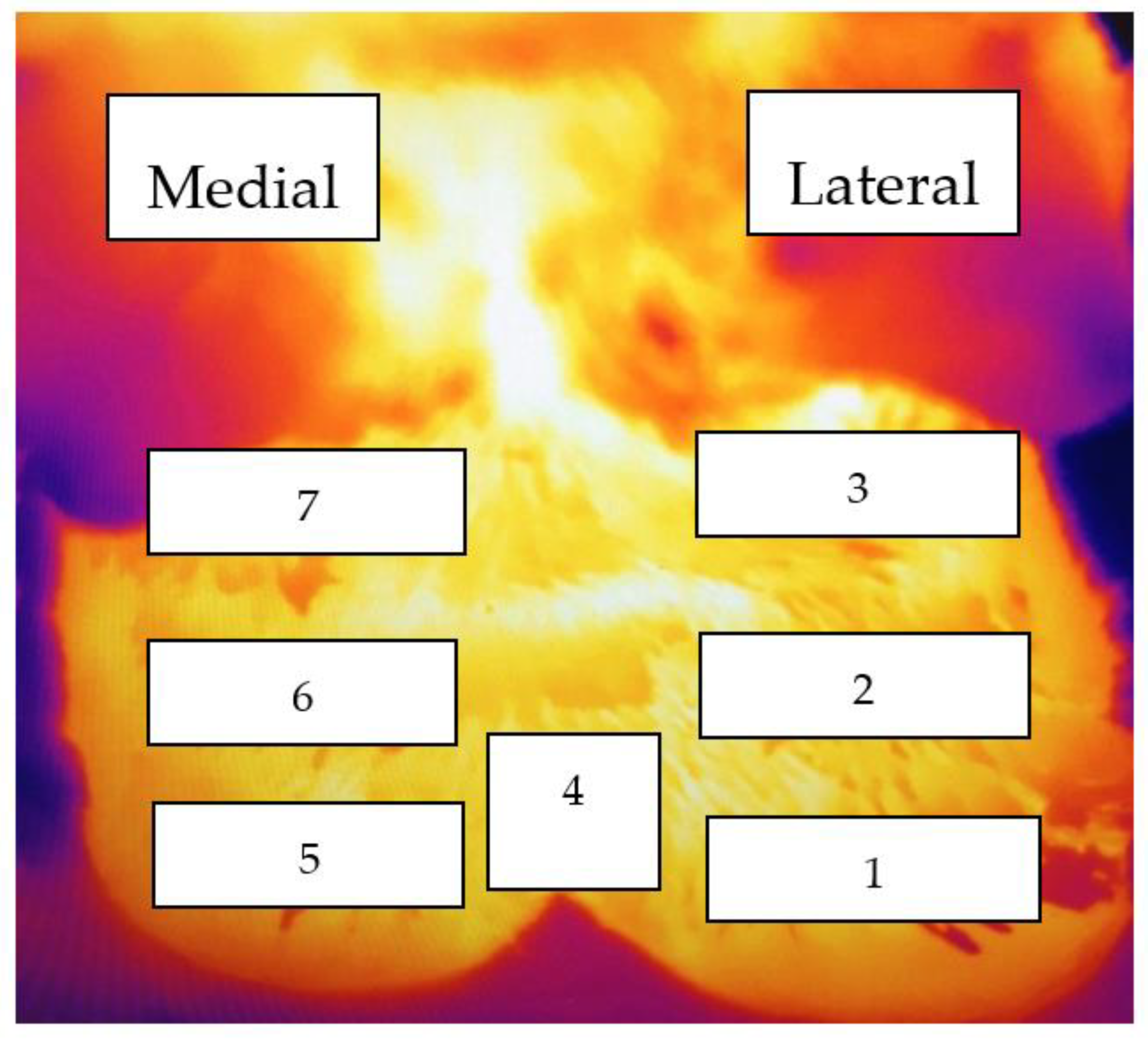
| Foot/Zone * | Description |
|---|---|
| Mean temperature | Average temperature, across both feet (all 14 zones) |
| Hottest zone | Highest zone temperature, across both feet (all 14 zones) |
| Hottest zone 4 | The highest zone 4 temperature on either foot |
| Hottest coronary band (CB) | The highest zone 1 or 5 temperature on either foot |
| Hottest above the coronary band zone (ACB) | The highest zone 2 or 6 temperature on either foot |
| Hottest zone below the accessory digit (BAD) | The highest zone 3 or 7 temperature on either foot |
| Zone | Mean | 95% Confidence Interval | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| 1 | 33.51 | 33.375 | 33.646 |
| 2 | 33.03 | 32.889 | 33.165 |
| 3 | 33.65 | 33.507 | 33.787 |
| 4 | 34.12 | 33.986 | 34.250 |
| 5 | 33.46 | 33.323 | 33.596 |
| 6 | 33.01 | 32.869 | 33.148 |
| 7 | 33.55 | 33.414 | 33.691 |
| Model Parameter | 95% Confidence Interval | ||
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| Mean temperature (intercept) | 34.877 | 34.614 | 35.140 |
| Locomotion score * | 0.944 | 0.781 | 1.141 |
| Hottest coronary band (intercept) | 35.483 | 35.199 | 35.768 |
| Locomotion score * | 1.067 | 0.883 | 1.289 |
| Temperature Measure 1 | Optimal Threshold (°C) | AUC (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | PPV (95% CI) * | NPV (95% CI) * |
|---|---|---|---|---|---|---|
| Mean Temperature | 34.5 | 0.91 (0.84–0.97) | 92.4 (87.63–95.80) | 80.0 (61.43–92.29) | 63.3 (50.21–74.60) | 96.6 (93.27–98.31) |
| Hottest coronary band | 35.1 | 0.87 (0.80–0.95) | 85.4 (79.48–90.16) | 76.7 (57.72–90.07) | 46.1 (36.42–56.07) | 95.7 (92.14–97.73) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werema, C.W.; Laven, L.; Mueller, K.; Laven, R. Evaluating Alternatives to Locomotion Scoring for Lameness Detection in Pasture-Based Dairy Cows in New Zealand: Infra-Red Thermography. Animals 2021, 11, 3473. https://doi.org/10.3390/ani11123473
Werema CW, Laven L, Mueller K, Laven R. Evaluating Alternatives to Locomotion Scoring for Lameness Detection in Pasture-Based Dairy Cows in New Zealand: Infra-Red Thermography. Animals. 2021; 11(12):3473. https://doi.org/10.3390/ani11123473
Chicago/Turabian StyleWerema, Chacha Wambura, Linda Laven, Kristina Mueller, and Richard Laven. 2021. "Evaluating Alternatives to Locomotion Scoring for Lameness Detection in Pasture-Based Dairy Cows in New Zealand: Infra-Red Thermography" Animals 11, no. 12: 3473. https://doi.org/10.3390/ani11123473
APA StyleWerema, C. W., Laven, L., Mueller, K., & Laven, R. (2021). Evaluating Alternatives to Locomotion Scoring for Lameness Detection in Pasture-Based Dairy Cows in New Zealand: Infra-Red Thermography. Animals, 11(12), 3473. https://doi.org/10.3390/ani11123473






