Imaging and Gross Pathological Appearance of Changes in the Parasagittal Grooves of Thoroughbred Racehorses
Simple Summary
Abstract
1. Introduction
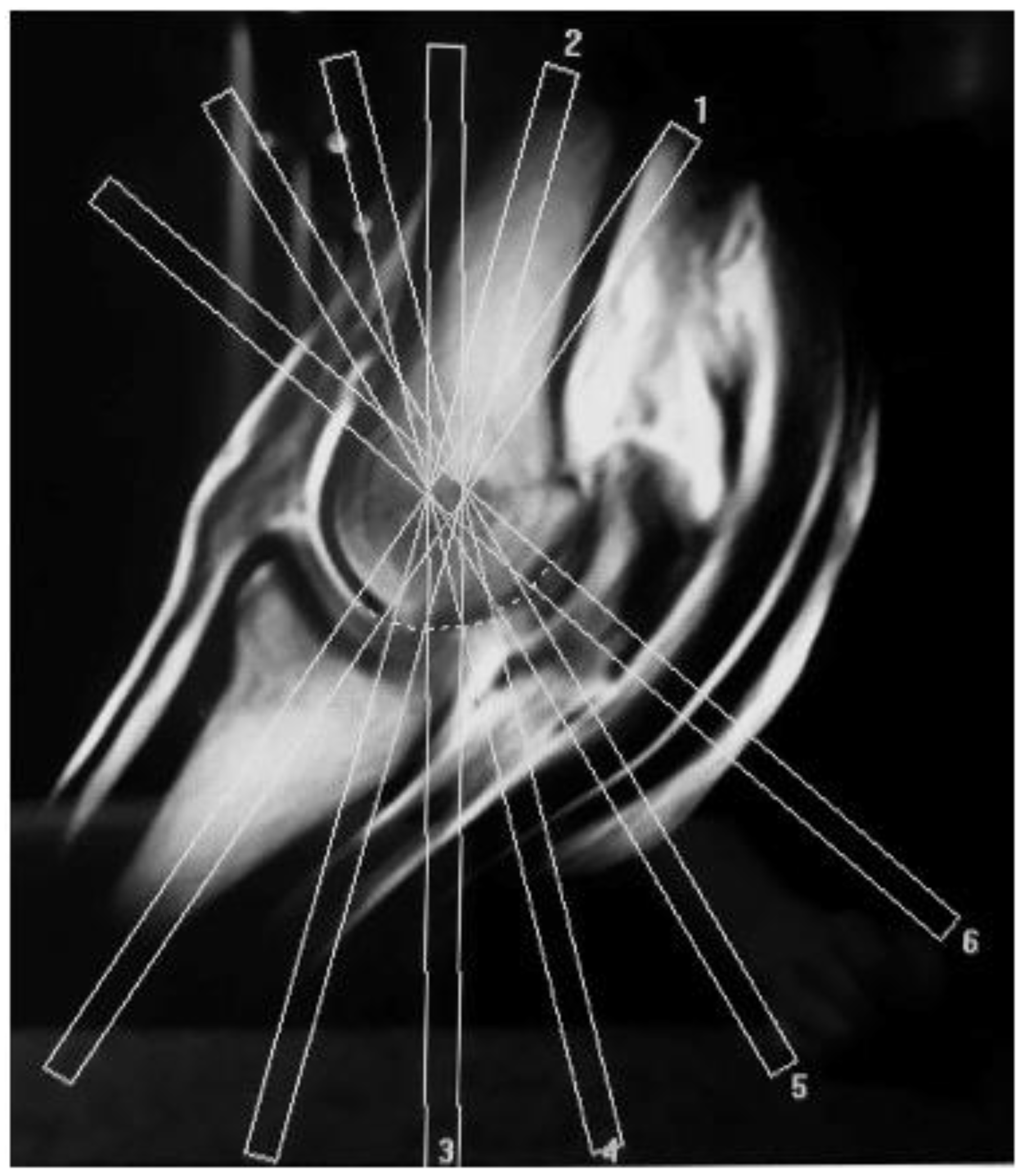
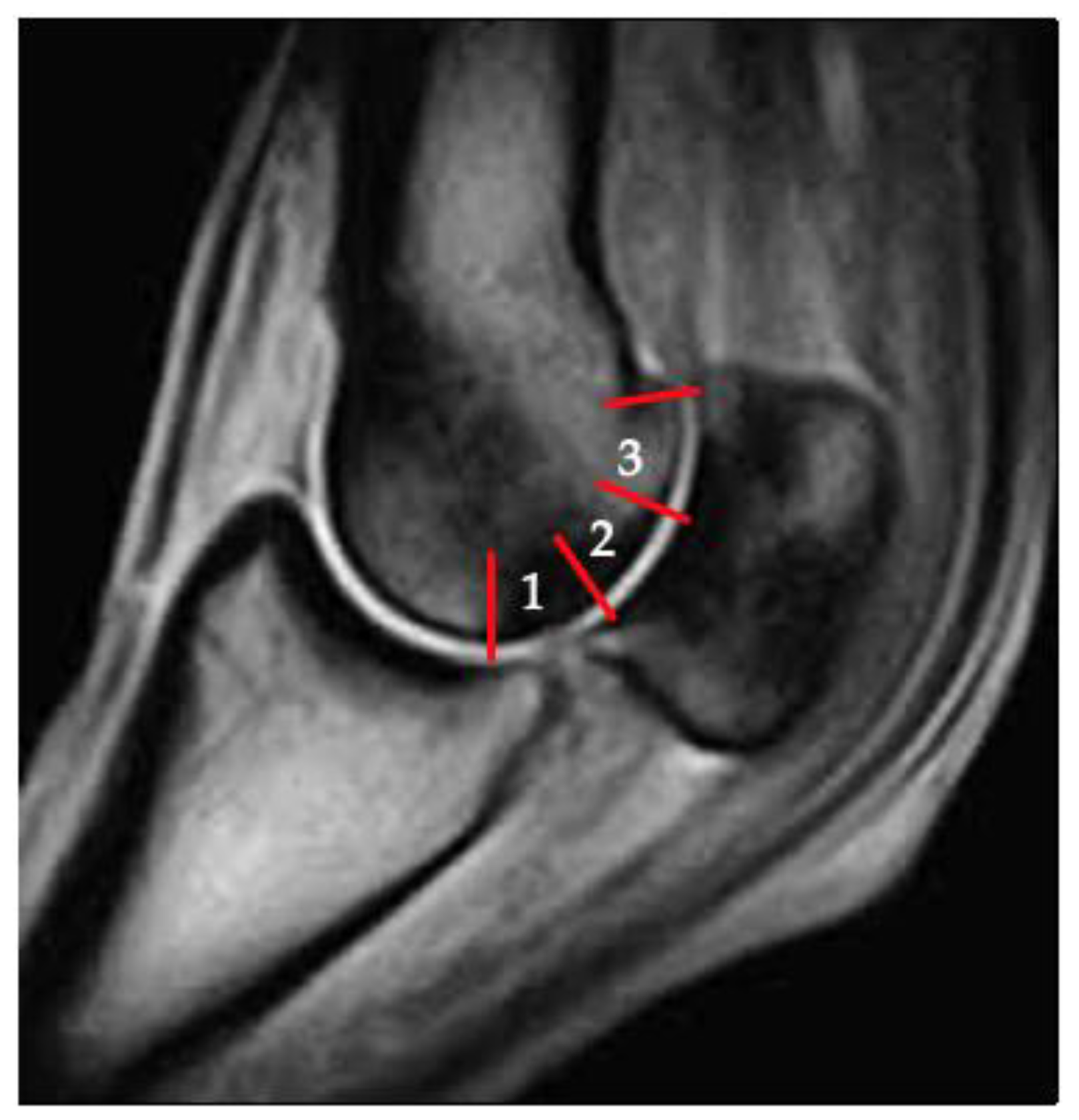
2. Materials and Methods
Data Analysis
3. Results
3.1. Horse Data
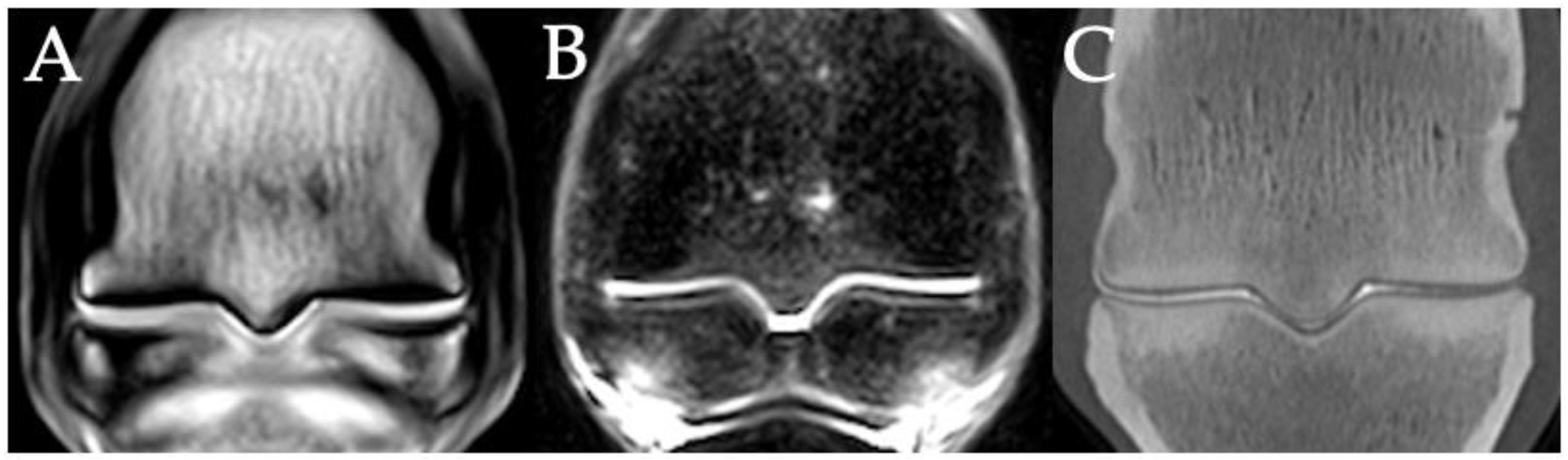
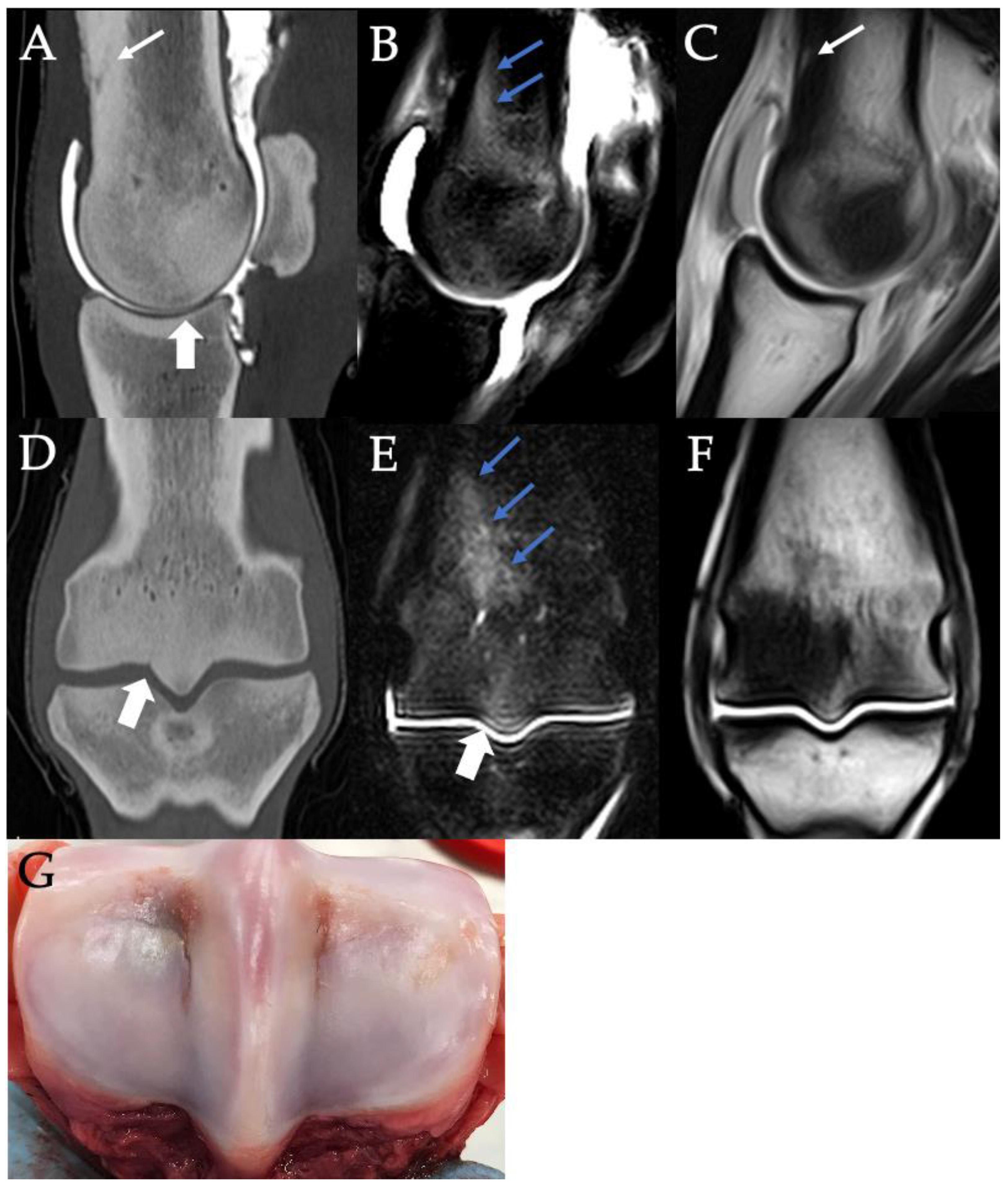
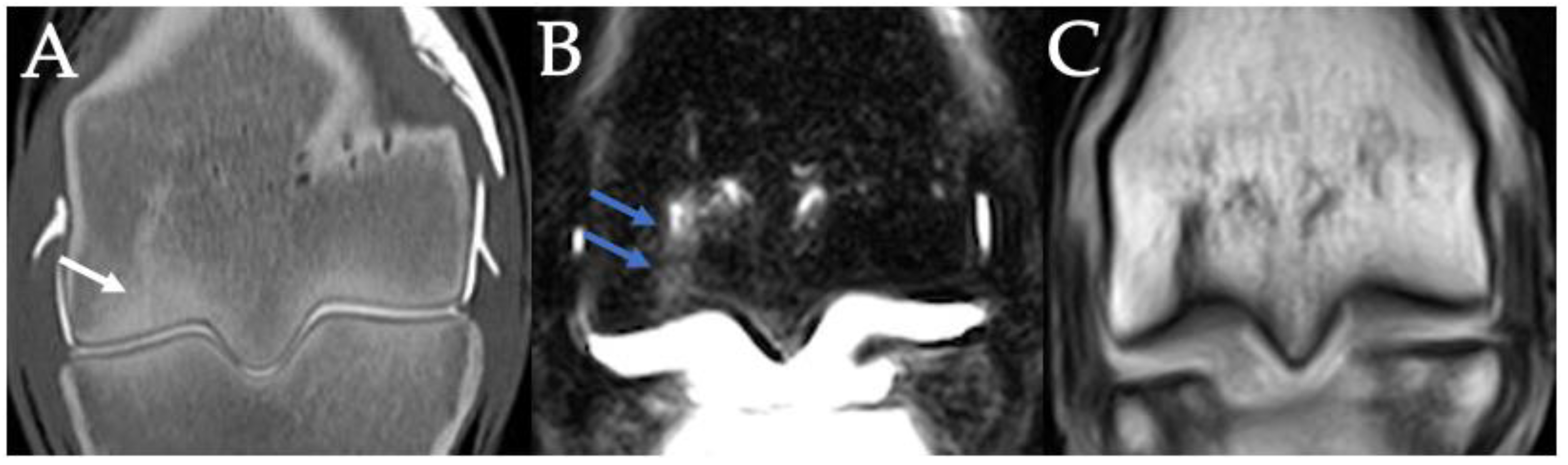
3.2. Sclerosis of the Subchondral and Adjacent Medullary Bone of the PSGs
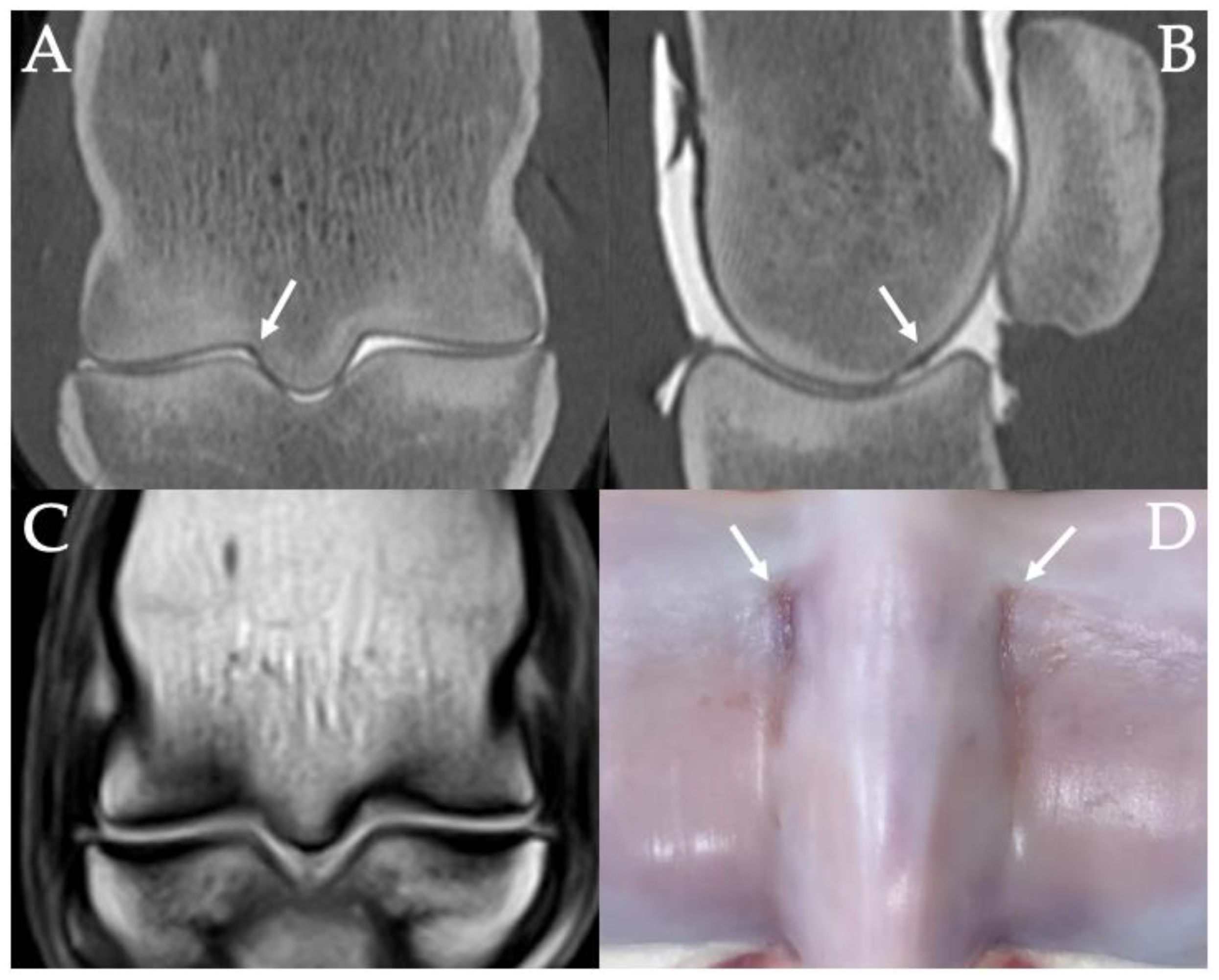
3.3. PSG Subchondral Bone Lysis
3.4. PSG Subchondral Bone Defects
3.5. PSG Cartilage Defects
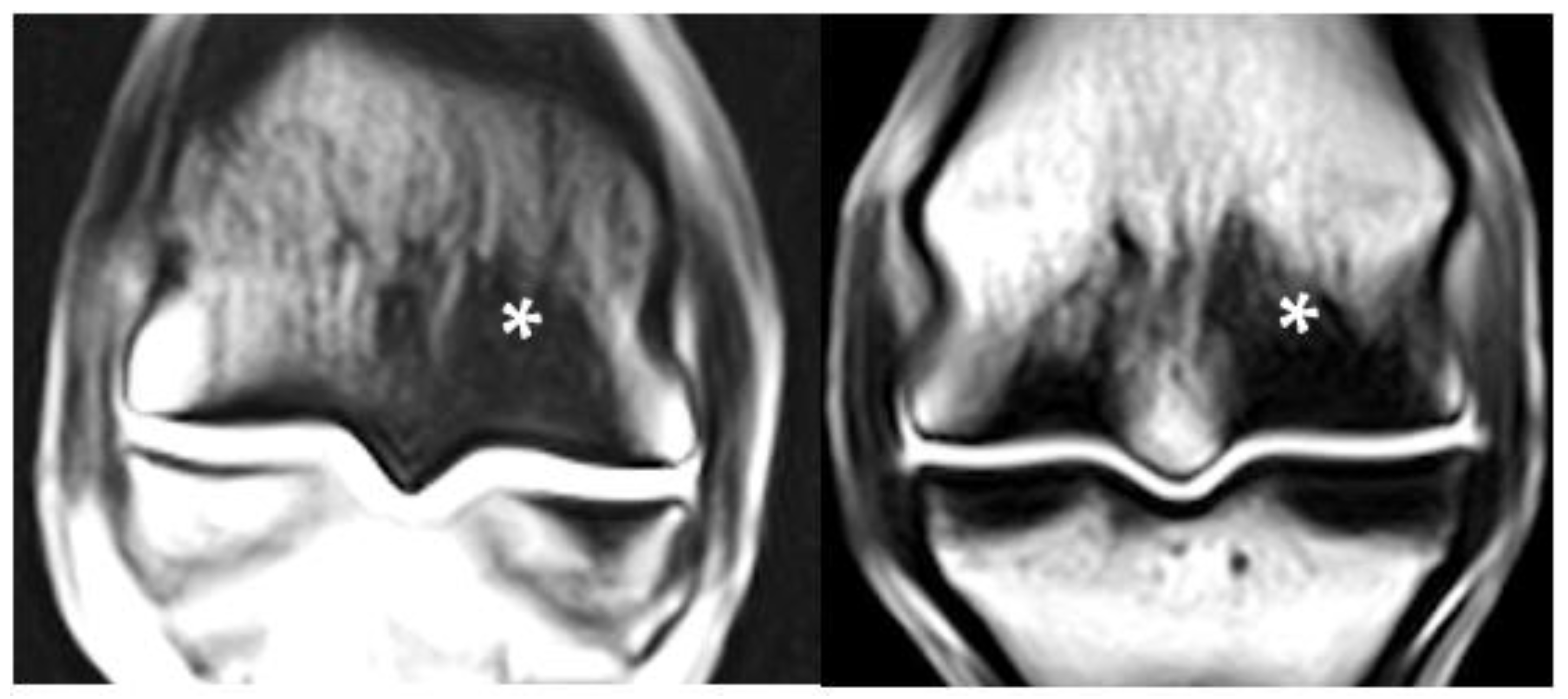
3.6. PSG STIR Hyperintensity
3.7. Combinations of PSG Pathology
3.8. Fetlocks with Possible Fracture Pathology
3.9. Histopathology
4. Discussion
4.1. Relevance to Fracture Risk
4.2. Imaging Techniques
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, S.E. Low-field standing magnetic resonance imaging findings of the metacarpo/metatarsophalangeal joint of racing Thoroughbreds with lameness localised to the region: A retrospective study of 131 horses. Equine Vet. J. 2012, 44, 169–177. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.; Baker, T.A.; Brounts, S.H.; Sample, S.; Markel, M.; Scollay, M.; Marquis, M.; Muir, P. Detection of articular pathology of the distal aspect of the third metacarpal bone in thoroughbred racehorses: Comparison of radiography, computed tomography and magnetic resonance imaging. Vet. Surg. 2011, 40, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Hontoir, F.; Nisolle, J.-F.; Meurisse, H.; Simon, V.; Tallier, M.; Vanderstricht, R.; Antoine, N.; Piret, J.; Clegg, P.; Vanderveert, M. A comparison of 3-T magnetic resonance imaging and computed tomography arthrography to identify structural cartilage defects of the fetlock joint in the horse. Vet. J. 2014, 199, 115–122. [Google Scholar] [CrossRef]
- Olive, J.; Serraud, N.; Vila, T.; Germain, J.-P. Metacarpophalangeal joint injury patterns on magnetic resonance imaging: A comparison in racing Standardbreds and Thoroughbreds. Vet. Radiol. Ultrasound 2017, 58, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Parkin, T.D.H.; Clegg, P.D.; French, N.P.; Proudman, C.J.; Riggs, C.M.; Singer, E.R.; Webbon, P.M.; Morgan, K.L. Risk of fatal distal limb fractures among Thoroughbreds involved in the five types of racing in the United Kingdom. Vet. Record. 2004, 154, 493–497. [Google Scholar] [CrossRef]
- Clegg, P.D. Musculoskeletal disease and injury, now and in the future. Part 1: Fractures and fatalities. Equine Vet. J. 2011, 43, 643–649. [Google Scholar] [CrossRef]
- Riggs, C.M. Aetiopathogenesis of parasagittal fractures of the distal condyles of the third metacarpal and third metatarsal bones–review of the literature. Equine Vet. J. 1999, 31, 116–120. [Google Scholar] [CrossRef]
- Turley, S.M.; Thambyah, A.; Rigss, M.; Firth, E.; Broom, D. Microstructural changes in cartilage and bone related to repetitive overloading in an equine athlete model. J. Anat. 2014, 224, 647–658. [Google Scholar] [CrossRef]
- Bani Hassan, E.; Mirams, M.; Mackie, E.J.; Whitton, R.C. Prevalence of subchondral bone pathological changes in the distal metacarpi/metatarsi of racing Thoroughbred horses. Aust Vet. J. 2017, 95, 362–369. [Google Scholar] [CrossRef]
- Radtke, C.K.; Danova, N.A.; Scollay, M.C.; Santschi, E.M.; Markel, M.D.; Da Costa, G.T.; Muir, P. Macroscopic changes in the distal ends of the third metacarpal and metatarsal bones of Thoroughbred racehorses with condylar fractures. Am. J. Vet. Res. 2003, 64, 1110–1116. [Google Scholar] [CrossRef]
- Morgan, J.W.; Santschi, E.M.; Zekas, L.J.; Scollay-Ward, M.C.; Markel, M.D.; Radtke, C.L.; Sample, S.J.; Keuler, N.S.; Muir, P. Comparison of radiography and computed tomography to evaluate metacarpo/metatarsophalangeal joint pathology of paired limbs of thoroughbred racehorses with severe condylar fracture. Vet. Surg. 2006, 35, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Tranquille, C.A.; Murray, R.C.; Parkin, T.D. Can we use subchondral bone thickness on high-field magnetic resonance images to identify Thoroughbred racehorses at risk of catastrophic lateral condylar fracture? Equine Vet. J. 2017, 49, 67–171. [Google Scholar] [CrossRef] [PubMed]
- Trope, G.D.; Zadeh, A.; Anderson, S.; Mackie, E.; Whitton, R. Can high-resolution peripheral quantitative computed tomography imaging of subchondral and cortical bone predict condylar fracture in Thoroughbred racehorses? Equine Vet. J. 2015, 47, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Riggs, C.M.; Whitehouse, G.H.; Boyde, A. Pathology of the distal condyles of the third metacarpal and third metatarsal bones of the horse. Equine Vet. J. 1999, 31, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Janes, J.G.; Kennedy, L.A.; Garrett, K.S.; Engiles, J.B. Common lesions of the distal end of the third metacarpal/metatarsal bone in racehorse catastrophic breakdown injuries. J. Vet. Diagn. Investig. 2017, 29, 431–436. [Google Scholar] [CrossRef]
- Whitton, R.C.; Ayodele, B.A.; Hitchens, P.L.; Mackie, E.J. Subchondral bone microdamage accumulation in distal metacarpus of Thoroughbred racehorses. Equine Vet. J. 2018, 50, 766–773. [Google Scholar] [CrossRef]
- Whitton, R.C.; Trope, G.D.; Ghasem-Zadeh, A.; Anderson, G.A.; Parkin, T.D.H.; Mackie, E.J.; Seeman, E. Third metacarpal condylar fatigue fracture in equine athletes occur within previously modelled subchondral bone. Bone 2010, 47, 826–831. [Google Scholar] [CrossRef]
- Whitton, R.C.; Mirams, M.; Mackie, E.J.; Anderson, G.A.; Seeman, E. Exercise-induced inhibition of remodelling is focally offset with fatigue fracture in racehorses. Osteoporos. Int. 2013, 24, 2043–2048. [Google Scholar] [CrossRef]
- Peloso, J.G.; Cohen, N.D.; Vogler, J.B.; Marquis, P.A.; Hilt, L. Association of catastrophic condylar fracture with bony changes of the third metacarpal bone identified by use of standing magnetic resonance imaging in forelimbs form cadavers of Thoroughbred racehorses in the United States. Am. J. Vet. Res. 2019, 80, 178–188. [Google Scholar] [CrossRef]
- Denoix, J.M.; Coudry, V. Clinical insights: Imaging of the equine fetlock in Thoroughbred racehorses: Identification of imaging changes to predict catastrophic injury. Equine Vet. J. 2020, 52, 342–343. [Google Scholar] [CrossRef]
- Olive, J.; D’Anjou, M.-A.; Alexander, K.; Laverty, S.; Theoret, C. Comparison of Magnetic Resonance Imaging, Computed Tomography, and Radiography for Assessment of Noncartilaginous Changes in Equine Metacarpophalangeal Osteoarthritis. Vet. Radiol. Ultrasound 2010, 51, 267–279. [Google Scholar] [CrossRef]
- Barr, E.D.; Pinchbeck, G.L.; Clegg, P.D.; Boyde, A.; Riggs, C.M. Post-mortem evaluation of palmar osteochondral disease (traumatic osteochondrosis) of the metacarpo/metatarsophalangeal joint in Thoroughbred racehorses. Equine Vet. J. 2009, 41, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Stepnik, M.W.; Radtke, C.K.; Scollay, M.C.; Oshel, P.E.; Albrecht, R.M.; Santschi, A.M.; Markel, M.D.; Muir, P. Scanning electron microscopic examination of third metacarpal/third metatarsal bone failure surfaces in Thoroughbred racehorses with condylar fracture. Vet. Surg. 2004, 33, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, P.H.; Palmer, L.; Powell, S.E. Unicortical condylar fracture of the Thoroughbred fetlock: 45 cases (2006–2013). Equine Vet. J. 2015, 47, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.L.; Finnane, A.; Phillips, C.J.C.; Greer, R.M.; Woldeyohannes, S.M.; Perkins, N.R.; Kidd, L.J.; Ahern, B.J. The Risk Factors for Musculoskeletal Injuries in Thoroughbred Racehorses in Queensland, Australia: How These Vary for Two-Year-Old and Older Horses and with Type of Injury. Animals 2021, 11, 270. [Google Scholar] [CrossRef]
- Stover, S.M. Diagnostic Workup of Upper-Limb Stress Fractures and Proximal Sesamoid Bone Stress Remodelling. AAEP Proc. 2013, 59, 427–435. [Google Scholar]
- Shaffer, S.K.; To, C.; Garcia, T.C.; Fyhrie, D.P.; Uzal, F.A.; Stover, S.M. Subchondral focal osteopenia associated with proximal sesamoid bone fracture in Thoroughbred racehorses. Equine Vet. J. 2021, 53, 294–305. [Google Scholar] [CrossRef]
- Anthenill, L.A.; Stover, S.M.; Gardner, I.A.; Hill, A.E.; Lee, C.M.; Anderson, M.L.; Barr, B.C.; Read, D.H.; Johnson, B.J.; Woods, L.W.; et al. Association between findings on palmarodorsal radiographic images and detection of a fracture in the proximal sesamoid bones of forelimbs obtained from cadavers of racing Thoroughbreds. Am. J. Vet. Res. 2006, 67, 858–868. [Google Scholar] [CrossRef]
- Jacklin, B.D.; Wright, I.M. Frequency distributions of 174 fractures of the distal condyles of the third metacarpal and metatarsal bones in 167 Thoroughbred racehorses (1999–2009). Equine Vet. J. 2012, 44, 707–713. [Google Scholar] [CrossRef]
- Easton, K. Effect of Bone Geometry on Stress Distribution Patterns in the Equine Metacarpophalangeal Joint. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2012. [Google Scholar]
- Tranquille, C.; Parkin, T.; Murray, C. Magnetic resonance imaging-detected adaptation and pathology in the distal condyles of the third metacarpus, associated with lateral condylar fracture in Thoroughbred racehorses. Equine Vet. J. 2012, 44, 699–706. [Google Scholar] [CrossRef]
- Anne-Archard, N.; Martel, G.; Fogarty, U.; Richard, H.; Beauchamp, G.; Laverty, S. Differences in third metacarpal trabecular microarchitecture between the parasagittal groove and condyle at birth and in adult racehorses. Equine Vet. J. 2019, 51, 115–122. [Google Scholar] [CrossRef]
- Liley, H.; Davies, H.; Firth, E.; Besier, T.; Fernandez, J. The effect of the sagittal ridge angle on cartilage stress in the equine metacarpophalangeal (fetlock) joint. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Kawcak, C.; McIlwraith, C.; Parkin, T.; Easton, K. The effects of third metacarpal geometry on the incidence of condylar fractures in thoroughbred racehorses. Osteoarthr. Cartil. 2009, 17, 237–238. [Google Scholar] [CrossRef]
- Mccarty, C.A.; Thomason, J.J.; Gordon, K.D.; Burkhart, T.A.; Milner, J.S.; Holdsworth, D.W. Finite-Element Analysis of Bone Stresses on Primary Impact in a Large-Animal Model: The Distal End of the Equine Third Metacarpal. PLoS ONE 2016, 11, e0159541. [Google Scholar]
- Wright, I.M.; Nixon, A.J. Fractures of the condyles of the third metacarpal and metatarsal bones. In Equine Fract. Repair, 2nd ed.; Nixon, A.J., Ed.; Wiley: Saunders, PA, USA, 2012. [Google Scholar]
- Wulster, K.; Ortved, K.; Curtiss, A.; Richardson, D. Response to Letter to the Editor: Validation of standing cone beam computed tomography for diagnosing subchondral fetlock pathology in the Thoroughbred racehorse. Equine Vet. J. 2021, 53, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Anthenill, L.A.; Gardner, I.A.; Pool, R.R.; Garcia, T.C.; Stover, S.M. Comparison of macrostructural and microstructural bone features in Thoroughbred racehorses with and without midbody fracture of the proximal sesamoid bone. Am. J. Vet. Res. 2010, 71, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Peloso, J.G.; Vloger, J.; Cohen, N.; Marquis, N.; Hilt, N. Association of catastrophic biaxial fracture of the proximal sesamoid bones with bony changes of the metacarpophalangeal joint identified by standing magnetic resonance imaging in cadaveric forelimbs of Thoroughbred racehorses. J. Am. Vet. Med. Assoc. 2015, 246, 661–673. [Google Scholar] [CrossRef]
- Gray, S.N.; Spriet, M.; Garcia, T.C.; Uzal, F.A.; Stover, S.M. Preexisting lesions associated with complete diaphyseal fractures of the third metacarpal bone in 12 Thoroughbred racehorses. J. Vet. Diagn Investig. 2017, 29, 437–441. [Google Scholar] [CrossRef]
- Firth, E.C.; Doube, M.; Boyde, A. Changes in mineralised tissue at the site of origin of condylar fracture are present before athletic training in Thoroughbred horses. N. Z. Vet. J. 2009, 57, 278–283. [Google Scholar] [CrossRef]
- Olive, J.; D’Anjou, M.A.; Girard, C.; Laverty, S.; Theoret, C. Imaging and histological features of central subchondral osteophytes in racehorses with metacarpophalangeal joint osteoarthritis. Equine Vet. J. 2009, 41, 859–864. [Google Scholar] [CrossRef]
- Ramzan, P.H.L. Letter to the Editor: Validation of standing cone beam computed tomography for diagnosing subchondral fetlock pathology in the Thoroughbred racehorse. Equine Vet. J. 2021, 53, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Colgate, V.A.; Carpenter, R.; Kawcak, C.; Muir, P.; Palmer, S.; Parkin, T.; Powell, S.E.; Ramzan, P.; Riggs, C.M.; Spriet, M.; et al. Science-in-brief: Risk assessment for reducing injuries of the fetlock bones in Thoroughbred racehorses. Equine Vet. J. 2020, 52, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.G.; Winter, M.D.; Sheppard, B.J.; Berry, C.R.; Hernandez, J.A. Correlation of articular cartilage thickness measurements made with magnetic resonance imaging, magnetic resonance arthrography, and computed tomographic arthrography with gross articular cartilage thickness in the equine metacarpophalangeal joint. Vet. Radiol. Ultrasound 2016, 57, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.B.; Kawcak, C.E.; Barrett, M.F.; McIlwraith, C.W.; Grinstaff, M.W.; Goodrich, L.R. Recent advances in articular cartilage evaluation using computed tomography and magnetic resonance imaging. Equine Vet. J. 2018, 50, 564–579. [Google Scholar] [CrossRef]
- Murray, R.C.; Mair, T.S.; Sherlock, C.E.; Blunden, A.S. Comparison of high-field and low-field magnetic resonance images of cadaver limbs of horses. Vet. Rec. 2009, 165, 281–288. [Google Scholar] [CrossRef]
- Smith, M.A.; Dyson, S.J.; Murray, R.C. The appearance of the equine metacarpophalangeal region on high-field vs. standing low-field magnetic resonance imaging. Vet. Radiol. Ultrasound 2011, 52, 61–70. [Google Scholar] [CrossRef]
- Werpy, N.M.; Ho, C.P.; Pease, A.P.; Kawcak, C.E. The effect of sequence selection and field strength on detection of osteochondral defects in the metacarpophalangeal joint. Vet. Radiol. Ultrasound 2011, 52, 154–160. [Google Scholar] [CrossRef]
- Curtiss, A.L.; Ortved, K.F.; Dallap-Schaer, B.; Gouzeev, S.; Stefanovski, D.; Richardson, D.W.; Wulster, K.B. Validation of standing cone beam computed tomography for diagnosing subchondral fetlock pathology in the Thoroughbred racehorse. Equine Vet. J. 2020, 53, 510–523. [Google Scholar] [CrossRef]
- Schramme, M.C.; Kerekes, Z.; Hunter, S.; Kerekes, Z.; Hunter, S.; Nagy, K.; Pease, A. Improved identification of the palmar fibrocartilage of the navicular bone with saline magnetic resonance bursography. Vet. Radiol. Ultrasound 2009, 50, 606–614. [Google Scholar] [CrossRef]
- Pease, A.; Marr, C.M. How to publish diagnostic imaging studies: Common mistakes and recommendations. Equine Vet. J. 2019, 51, 7–10. [Google Scholar] [CrossRef]
- Reesink, H.L.; Palmer, S.E. Letter to the Editor: Selection of appropriate controls for studying fatal musculoskeletal injury in racehorses. Equine Vet. J. 2019, 51, 559–560. [Google Scholar] [CrossRef] [PubMed]




| Overall | FF | OF | NF | Adult | Juvenile | CL | Control | |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| CT—Lateral PSG | ||||||||
| Frontal | 53 (70%) | 10 (63%) | 17 (71%) | 26 (72%) | 19 (70%) | 34 (69%) | 7 (64%) | 26 (72%) |
| Condylar | 60 (79%) | 10 (63%) | 21 (88%) | 29 (81%) | 23 (85%) | 37 (76%) | 8 (73%) | 29 (81%) |
| CT—Medial PSG | ||||||||
| Frontal | 74 (97%) | 16 (100%) | 23 (96%) | 35 (97%) | 27 (100%) | 47 (96%) | 10 (91%) | 35 (97%) |
| Condylar | 75 (99%) | 16 (100%) | 23 (96%) | 36 (100%) | 27 (100%) | 48 (98%) | 10 (91%) | 36 (100%) |
| MRI—Lateral PSG | ||||||||
| Frontal | 34 (45%) | 7 (44%) | 9 (38%) | 18 (50%) | 13 (48%) | 21 (43%) | 5 (45%) | 18 (50%) |
| Condylar | 39 (51%) | 7 (44%) | 11 (46%) | 21 (58%) | 12 (44%) | 27 (55%) | 5 (45%) | 21 (58%) |
| MRI—Medial PSG | ||||||||
| Frontal | 67 (88%) | 16 (100%) | 19 (79%) | 32 (89%) | 22 (81%) | 45 (92%) | 10 (91%) | 32 (89%) |
| Condylar | 67 (88%) | 15 (94%) | 21 (88%) | 31 (86%) | 22 (81%) | 45 (92%) | 9 (82%) | 31 (86%) |
| Radiography—Lateral PSG | ||||||||
| 6 (8%) | 2 (13%) | 0 (0%) | 4 (11%) | 3 (11%) | 3 (6%) | 4 (11%) | 0 (0%) | |
| Radiography—Medial PSG | ||||||||
| 10 (13%) | 2 (13%) | 3 (13%) | 5 (14%) | 6 (22%) | 4 (8%) | 5 (14%) | 1 (9%) | |
| Overall | FF | OF | NF | Adult | Juvenile | Control | Contralateral |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| CT—Lateral PSG | |||||||
| 41 (54%) | 9 (56%) | 14 (58%) | 18 (50%) | 12 (44%) | 29 (59%) | 18 (50%) | 8 (73%) |
| CT—Medial PSG | |||||||
| 45 (59%) | 10 (62%) | 15 (62%) | 20 (56%) | 15 (56%) | 30 (61%) | 20 (56%) | 6 (55%) |
| MRI—Lateral PSG | |||||||
| 27 (36%) | 5 (31%) | 11 (46%) | 11 (31%) | 10 (37%) | 17 (35%) | 11 (31%) | 3 (27%) |
| MRI—Medial PSG | |||||||
| 45 (59%) | 12 (75%) | 14 (58%) | 19 (53%) | 17 (63%) | 28 (57%) | 19 (53%) | 5 (45%) |
| Radiography—Lateral PSG | |||||||
| 12 (16%) | 1 (6%) | 4 (17%) | 7 (19%) | 5 (19%) | 7 (14%) | 7 (19%) | 0 (0%) |
| Radiography—Medial PSG | |||||||
| 26 (34%) | 9 (56%) | 8 (33%) | 9 (25%) | 9 (33%) | 17 (35%) | 9 (25%) | 4 (36%) |
| Location | Overall | FF | OF | NF | Adult | Juvenile | Contralateral | Control |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Lateral PSG | 30 (44%) | 5 (36%) | 12 (55%) | 13 (41%) | 13 (52%) | 17 (40%) | 6 (60%) | 13 (41%) |
| Medial PSG | 20 (29%) | 4 (29%) | 7 (32%) | 9 (28%) | 11 (44%) | 9 (21%) | 3 (30%) | 9 (28%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, G.C.A.; Ahern, B.J.; Palmieri, C.; Young, A.C. Imaging and Gross Pathological Appearance of Changes in the Parasagittal Grooves of Thoroughbred Racehorses. Animals 2021, 11, 3366. https://doi.org/10.3390/ani11123366
Johnston GCA, Ahern BJ, Palmieri C, Young AC. Imaging and Gross Pathological Appearance of Changes in the Parasagittal Grooves of Thoroughbred Racehorses. Animals. 2021; 11(12):3366. https://doi.org/10.3390/ani11123366
Chicago/Turabian StyleJohnston, Georgina C. A., Benjamin J. Ahern, Chiara Palmieri, and Alex C. Young. 2021. "Imaging and Gross Pathological Appearance of Changes in the Parasagittal Grooves of Thoroughbred Racehorses" Animals 11, no. 12: 3366. https://doi.org/10.3390/ani11123366
APA StyleJohnston, G. C. A., Ahern, B. J., Palmieri, C., & Young, A. C. (2021). Imaging and Gross Pathological Appearance of Changes in the Parasagittal Grooves of Thoroughbred Racehorses. Animals, 11(12), 3366. https://doi.org/10.3390/ani11123366






