1. Introduction
Animal nutrition plays an important role in the treatment of many diseases. Pathological changes lead to specific disturbances in the use of nutrients, which may lead to certain metabolic disorders. The idea behind a therapeutic diet is to match the energy and nutrient requirements during a disease state in such a way as to compensate for these disorders [
1].
It is known that cardiac failure activates compensatory mechanisms. One of the most important of these is the renin-angiotensin-aldosterone axis, which is responsible for an increase in blood pressure. The main mechanism involved in the development of hypertension is sodium retention associated with simultaneous potassium loss. Therefore, the recommendation for functional foods in a prescription diet for cardiopathy according to the European Union is te limitation of sodium content to a value below 2.6 g/kg of a complete diet with a moisture content of 12% for pets [
2,
3]. So far, this is the sole “cardiologic” requirement for an applied diet that has been recommended.
There are, however, several other important nutritional ingredients, e.g., the long-chain fatty acids: eicosapentaenoic acid (EPA, 20:5 (
n-3)) or docosahexaenoic acid (DHA 22:6 (
n-3)), which can significantly support prescription diet for cardiopathy [
4,
5]. The reduced concentration of fatty acids (FA) in the blood serum increased after eating a fish-meat diet. Such a diet increases the concentration of fatty acids in blood serum, cells and tissues in humans and dogs with heart disease [
5,
6]. Metabolomic studies reflect the species-specific physiological and biochemical processes occurring during the development of heart failure and responses to nutrition change. Currently, two types of metabolomics analyses are performed: metabolomic fingerprinting and targeted metabolomics analysis. The evaluation of metabolite levels is a very sensitive tool since the current methodology allows for the detection of very small differences in their concentrations. Protein metabolites or simply amino acids have been found in many studies searching for biomarkers of specific diseases. Quite recently, using metabolomic methodologies, it was possible to identify metabolic inflexibility to food intake and lower carnitine concentrations in overweight Labrador Retrievers [
7,
8]. Up-to-date published papers revealed many different factors: feeding methods, nutritional habits of owners, food preparation methods, etc. influencing animal plasma or urine metabolomic response. One metabolomics study with serum transcriptomic analyzes of serum identified cellular and metabolic pathways that play a role in myxomatous mitral valve disease (MMVD). The study results identified 41 known and 13 unknown serum metabolites that were significantly different between healthy and MMVD dogs, representing alterations in fatty acid and glucose energy metabolism, oxidative stress, and other pathways [
9]. There is very limited information on the influence of supplementation of unsaturated fatty acids in dogs with MMVD and only one of them establishes the impact of diet modification on the metabolomic profile in MMVD dogs [
10].
The aim of this study was to assess whether a 6-month administration of a diet enriched with EPA + DHA from fish meat in dogs suffering from heart failure due to MMVD improves their metabolic profile and clinical status.
4. Discussion
Our research presents the first contribution on the impact of supplementation of DHA + EPA from fish meat on metabolism in dogs with MMVD stage B2 and C2. There are many reports that polyunsaturated omega-3 and omega-6 fatty acids have a very beneficial effect on the metabolic functions of the body. In both humans and animals receiving an enriched diet with essential fatty acid, especially omega-3 fatty acids, a reduced morbidity and lower mortality have been noted in people in the course of heart disease [
16,
17,
18]. Omega-3 polyunsaturated fatty acids must be systematically supplied in the diet because they belong to exogenous compounds, which cannot be synthesize de novo in the mammalian body. The precursor of the omega-3 family is alpha-linolenic acid (18:3 omega-3), which by elongation and desaturation of the carbon hydrate chain is converted into a biologically more active EPA (20:5 omega-3) and DHA (22:6 omega-3). The metabolism of alpha-linolenic acid is subject to large individual fluctuations, but generally is inefficient because no more than 5% is converted to EPA and less than 1% to DHA [
5]. Therefore, increasing tissue EPA and especially DHA levels via dietary supplementation would be more effective to the sick dogs. Depending on the source of origin, fatty acids differ in quantity and composition: fish and fish oils are rich in long chain
n-3 FA, mostly EPA and DHA, whereas terrestrial plants only provide alpha linolenic acid [
19,
20]. Polyunsaturated fatty acid (PUFA) omega-3 and omega-6 metabolites have a significant, but different, effect on cellular biochemical processes, therefore the proper ratio between both acids should be retained in the diet. The optimal ratio of omega-6 PUFA to omega-3 PUFA in healthy dogs should be 5–10:1 [
21]. When this ratio is inappropriate, the effect may be counterproductive. In a typical human “western diet” the ratio of omega-6 to omega-3 PUFA increases to 15–16.7/1 and can be an independent factor in the development of heart failure [
21,
22]. Several strategies are used to increase the amount of
n-3 PUFAs in the bloodstream: (1) increased consumption of fish fats or their meat rich in
n-3 PUFAs, (2) enrichment of food products with fish oil and alpha-oleic acid, (3) enrichment of PUFAs in meat of farmed animals by using a diet rich in
n-3 PUFAs, (4) increasing the amount of
n-3 PUFAs in oilseed crops by genetic engineering [
23,
24,
25,
26].
The first strategy increasing the intake of n-3 PUFAs (DHA + EPA) by increasing the amount of fish fat and meat was used. Despite the fact that dogs from both experimental groups accepted their diets, a decrease of body weight and fat index was observed in both groups. A decrease of subcutaneous fat was more visible after 3 months than at the end of the study, but the differences between groups were not significant. Owners considered the diet enriched in DHA + EPA (from fish meat) as tasty for their pets and it was very readily accepted by dogs (several owners noticed improvement of coat, and an increase of vitality). However, some animals lost their initial enthusiasm after a few weeks on the diet. We suspect that loss of body weight may be related to the categorical prohibition of giving dogs snacks during the course of the study and strictly limiting them to the tested diet. The average weight loss was more pronounced in the group of dogs receiving the standard diet, but the differences between groups was not significant.
Previous studies have shown that omega-3 PUFAs have many beneficial effects in human heart failure including inhibition of proinflammatory response in patients with increased concentration of TNF-alfa, IL-1 and IL-6 [
27]. This effect is secondary to decreased activity of transcriptional factor NF-kappaB, which controls cytokine synthesis (and whose activity increases pathologically in heart failure) [
28,
29]. In the canine MMVD transcriptome there is a consistently increased expression of inflammatory genes; predominantly the expression of toll-like receptors and interleukins, which are involved in both the control of inflammation as well as other biological pathways in dogs [
10] and people [
30,
31]. Earlier research revealed that the equilibration process of
n-6 FA is slow and the beneficial anti-inflammatory effects of dietary
n-3 FA supplementation in dogs takes a few weeks [
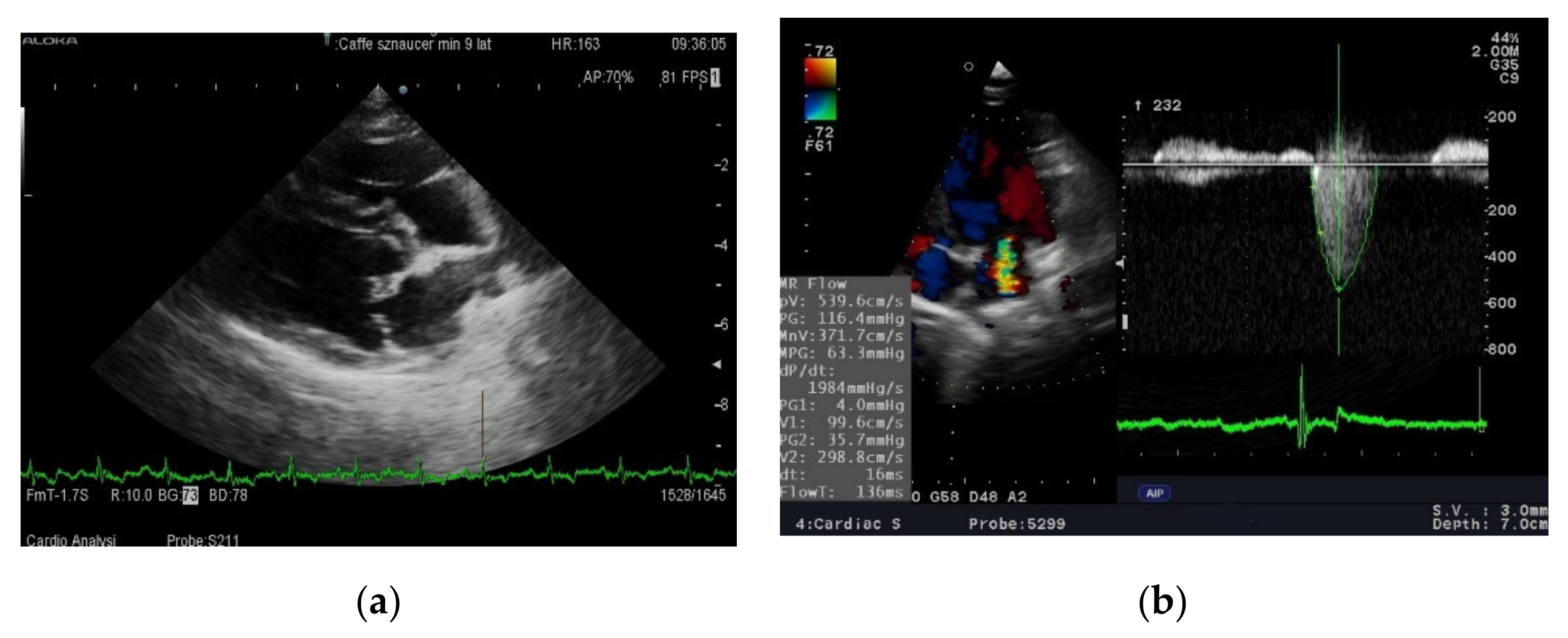
32]. In our study, despite a 6 month feeding period, neither diet was found to affect leukocyte counts or CRP levels with values remaining within normal ranges throughout the observation period in both groups. It seems, therefore, that at the B2 and Cc stages of MMVD the activation of inflammatory processes is too low for the effect of anti-inflammatory diets to be seen. Studies documenting the increased activity of proinflammatory factors were performed in dogs asleep due to advanced heart disease. Neither electrocardiographic nor echocardiographic parameters revealed any changes. This finding is different from the results of Li et al. [
9]. However, in this experiment, a statistically significant reduction in left atrium size was only observed in a group of previously untreated dogs. The reduction in left atrium size was correlated with a drop in blood pressure and was not significant in dogs that had previously received angiotensin converting enzyme inhibitor (ACE-I). In our study all dogs were treated, which may explain the difference in results. There was a clear (slightly below statistical significance) increase in left ventricular contractility as measured by the shortening and ejection fraction in dogs fed a diet rich in unsaturated fatty acids after 3 months. The difference disappeared after 6 months and the systolic function in both groups was almost identical. Routine diagnostic tests were not sensitive enough to assess whether feeding with a diet enriched in DHA + EPA inhibits or slows down pathological processes occurring in valves or heart muscle in dogs with MMVD. Therefore, it was decided to evaluate the effect of the studied diet on the metabolomic image.
To explain the metabolic changes that occurred during the study, a widely used methodology for serum metabolomic profiling using liquid chromatography coupled with mass spectroscopy was chosen. There are many examples in the literature of the use of metabolomics in the diagnosis of human cardiovascular disease caused by atherosclerosis [
28], swine model of atherosclerosis [
33,
34], coronary heart disease [
35,
36,
37] and hypertension [
38]. This method has also been used successfully in studies on animals: rats with hypertension and myocardial infarction, pigs with myocardial infarct and dogs with MMVD [
39,
40,
41].
In our dogs, the results of the analyses show that the effect of diet on metabolism increases over time. The differences after 6 months were significantly greater than after 3 months. Of the many metabolic characteristics determined by mass spectrometry, accurate assignment of names to compounds involved in the differentiation of both groups of animals was possible for only six metabolites. It should be noted that these are not the only variables-metabolites generating separation between observations,
Table 6. Metabolites that increased after 6 months of feeding dogs with a diet rich in DHA + EPA included: lactate, pyruvate and aspartate, while reduced values were noted for alanine, xanthine and glycerophosphocholine. Although the diet enriched with an additional source of omega-3 DHA and EPA should have a positive effect on changes in metabolism, an increase in blood lactate is observed.
As it has been shown in very extensive studies devoted to DHA and EPA influence on Atlantic salmon fish, both omega-3 acids divergently induced gene expression associated with glucose metabolism. DHA is responsible for glycolytic pathways with the production of pyruvate and lactate. While EPA and EPA + DHA was positively associated with glycogen degradation to glucose, simultaneously EPA was inducing gluconeogenesis by specific gene glucose-6-phosphatase which release glucose. An increase in lactate and pyruvate may be primarily associated with accelerated glycolytic pathways of DHA (anaerobic glycolysis) [
42]. An increase in lactate is usually associated with a decrease in glucose. However, in dogs fed a diet enriched with DHA + EPA (from fish meat), glucose levels were stable (and even increased statistically insignificantly compared to the first study), which can be associated with EPA. This indicates that despite increased glycolysis, the mechanisms that control blood glucose remain functional. A decrease in pH associated with increase in lactate helps to increase the use of fatty acids in all myocytes as an energy source via increase pyruvate dehydrogenase activity [
43]. It has been proven in human studies with the use of isotope techniques that the increase in the metabolic rate of PUFA has a “sparing” effect on the use of intramuscular glycogen stores (promotes the reconstruction of glycogen reserves in muscles) and may also occur in conditions of no changes in blood glucose [
44].
Increased asparagine levels in the group of dogs fed the DHA + EPA diet may indicate increased nitrogen transport from cells. Asparagine can be converted into aspartate, and this—as a glucogenic amino acid—can be transaminated into oxaloacetate (a key regulator of levels of Krebs cycle intermediates). This reaction is critical because it allows aspartate and asparagine stores to serve as absorbers for excess oxaloacetate produced from supplemented α-ketoglutarate precursors.
Dogs fed a diet enriched with DHA + EPA (from fish meat) had decreased levels of alanine. Alanine metabolism is tightly associated with gluconeogenesis, which can be directly influenced by tricarboxylic acid cycle via pyruvate [
40]. The decrease in alanine levels correlates with increased amounts of lactate and pyruvate. This fact can confirm the assumption that despite using a diet rich in omega-3 fatty acids, heart disease increases glycolysis, at the expense of oxygen metabolism of fatty acids. A reduced level of glycerophosphocholine, a phospholipid precursor, is also noteworthy. Reports suggest that glycerophosphocholine metabolites may be closely related to the risk of heart disease [
45]. Therefore, its lowered serum level may be the first positive clinical sigh of diet enriched DHA + EPA (from fish meat).
As documented in humans the second beneficial effect is decrease of xantine, which is an intermediate product in purine catabolism. Purines have an influence on receptors (in particular the different types of P2Y-receptors) in blood vessels and the heart, where they are involved in the progression of heart failure [
46]. Increased levels of xantine were observed in patients with acute coronary syndrome and arteriosclerosis [
47,
48].
We suppose that such a negligible (contrary to literature data) beneficial effect of a diet rich in DHA + EPA was the result of including dogs in a relatively early stage of heart failure [
4,
5,
6,
16,
24,
28,
29]. During early stages of heart disease, metabolic disturbances can be almost completely counterbalanced by compensatory mechanisms. It is worth noting that heart failure is dominant in dogs over 7 years of age, while better effects of increasing the concentration of (
n-3) FA were observed in younger than older dogs [
49]. Only one metabolic analysis of the serum of dogs with MMVD has been published showing an increase of 102 metabolites including arginine, α-aminobutyrate, citrulline, caprate, deoxycarnitine and sphingomyelin. The margarate and methyl palmitate levels decreased [
10]. Our research confirms and complements this knowledge regarding the effects of unsaturated fats on the metabolism of dogs with MMVD, although the studies carried out have a number of limitations. First of all, there was a relatively small number of dogs in the groups, and secondly, the differences in the composition of the diet caused by the addition of fish meat. Moreover, we could not distinguish the effect of fatty acids from the effect of other nutrients in fish meat and it cannot be ruled out that the effects of diet enriched in DHA + EPA (from fish meat) in dogs with more advanced stages of heart failure may not be the same.