The Design of a New Catheter for Transcervical Artificial Insemination in Ewes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
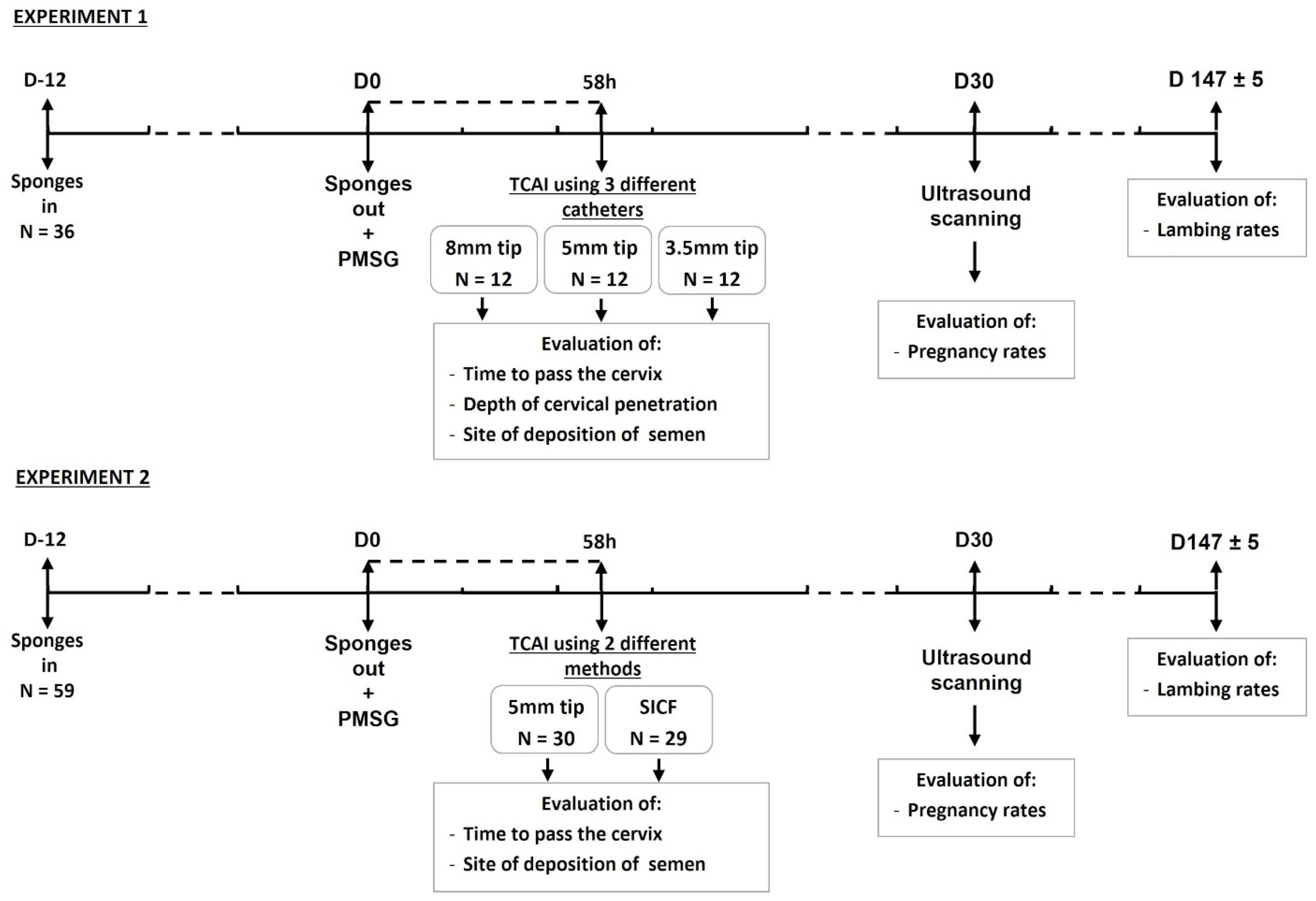
2.1. Experimental Design
2.2. Estrous Synchronization
2.3. Semen Collection and Preparation
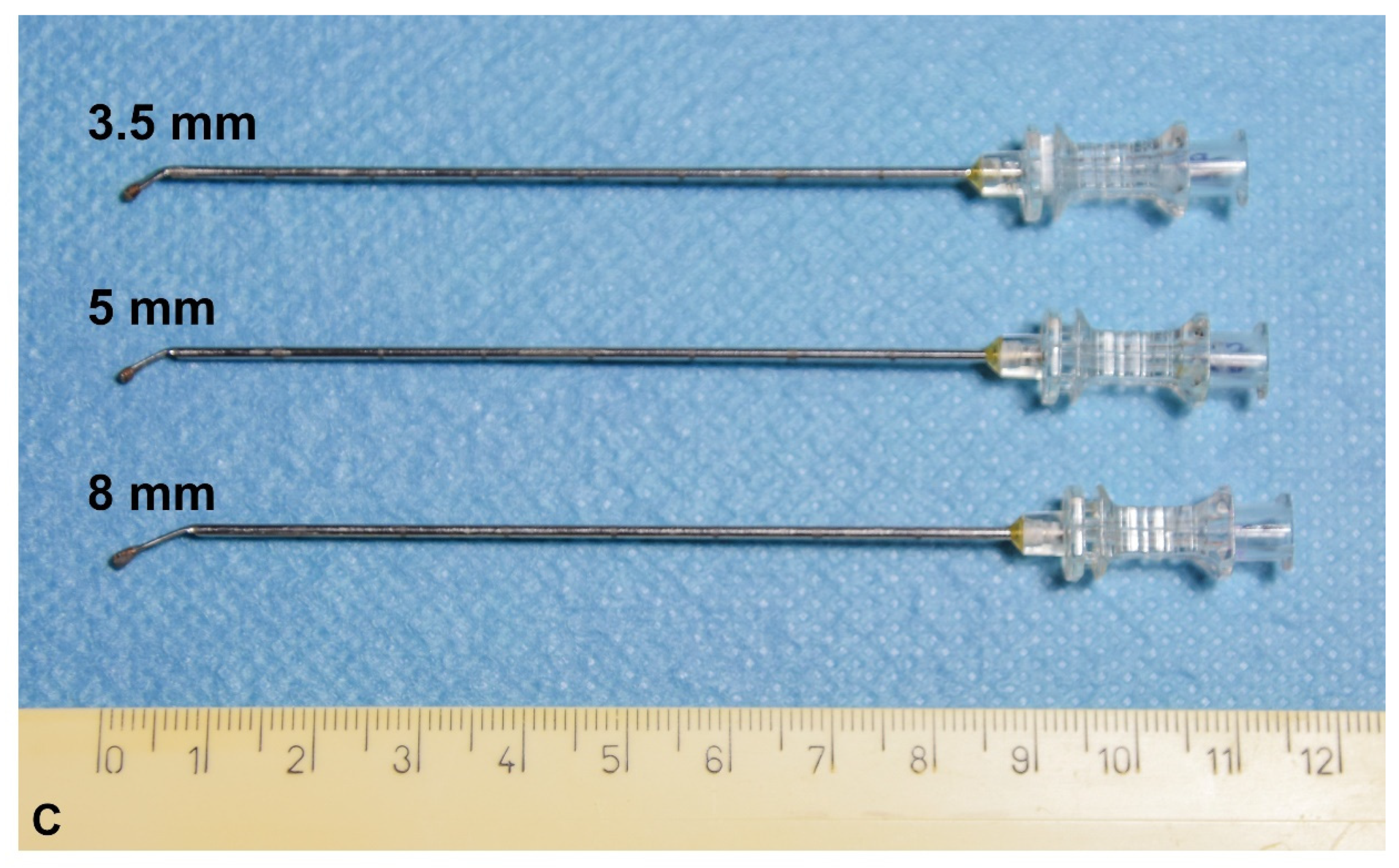
2.4. Experiment 1
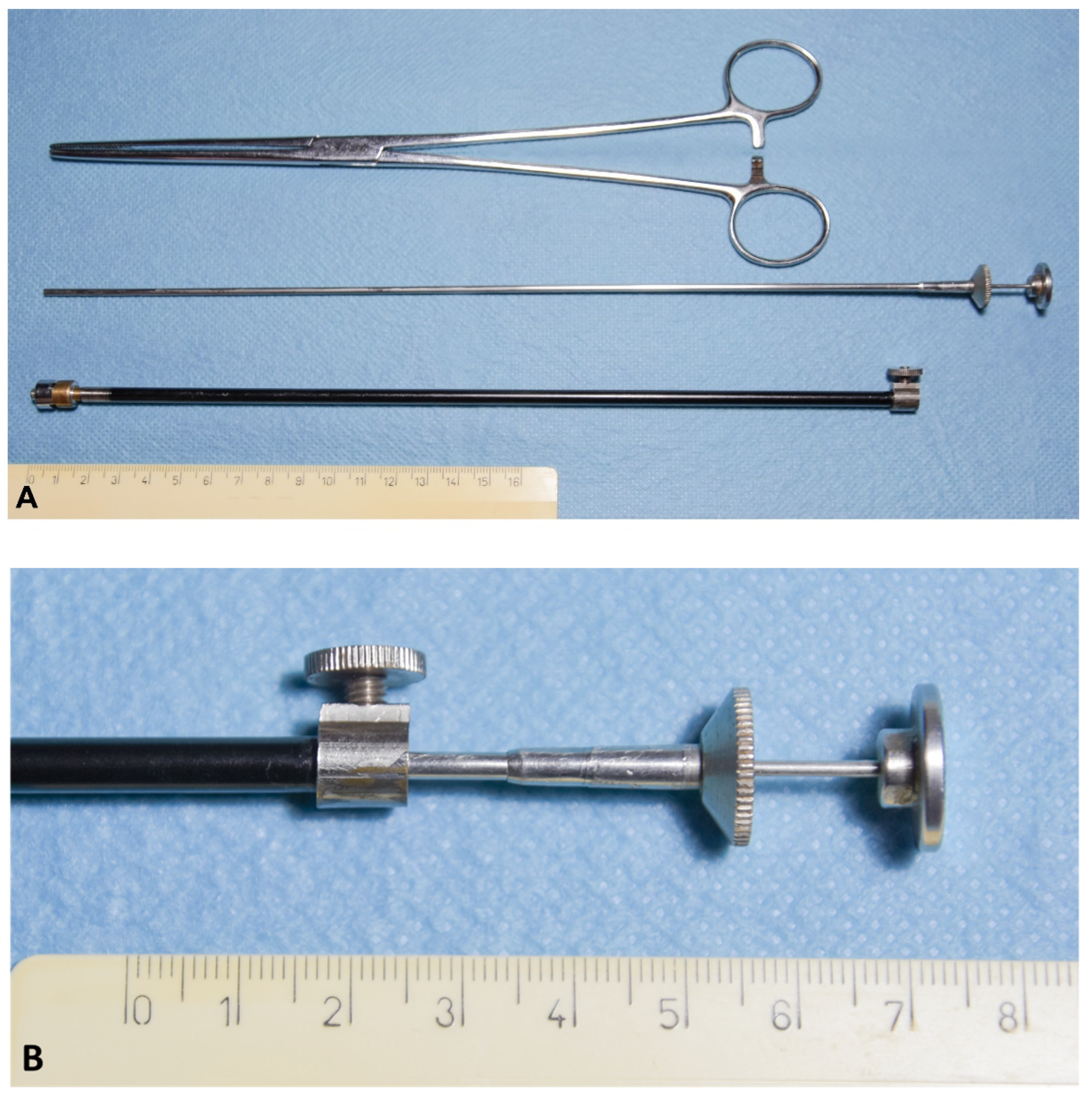
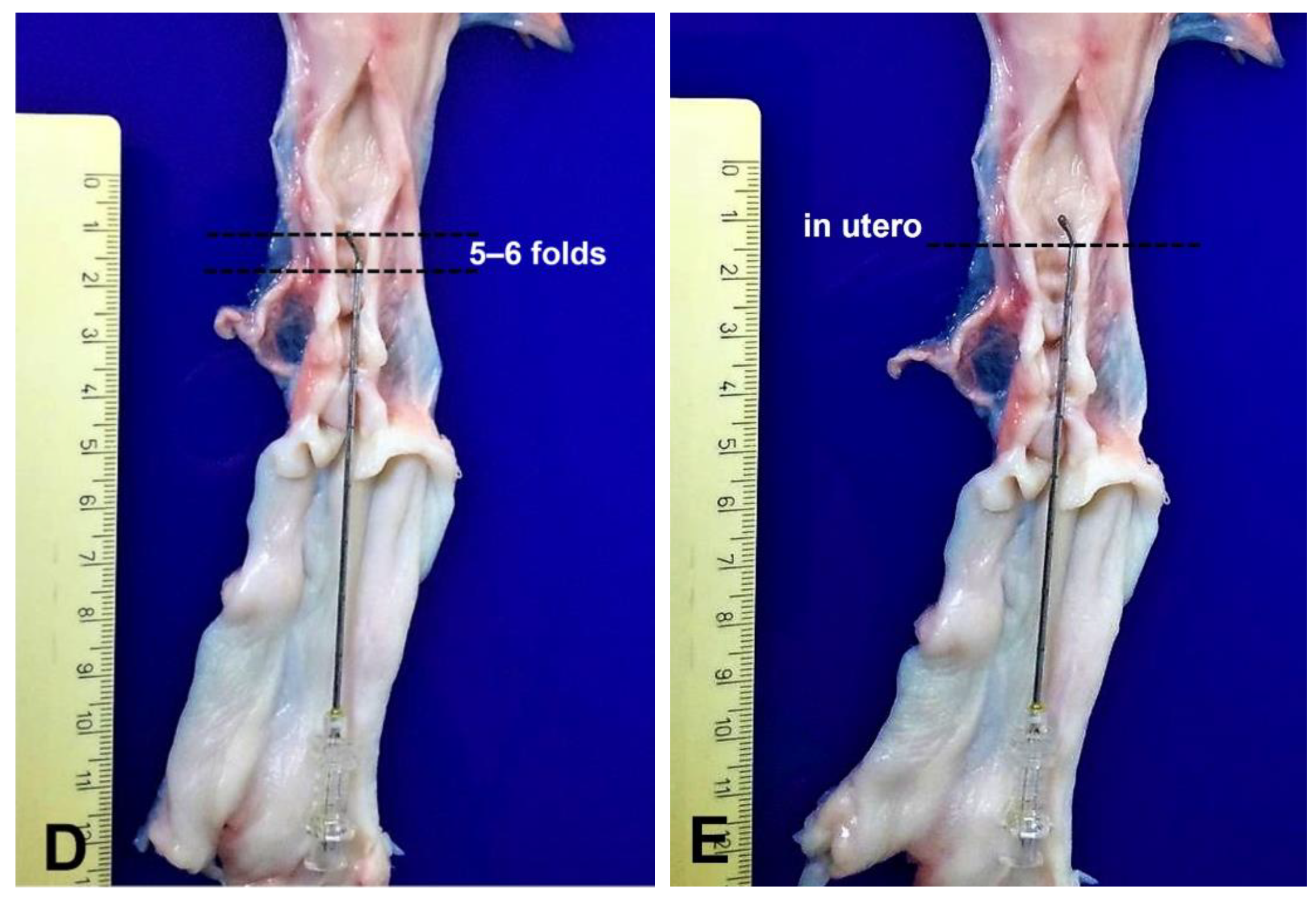
Catheter Design
2.5. Experiment 2
2.6. Ewe Restraint and Cervical Manipulation for TCAI
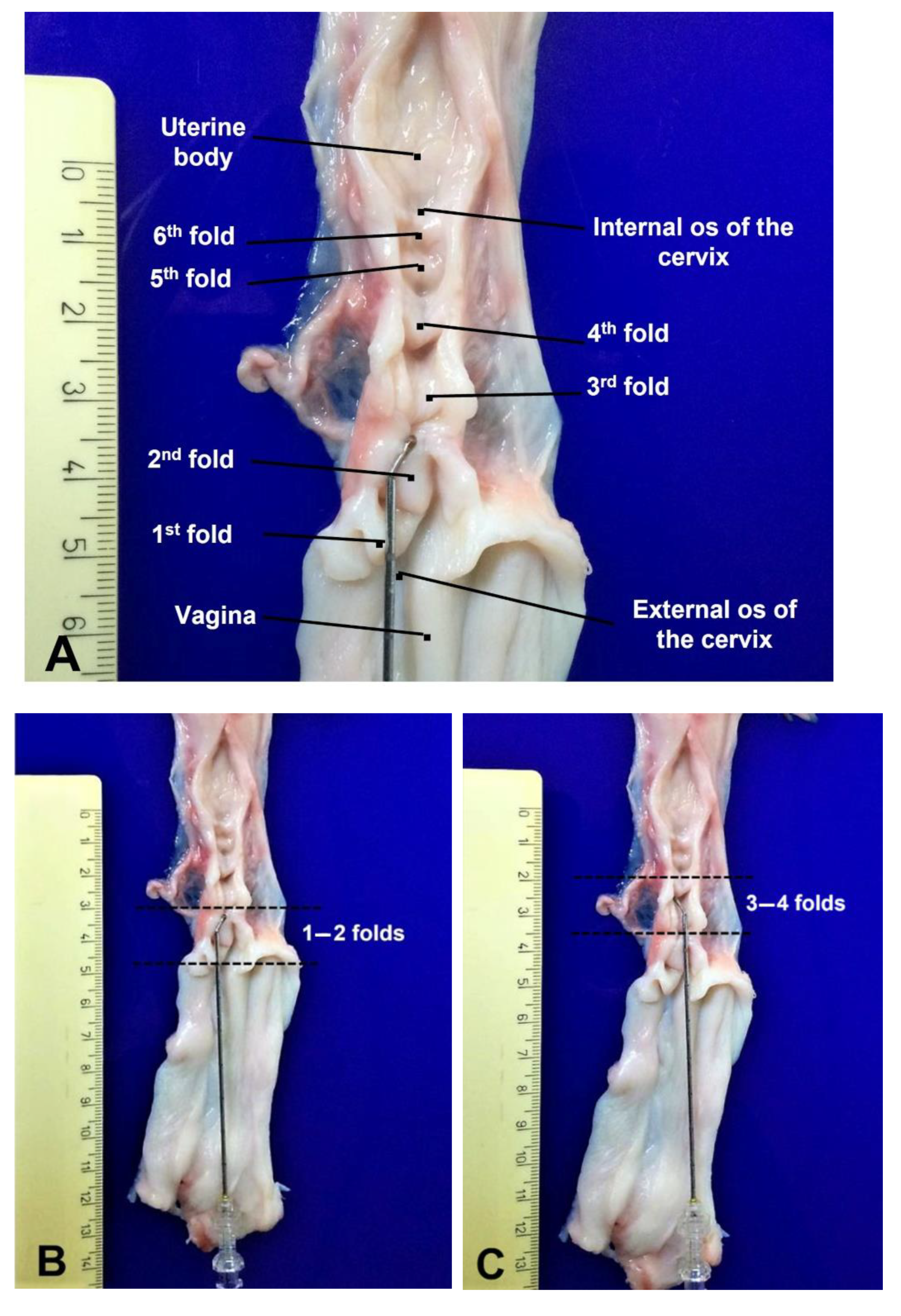
2.7. Data Collection
2.8. Statistical Analyses
3. Results
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kershaw, C.M.; Khalid, M.; McGowan, M.R.; Ingram, K.; Leethongdee, S.; Wax, G.; Scaramuzzi, R.J. The anatomy of the sheep cervix and its influence on the transcervical passage of an inseminating pipette into the uterine lumen. Theriogenology 2005, 64, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Kaabi, M.; Alvarez, M.; Anel, E.; Chamorro, C.A.; Boixo, J.C.; de Paz, P.; Anel, L. Influence of breed and age on morphometry and depth of inseminating catheter penetration in the ewe cervix: A postmortem study. Theriogenology 2006, 66, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Eppleston, J.; Salamon, S.; Moore, N.W.; Evans, G. The depth of cervical insemination and site of intrauterine insemination and their relationship to the fertility of frozen-thawed ram semen. Anim. Reprod. Sci. 1994, 36, 211–225. [Google Scholar] [CrossRef]
- Hiwasa, M.; Kohno, H.; Togari, T.; Okabe, K.; Fukui, Y. Fertility after different artificial insemination methods using a synthetic semen extender in sheep. J. Reprod. Dev. 2009, 55, 50–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Masoudi, R.; Zare Shahneh, A.; Towhidi, A.; Kohram, H.; Akbarisharif, A.; Sharafi, M. Fertility response of artificial insemination methods in sheep with fresh and frozen-thawed semen. Cryobiology 2017, 74, 77–80. [Google Scholar] [CrossRef]
- Richardson, L.; Hanrahan, J.P.; Donovan, A.; Martí, J.I.; Fair, S.; Evans, A.C.O.; Lonergan, P. Effect of site of deposition on the fertility of sheep inseminated with frozen-thawed semen. Anim. Reprod. Sci. 2012, 131, 160–164. [Google Scholar] [CrossRef]
- Windsor, D.P.; Szell, A.Z.; Buschbeck, C.; Edward, A.Y.; Milton, J.T.; Buckrell, B.C. Transcervical artificial insemination of Australian Merino ewes with frozen-thawed semen. Theriogenology 1994, 42, 147–157. [Google Scholar] [CrossRef]
- Fair, S.; Hanrahan, J.P.; O’Meara, C.M.; Duffy, P.; Rizos, D.; Wade, M.; Donovan, A.; Boland, M.P.; Lonergan, P.; Evans, A.C. Differences between Belclare and Suffolk ewes in fertilization rate, embryo quality and accessory sperm number after cervical or laparoscopic artificial insemination. Theriogenology 2005, 63, 1995–2005. [Google Scholar] [CrossRef]
- Pau, S.; Falchi, L.; Ledda, M.; Pivato, I.; Valentino, M.; Bogliolo, L.; Ariu, F.; Zedda, M.T. Reproductive Performance Following Transcervical Insemination with Frozen Thawed Semen in Ewes Submitted to Surgical Incision of Cervical Folds (SICF): Comparison with Laparoscopic Artificial Insemination. Animals 2020, 10, 108. [Google Scholar] [CrossRef]
- Alvarez, M.; Anel-Lopez, L.; Boixo, J.C.; Chamorro, C.; Neila-Montero, M.; Montes-Garrido, R.; de Paz, P.; Anel, L. Current challenges in sheep artificial insemination: A particular insight. Reprod. Domest. Anim. 2019, 54, 32–40. [Google Scholar] [CrossRef]
- Pau, S.; Falchi, L.; Ledda, M.; Bogliolo, L.; Ariu, F.; Zedda, M.T. Surgery on cervical folds for transcervical intrauterine artificial insemination with frozen-thawed semen enhances pregnancy rates in the sheep. Theriogenology 2019, 126, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Taema, M.; La Clanche, S.; Scaramuzzi, R.J. The pattern of cervical penetration and the effect of topical treatment with prostaglandin and/or FSH and oxytocin on the depth of cervical penetration in the ewe during the peri-ovulatory period. Theriogenology 2012, 78, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, R.M.; Sayre, B.L.; Lewis, G.S. Exogenous oxytocin dilates the cervix in ewes. J. Anim. Sci. 1992, 70, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Stellflug, J.N.; Wulster-Radcliffe, M.C.; Hensley, E.L.; Cowardin, E.A.; Seals, R.C.; Lewis, G.S. Oxytocin-induced cervical dilation and cervical manipulation in sheep: Effects on laparoscopic artificial insemination. J. Anim. Sci. 2001, 79, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Wulster-Radcliffe, M.C.; Costine, B.A.; Lewis, G.S. Estradiol-17 beta-oxytocin-induced cervical dilation in sheep: Application to transcervical embryo transfer. J. Anim. Sci. 1999, 77, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Akinbami, M.A.; Meredith, S.; Warren, J.E.; Anthony, R.V.; Day, B.N. Cervical dilation, conception rate, and concentrations of progesterone and estradiol-17B in postpartum ewes treated with porcine relaxin. Theriogenology 1990, 34, 927–940. [Google Scholar] [CrossRef]
- Bartlewski, P.M.; Candappa, I.B. Assessing the usefulness of prostaglandin E2 (Cervidil) for transcervical artificial insemination in ewes. Theriogenology 2015, 84, 1594–1602. [Google Scholar] [CrossRef]
- Leethongdee, S.; Khalid, M.; Bhatti, A.; Ponglowhapan, S.; Kershaw, C.M.; Scaramuzzi, R.J. The effects of the prostaglandin E analogue Misoprostol and follicle-stimulating hormone on cervical penetrability in ewes during the peri-ovulatory period. Theriogenology 2007, 67, 767–777. [Google Scholar] [CrossRef]
- Croy, B.A.; Prudencio, J.; Minhas, K.; Ashkar, A.A.; Galligan, C.; Foster, R.A.; Buckrell, B.; Coomber, B.L. A preliminary study on the usefulness of huIL-8 in cervical relaxation of the ewe for artificial insemination and for embryo transfer. Theriogenology 1999, 52, 271–287. [Google Scholar] [CrossRef]
- Santos, K.C.; Monte, A.P.; Lima, J.T.; Ribeiro, L.A.; Palheta Junior, R.C. Role of NO-cGMP pathway in ovine cervical relaxation induced by Erythroxylum caatingae Plowman. Anim. Reprod. Sci. 2016, 164, 23–30. [Google Scholar] [CrossRef]
- Perry, K.; Haresign, W.; Wathes, D.C.; Khalid, M. Intracervical application of hyaluronan improves cervical relaxation in the ewe. Theriogenology 2010, 74, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, M.C.; Turna, Ö.; Cirit, Ü.; Uçmak, M.; Tek, Ç.; Sabuncu, A.; Bacınoğlu, S. Lambing rates and litter size following carazolol administration prior to insemination in Kivircik ewes. Anim. Reprod. Sci. 2010, 118, 32–36. [Google Scholar] [CrossRef]
- Álvarez, M.; Chamorro, C.A.; Kaabi, M.; Anel-López, L.; Boixo, J.C.; Anel, E.; Anel, L.; de Paz, P. Design and “in vivo” evaluation of two adapted catheters for intrauterine transcervical insemination in sheep. Anim. Reprod. Sci. 2012, 131, 153–159. [Google Scholar] [CrossRef]
- Macías, A.; Ferrer, L.M.; Ramos, J.J.; Lidón, I.; Rebollar, R.; Lacasta, D.; Tejedor, M.T. Technical Note: A new device for cervical insemination of sheep—Design and field test. J. Anim. Sci. 2017, 95, 5263–5269. [Google Scholar] [CrossRef] [PubMed]
- Wulster-Radcliffe, M.C.; Lewis, G.S. Development of a new transcervical artificial insemination method for sheep: Effects of a new transcervical artificial insemination catheter and traversing the cervix on semen quality and fertility. Theriogenology 2002, 58, 1361–1371. [Google Scholar] [CrossRef]
- Halbert, G.W.; Dobson, H.; Walton, J.S.; Buckrell, B.C. A technique for transcervical intrauterine insemination of ewes. Theriogenology 1990, 33, 993–1010. [Google Scholar] [CrossRef]
- Halbert, G.W.; Dobson, H.; Walton, J.S.; Sharpe, P.; Buckrell, B.C. Field evaluation of a technique for transcervical intrauterine insemination of ewes. Theriogenology 1990, 33, 1231–1243. [Google Scholar] [CrossRef]
- Scaramuzzi, R.J.; Martin, G.B. The importance of interactions among nutrition, seasonality and socio-sexual factors in the development of hormone-free methods for controlling fertility. Reprod. Domest. Anim. 2008, 43, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kadirvel, G.; Kumar, S.; Kumaresan, A. Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Anim. Reprod. Sci. 2009, 114, 125–134. [Google Scholar] [CrossRef]
- Abril-Parreño, L.; Krogenæs, A.K.; Byrne, C.J.; Donovan, A.; Stuen, S.; Caldas, E.; Diskin, M.; Druart, X.; Fair, S. Ewe breed differences in cervical anatomy and cervicovaginal mucus properties: An international study. Theriogenology 2021, 160, 18–25. [Google Scholar] [CrossRef]
- El Khalil, K.; Allai, L.; Fatet, A.; Benmoula, A.; Hamidallah, N.; Badi, A.; Moussafir, Z.; Ibnelbachyr, M.; El Amiri, B. Morphometry and depth of inseminating catheter penetration in prolific and non- prolific ewes at different ages: A post mortem study. Anim. Reprod. Sci. 2018, 196, 43–47. [Google Scholar] [CrossRef]
- Rickard, J.P.; Pool, K.R.; Druart, X.; de Graaf, S.P. The fate of spermatozoa in the female reproductive tract: A comparative review. Theriogenology 2019, 137, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Windsor, D.P. Factors influencing the success of transcervical insemination in Merino ewes. Theriogenology 1995, 43, 1009–1018. [Google Scholar] [CrossRef]
- Buckrell, B.C.; Buschbeck, C.; Gartley, C.J.; Kroetsch, T.; McCutcheon, W.; Martin, J.; Penner, W.K.; Walton, J.S. Further development of a transcervical technique for artificial insemination in sheep using previously frozen semen. Theriogenology 1994, 42, 601–611. [Google Scholar] [CrossRef]
- Casali, R.; Pinczak, A.; Cuadro, F.; Guillen-Munoz, J.M.; Mezzalira, A.; Menchaca, A. Semen deposition by cervical, transcervical and intrauterine route for fixed-time artificial insemination (FTAI) in the ewe. Theriogenology 2017, 103, 30–35. [Google Scholar] [CrossRef] [PubMed]
| Insemination Catheter Type | Ewes | PR (%) | LR (%) |
|---|---|---|---|
| 8.0 mm | 12 | 1 (8.3) a | 1 (8.3) a |
| 5.0 mm | 12 | 7 (58.3) b | 7 (58.3) b |
| 3.5 mm | 12 | 2 (16.6) ab | 2 (16.6) ab |
| Total | 36 | 10 (27.7) | 10 (27.7) |
| Insemination Catheter Type | Site of Semen Deposition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–2 Folds | 3–4 Folds | 5–6 Folds | In Utero | |||||||||
| Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | |
| 8.0 mm | 2 | 0 (0) | 0 (0) | 5 a | 1 (20.0) | 1(20.0) | 4 | 0 (0) | 0 (0) | 1 a | 0 (0) a | 0 (0) a |
| 5.0 mm | 0 | - | - | 0 b | - | - | 3 | 0 (0) | 0 (0) | 9 b | 7 (77.8) b | 7 (77.8) b |
| 3.5 mm | 0 | - | - | 4 a | 0 (0) | 0 (0) | 5 | 1 (20.0) | 1(20.0) | 3 a | 1 (33.3) a | 1 (33.3) a |
| Overall | 2 | 0 (0) x | 0 (0) x | 9 | 1 (11.1) x | 1 (11.1) x | 13 | 1 (7.7) x | 1 (7.7) x | 12 | 8 (66.7) y | 8 (66.7) y |
| Insemination Catheter Type | Time to Reach the Cervical Lumen | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <10 s | 10–30 s | 30–60 s | >60 s | |||||||||
| Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | |
| 8.0 mm | 0 a | 0 (0) | 0 (0) | 1 | 0 (0) | 0 (0) | 0 | 0 (0) | 0 (0) | 11 a | 1 (9.1) | 1 (9.1) |
| 5.0 mm | 4 b | 3 (75.0) | 3 (75.0) | 3 | 3 (100) | 3 (100) | 2 | 1 (50.0) | 1 (50.0) | 3 b | 0 (0) | 0 (0) |
| 3.5 mm | 1 ab | 1 (100) | 1 (100) | 0 | 0 (0) | 0 (0) | 2 | 0 (0) | 0 (0) | 9 a | 1 (11.1) | 1 (11.1) |
| Overall | 5 | 4 (80.0) x | 4 (80) x | 4 | 3 (75.0) xy | 3 (75.0) xy | 4 | 1 (25.0) yz | 1 (25.0) yz | 23 | 2 (8.7) z | 2 (8.7) z |
| Transcervical Artificial Insemination Method | Ewes | PR (%) | LR (%) |
|---|---|---|---|
| Catheter 5.0 mm tip | 30 | 19 (63.3) | 16 (53.3) |
| Surgical incision of cervical folds | 29 | 23 (79.3) | 21 (72.4) |
| Overall | 59 | 42 (71.2) | 37 (62.7) |
| Transcervical Artificial Insemination Method | Time to Reach The Cervical Lumen | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <10 s | 10–30 s | 30–60 s | >60 s | |||||||||
| Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | |
| Catheter 5.0 mm tip | 11 | 8 (72.7) | 6 (54.5) | 11 | 11 (100) | 10 (90.9) | 4 | 0 (0) | 0 (0) | 4 | 0 (0) | 0 (0) |
| Surgical incision of cervical folds | 17 | 14 (82.4) | 14 (82.4) | 6 | 6 (100) | 5 (83.3) | 5 | 3 (60.0) | 1 (20.0) | 1 | 1 (100) | 1 (100) |
| Overall | 28 | 22 (78.6) | 20 (71.4) | 17 | 17 (100) | 15 (88.2) | 9 | 3 (33.3) | 1 (11.1) | 5 | 1 (20.0) | 1 (20.0) |
| Transcervical Artificial Insemination Method | Site of Semen Deposition | |||||
|---|---|---|---|---|---|---|
| In Cervix | In Utero | |||||
| Ewes | PR (%) | LR (%) | Ewes | PR (%) | LR (%) | |
| Catheter 5.0 mm tip | 4 | 0 (0) | 0 (0) | 26 | 19 (73.1) | 16 (61.5) |
| Surgical incision of cervical folds | 4 | 1 (25.0) | 1 (25.0) | 25 | 22 (84.6) | 20 (80.0) |
| Total | 8 | 1 (12.5) | 1 (12.5) | 51 | 41 (80.4) | 36 (70.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falchi, L.; Zedda, M.T.; Pau, S.; Ledda, M.; Melosu, V.; Rassu, S.P.G. The Design of a New Catheter for Transcervical Artificial Insemination in Ewes. Animals 2021, 11, 3348. https://doi.org/10.3390/ani11123348
Falchi L, Zedda MT, Pau S, Ledda M, Melosu V, Rassu SPG. The Design of a New Catheter for Transcervical Artificial Insemination in Ewes. Animals. 2021; 11(12):3348. https://doi.org/10.3390/ani11123348
Chicago/Turabian StyleFalchi, Laura, Maria Teresa Zedda, Salvatore Pau, Mauro Ledda, Valentino Melosu, and Salvatore Pier Giacomo Rassu. 2021. "The Design of a New Catheter for Transcervical Artificial Insemination in Ewes" Animals 11, no. 12: 3348. https://doi.org/10.3390/ani11123348
APA StyleFalchi, L., Zedda, M. T., Pau, S., Ledda, M., Melosu, V., & Rassu, S. P. G. (2021). The Design of a New Catheter for Transcervical Artificial Insemination in Ewes. Animals, 11(12), 3348. https://doi.org/10.3390/ani11123348





