Differential Metabolic and Transcriptional Responses of Gilthead Seabream (Sparus aurata) Administered with Cortisol or Cortisol-BSA
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurement of Plasma Cortisol and Tissue Metabolites
2.3. RNA Extraction and cDNA Synthesis
2.4. Real Time-PCR
2.5. Statistical Analysis
3. Results
3.1. Plasma Indicators in S. aurata Administered with Cortisol or Cortisol-BSA
3.2. Energetic Metabolites in Liver and Skeletal Muscle of S. aurata Administered with Cortisol or Cortisol-BSA
3.3. Corticosteroid Receptor-Related Genes Expression in Liver and Skeletal Muscle of S. aurata Administered with Cortisol or Cortisol-BSA
3.4. Glucose Metabolism—And Atrophy-Related Genes in S. aurata Administered with Cortisol or Cortisol-BSA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Magalhães, C.S.F.R.; Cerqueira, M.A.C.; Schrama, D.; Moreira, M.J.V.; Boonanuntanasarn, S.; Rodrigues, P.M.L. A Proteomics and other Omics approach in the context of farmed fish welfare and biomarker discovery. Rev. Aquac. 2020, 12, 122–144. [Google Scholar] [CrossRef]
- De Mercado, E.; Larrán, A.M.; Pinedo, J.; Tomás-Almenar, C. Skin mucous: A new approach to assess stress in rainbow trout. Aquaculture 2018, 484, 90–97. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Mancera, J.M.; Prunet, P.; Magnoni, L.J. Welfare and stressors in fish: Challenges facing aquaculture. Front. Physiol. 2020, 11, 162. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Ruiz-Jarabo, I. Physiology: An Important Tool to Assess the Welfare of Aquatic Animals. Biology 2021, 10, 61. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Ellis, T.; Yildiz, H.Y.; López-Olmeda, J.; Spedicato, M.T.; Tort, L.; Øverli, Ø.; Martins, C.I. Cortisol and finfish welfare. Fish. Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Daskalova, A. Farmed fish welfare: Stress, post-mortem muscle metabolism, and stress-related meat quality changes. Int. Aquat. Res. 2019, 11, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Madsen, S.S. Cortisol treatment improves the development of hypoosmoregulatory mechanisms in the euryhaline rainbow trout, Salmo gairdneri. Fish Physiol. Biochem. 1990, 8, 45–52. [Google Scholar] [CrossRef]
- Tripathi, G.; Verma, P. Pathway-specific response to cortisol in the metabolism of catfish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 463–471. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Cádiz, L.; Gozdowska, M.; Kulczykowska, E.; Martínez-Rodríguez, G.; Mancera, J.M. Arginine vasotocin and cortisol co-regulate vasotocinergic, isotocinergic, stress, and thyroid pathways in the gilthead sea bream (Sparus aurata). Front. Physiol. 2019, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Laiz-Carrión, R.; Sangiao-Alvarellos, S.; Guzmán, J.M.; Del Río, M.P.M.; Míguez, J.M.; Soengas, J.L.; Mancera, J.M. Energy metabolism in fish tissues related to osmoregulation and cortisol action. Fish Physiol. Biochem. 2002, 27, 179–188. [Google Scholar] [CrossRef]
- Teles, M.; Boltaña, S.; Reyes-López, F.; Santos, M.A.; Mackenzie, S.; Tort, L. Effects of chronic cortisol administration on global expression of GR and the liver transcriptome in Sparus aurata. Mar. Biotechnol. 2013, 15, 104–114. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Gorissen, M.; Mancera, J.M.; Ruiz-Jarabo, I. What can we learn from glucocorticoid administration in fish? Effects of cortisol and dexamethasone on intermediary metabolism of gilthead seabream (Sparus aurata L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 231, 1–10. [Google Scholar] [CrossRef]
- Aluru, N.; Vijayan, M.M. Stress transcriptomics in fish: A role for genomic cortisol signaling. Gen. Comp. Endocrinol. 2009, 164, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Aedo, J.E.; Ruiz-Jarabo, I.; Martínez-Rodríguez, G.; Boltaña, S.; Molina, A.; Valdés, J.A.; Mancera, J.M. Contribution of non-canonical cortisol actions in the early modulation of glucose metabolism of gilthead sea bream (Sparus aurata). Front. Endocrinol. 2019, 10, 779. [Google Scholar] [CrossRef]
- Aedo, J.E.; Zuloaga, R.; Bastías-Molina, M.; Meneses, C.; Boltaña, S.; Molina, A.; Valdés, J.A. Early transcriptomic responses associated with the membrane-initiated action of cortisol in the skeletal muscle of rainbow trout (Oncorhynchus mykiss). Physiol. Genom. 2019, 51, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Thraya, M.; Vijayan, M.M. Nongenomic cortisol signaling in fish. Gen. Comp. Endocrinol. 2018, 265, 121–127. [Google Scholar] [CrossRef]
- Estensoro, I.; Ballester-Lozano, G.; Benedito-Palos, L.; Grammes, F.; Martos-Sitcha, J.A.; Mydland, L.T.; Calduch-Giner, J.A.; Fuentes, J.; Karalazos, V.; Ortiz, Á.; et al. Dietary butyrate helps to restore the intestinal status of a marine teleost (Sparus aurata) fed extreme diets low in fish meal and fish oil. PLoS ONE 2016, 11, e0166564. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D.; Decker, K. Glycogen determination with amyloglucosidase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 127–1131. [Google Scholar]
- Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Arjona, F.J.; Laiz-Carrión, R.; Flik, G.; Klaren, P.H.; Mancera, J.M. Energy metabolism of hyperthyroid gilthead sea bream Sparus aurata L. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 191, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yúfera, M.; Halm, S.; Beltran, S.; Fusté, B.; Planas, J.V.; Martínez-Rodríguez, G. Transcriptomic characterization of the larval stage in gilthead seabream (Sparus aurata) by 454 pyrosequencing. Mar. Biotechnol. 2012, 14, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Tsalafouta, A.; Sarropoulou, E.; Papandroulakis, N.; Pavlidis, M. Characterization and expression dynamics of key genes involved in the gilthead sea bream (Sparus aurata) cortisol stress response during early ontogeny. Mar. Biotechnol. 2018, 20, 611–622. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Galhardo, L.; Oliveira, R.F. Psychological stress and welfare in fish. Annu. Rev. Biomed. Sci. 2009, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Eliason, E.J.; Zolderdo, A.J.; Lapointe, D.; Best, C.; Gilmour, K.M.; Cooke, S.J. Cortisol modulates metabolism and energy mobilization in wild-caught pumpkinseed (Lepomis gibbosus). Fish Physiol. Biochem. 2019, 45, 1813–1828. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; Björnsson, B.T.; McCormick, S.D. Effects of long-term cortisol treatment on growth and osmoregulation of Atlantic salmon and brook trout. Gen. Comp. Endocrinol. 2021, 308, 113769. [Google Scholar] [CrossRef]
- Roy, B.; Rai, U. Genomic and non-genomic effect of cortisol on phagocytosis in freshwater teleost Channa punctatus: An in vitro study. Steroids 2009, 74, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, M.B.; Aedo, J.E.; Zuloaga, R.; Valenzuela, C.; Molina, A.; Valdés, J.A. Cortisol induces reactive oxygen species through a membrane glucocorticoid receptor in rainbow trout myotubes. J. Cell. Biochem. 2017, 118, 718–725. [Google Scholar] [CrossRef]
- Weiss, G.L.; Rainville, J.R.; Zhao, Q.; Tasker, J.G. Purity and stability of the membrane-limited glucocorticoid receptor agonist dexamethasone-BSA. Steroids 2019, 142, 2–5. [Google Scholar] [CrossRef]
- Laiz-carrión, R.; Martín Del Río, M.P.; Miguez, J.M.; Mancera, J.M.; Soengas, J.L. Influence of cortisol on osmoregulation and energy metabolism in gilthead seabream Sparus aurata. J. Exp. Zool. A Comp. Exp. Biol. 2003, 298, 105–118. [Google Scholar] [CrossRef]
- Arends, R.J.; Mancera, J.M.; Munoz, J.L.; Bonga, S.W.; Flik, G. The stress response of the gilthead sea bream (Sparus aurata L.) to air exposure and confinement. J. Endocrinol. 1999, 163, 149. [Google Scholar] [CrossRef]
- Calduch-Giner, J.A.; Davey, G.; Saera-Vila, A.; Houeix, B.; Talbot, A.; Prunet, P.; Cairns, M.T.; Pérez-Sánchez, J. Use of microarray technology to assess the time course of liver stress response after confinement exposure in gilthead sea bream (Sparus aurata L.). BMC Genom. 2010, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Jerez-Cepa, I.; Fernández-Castro, M.; Alameda-López, M.; González-Manzano, G.; Mancera, J.M.; Ruiz-Jarabo, I. Transport and recovery of gilthead seabream (Sparus aurata L.) sedated with AQUI-S® and etomidate: Effects on intermediary metabolism and osmoregulation. Aquaculture 2021, 530, 735745. [Google Scholar] [CrossRef]
- Montero, D.; Izquierdo, M.S.; Tort, L.; Robaina, L.; Vergara, J.M. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol. Biochem. 1999, 20, 53–60. [Google Scholar] [CrossRef]
- Aluru, N.; Vijayan, M.M. Hepatic transcriptome response to glucocorticoid receptor activation in rainbow trout. Physiol. Genom. 2007, 31, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Vijayan, M.M. Mechanisms of cortisol action in fish hepatocytes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 199, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Prunet, P.; Sturm, A.; Milla, S. Multiple corticosteroid receptors in fish: From old ideas to new concepts. Gen. Comp. Endocrinol. 2006, 147, 17–23. [Google Scholar] [CrossRef]
- Teles, M.; Tridico, R.; Callol, A.; Fierro-Castro, C.; Tort, L. Differential expression of the corticosteroid receptors GR1, GR2 and MR in rainbow trout organs with slow release cortisol implants. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Sakamoto, T. The role of ‘mineralocorticoids’ in teleost fish: Relative importance of glucocorticoid signaling in the osmoregulation and ‘central’ actions of mineralocorticoid receptor. Gen. Comp. Endocrinol. 2013, 181, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Liew, H.J.; Fazio, A.; Faggio, C.; Blust, R.; De Boeck, G. Cortisol affects metabolic and ionoregulatory responses to a different extent depending on feeding ration in common carp, Cyprinus carpio. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 189, 45–57. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Zuloaga, R.; Mercado, L.; Einarsdottir, I.E.; Björnsson, B.T.; Valdés, J.A.; Molina, A. Chronic stress inhibits growth and induces proteolytic mechanisms through two different nonoverlapping pathways in the skeletal muscle of a teleost fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R102–R113. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, B.M.; Evenhuis, J.P. Molecular characterization of atrogin-1/F-box protein-32 (FBXO32) and F-box protein-25 (FBXO25) in rainbow trout (Oncorhynchus mykiss): Expression across tissues in response to feed deprivation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Vélez, E.J.; Azizi, S.; Lutfi, E.; Capilla, E.; Moya, A.; Navarro, I.; Fernández-Borràs, J.; Blasco, J.; Gutiérrez, J. Moderate and sustained exercise modulates muscle proteolytic and myogenic markers in gilthead sea bream (Sparus aurata). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R643–R653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmerón, C.; Navarro, I.; Johnston, I.A.; Gutiérrez, J.; Capilla, E. Characterization and expression analysis of cathepsins and ubiquitin-proteasome genes in gilthead sea bream (Sparus aurata) skeletal muscle. BMC Res. Notes 2015, 8, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aravena-Canales, D.; Aedo, J.E.; Molina, A.; Valdés, J.A. Regulation of the early expression of MAFbx/atrogin-1 and MuRF1 through membrane-initiated cortisol action in the skeletal muscle of rainbow trout. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 253, 110565. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.; Akbarzadeh, A.; Mehrzad, J.; Noori, A.; Harsij, M. Effect of stocking density on growth performance, plasma biochemistry and muscle gene expression in rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 498, 271–278. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Ponce, C.; Zuloaga, R.; González, P.; Avendaño-Herrera, R.; Valdés, J.A.; Molina, A. Effects of crowding on the three main proteolytic mechanisms of skeletal muscle in rainbow trout (Oncorhynchus mykiss). BMC Vet. Res. 2020, 16, 294. [Google Scholar] [CrossRef]
- Wiseman, S.; Osachoff, H.; Bassett, E.; Malhotra, J.; Bruno, J.; Vanaggelen, G.; Mommsen, T.P.; Vijayan, M.M. Gene expression pattern in the liver during recovery from an acute stressor in rainbow trout. Comp. Biochem. Physiol. Part D Genom. Proteom. 2007, 2, 234–244. [Google Scholar] [CrossRef]
- Momoda, T.S.; Schwindt, A.R.; Feist, G.W.; Gerwick, L.; Bayne, C.J.; Schreck, C.B. Gene expression in the liver of rainbow trout, Oncorhynchus mykiss, during the stress response. Comp. Biochem. Physiol. Part D Genom. Proteom. 2007, 2, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W. The hypoxia signaling pathway and hypoxic adaptation in fishes. Sci. China Life. Sci. 2015, 58, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khansari, A.R.; Balasch, J.C.; Vallejos-Vidal, E.; Teles, M.; Fierro-Castro, C.; Tort, L.; Reyes-López, F.E. Comparative study of stress and immune-related transcript outcomes triggered by Vibrio anguillarum bacterin and air exposure stress in liver and spleen of gilthead seabream (Sparus aurata), zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 86, 436–448. [Google Scholar] [CrossRef] [PubMed]
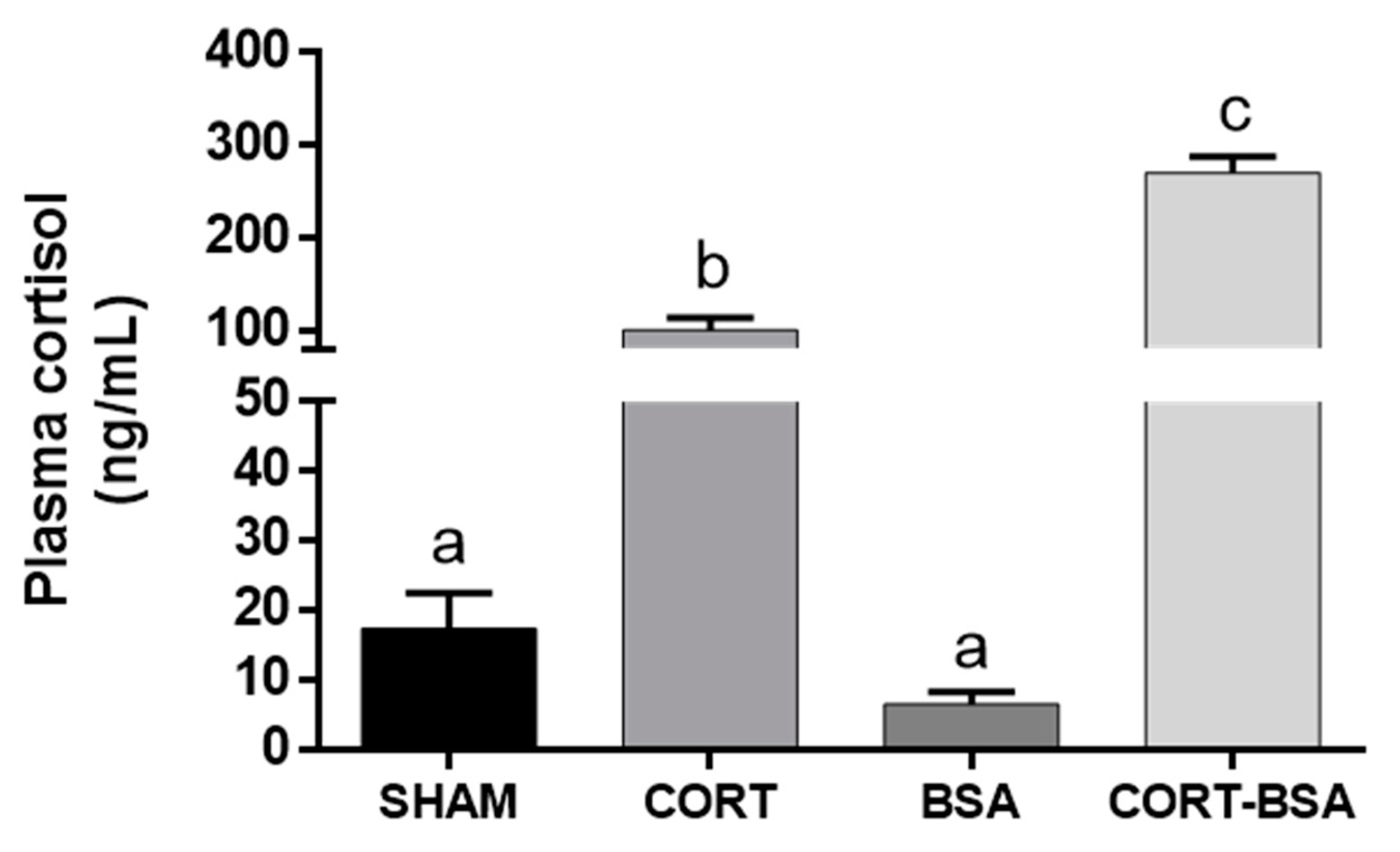
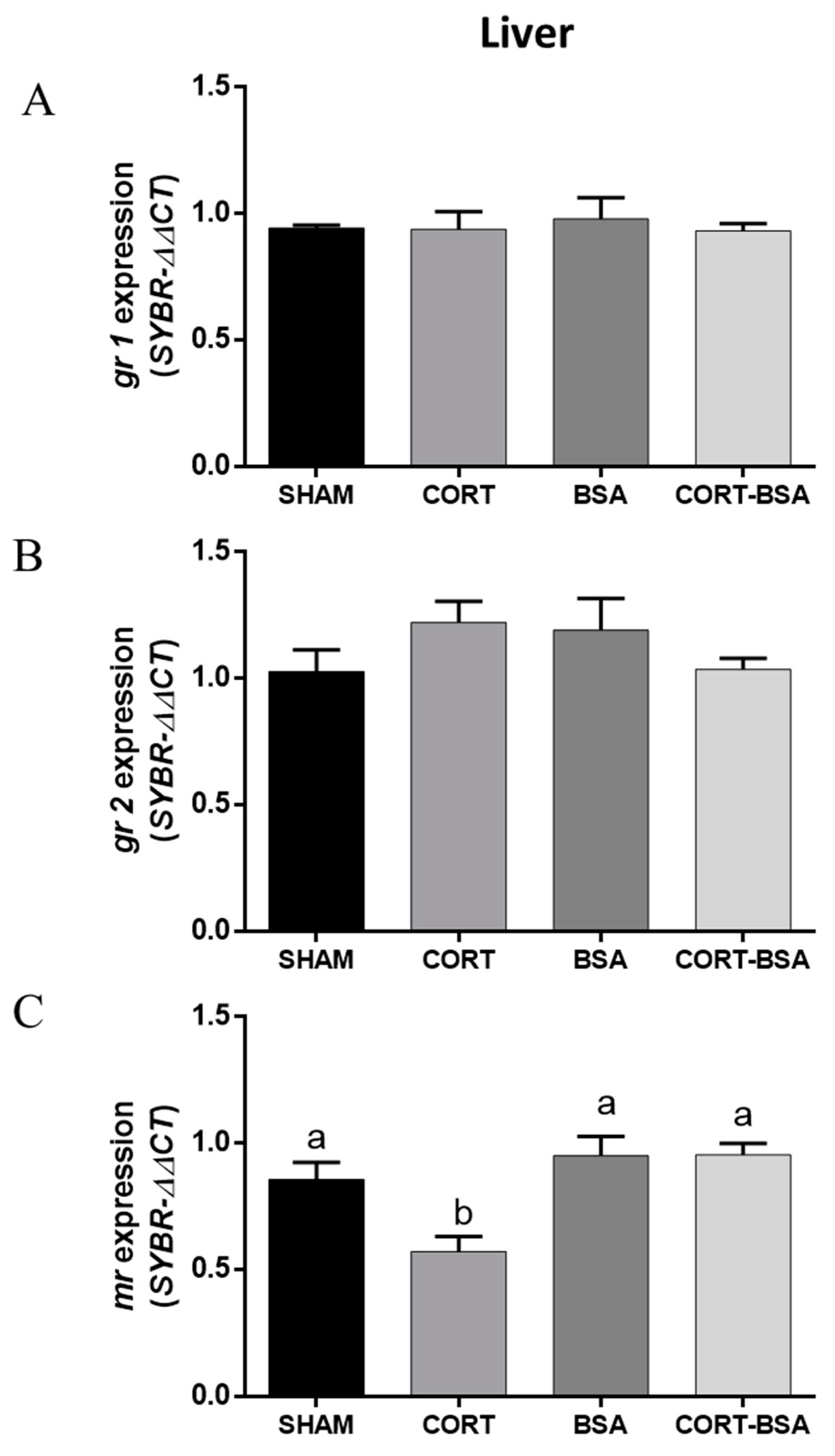

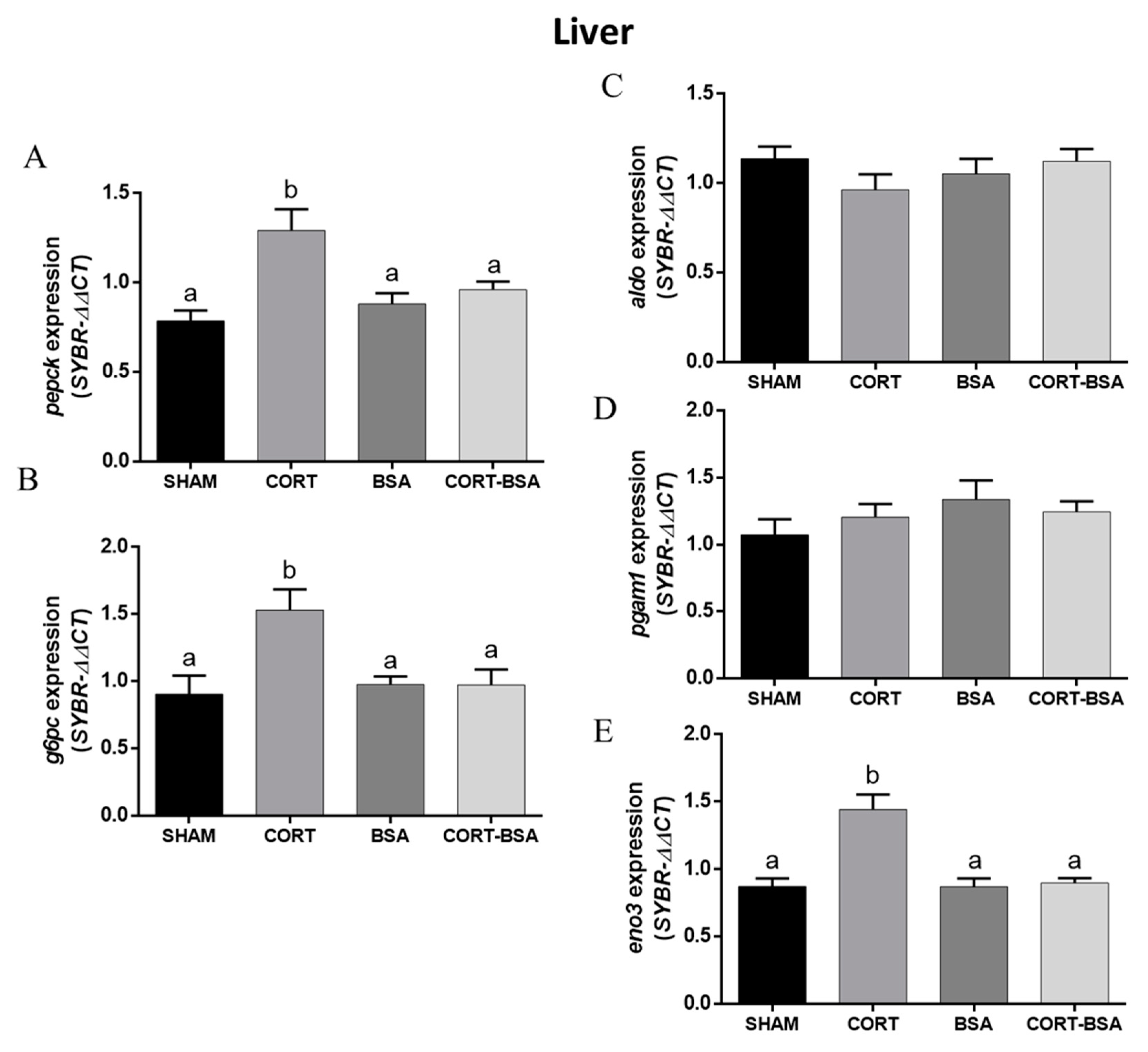


| Parameter | SHAM | CORT | BSA | CORT-BSA |
|---|---|---|---|---|
| Glucose (mmol/L) | 4.07 ± 0.19 a | 6.95 ± 0.39 b | 4.07 ± 0.25 a | 3.76 ± 0.16 a |
| Triacylglycerols (mg/dL) | 92.64 ± 14.12 a | 257.39 ± 24.64 b | 66.69 ± 2.76 a | 64.52 ± 6.69 a |
| Lactate (mmol/L) | 2.11 ± 0.28 | 2.58 ± 0.24 | 1.60 ± 0.26 | 1.66 ± 0.23 |
| Protein (mg/mL) | 31.76 ± 1.27 | 34.76 ± 1.16 | 28.64 ± 2.11 | 30.39 ± 0.91 |
| Parameter | SHAM | CORT | BSA | CORT-BSA |
|---|---|---|---|---|
| Glucose (mg/g) | 1.05 ± 0.17 | 1.16 ± 0.16 | 1.05 ± 0.03 | 1.16 ± 0.07 |
| Triacylglycerols (mg/g) | 0.14 ± 0.02 | 0.27 ± 0.08 | 0.12 ± 0.02 | 0.12 ± 0.01 |
| Lactate (mg/g) | 0.011 ± 0.005 | 0.014 ± 0.004 | 0.013 ± 0.006 | 0.009 ± 0.002 |
| Glycogen (mg/g) | 0.27 ± 0.11 a | 0.79 ± 0.09 b | 0.20 ± 0.07 a | 0.23 ± 0.06 a |
| Parameter | SHAM | CORT | BSA | CORT-BSA |
|---|---|---|---|---|
| Glucose (mg/g) | 2.16 ± 0.14 | 2.18 ± 0.08 | 1.94 ± 0.03 | 2.05 ± 0.09 |
| Triacylglycerols (mg/g) | 1.29 ± 0.54 | 2.94 ± 1.17 | 0.45 ± 0.15 | 1.17 ± 0.34 |
| Lactate (mg/g) | 0.29 ± 0.02 | 0.34 ± 0.03 | 0.24 ± 0.02 | 0.27 ± 0.02 |
| Glycogen (mg/g) | 0.66 ± 0.08 | 0.97 ± 0.12 | 0.33 ± 0.06 | 0.79 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aedo, J.; Aravena-Canales, D.; Ruiz-Jarabo, I.; Oyarzún, R.; Molina, A.; Martínez-Rodríguez, G.; Valdés, J.A.; Mancera, J.M. Differential Metabolic and Transcriptional Responses of Gilthead Seabream (Sparus aurata) Administered with Cortisol or Cortisol-BSA. Animals 2021, 11, 3310. https://doi.org/10.3390/ani11113310
Aedo J, Aravena-Canales D, Ruiz-Jarabo I, Oyarzún R, Molina A, Martínez-Rodríguez G, Valdés JA, Mancera JM. Differential Metabolic and Transcriptional Responses of Gilthead Seabream (Sparus aurata) Administered with Cortisol or Cortisol-BSA. Animals. 2021; 11(11):3310. https://doi.org/10.3390/ani11113310
Chicago/Turabian StyleAedo, Jorge, Daniela Aravena-Canales, Ignacio Ruiz-Jarabo, Ricardo Oyarzún, Alfredo Molina, Gonzalo Martínez-Rodríguez, Juan Antonio Valdés, and Juan Miguel Mancera. 2021. "Differential Metabolic and Transcriptional Responses of Gilthead Seabream (Sparus aurata) Administered with Cortisol or Cortisol-BSA" Animals 11, no. 11: 3310. https://doi.org/10.3390/ani11113310
APA StyleAedo, J., Aravena-Canales, D., Ruiz-Jarabo, I., Oyarzún, R., Molina, A., Martínez-Rodríguez, G., Valdés, J. A., & Mancera, J. M. (2021). Differential Metabolic and Transcriptional Responses of Gilthead Seabream (Sparus aurata) Administered with Cortisol or Cortisol-BSA. Animals, 11(11), 3310. https://doi.org/10.3390/ani11113310









