Staphylococcus-Induced Bacteriospermia In Vitro: Consequences on the Bovine Spermatozoa Quality, Extracellular Calcium and Magnesium Content
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Collection and Gradient Separation
2.2. Study Design
2.3. Sperm Motility Analysis
2.4. Plasma Membrane Integrity
2.5. Mitochondrial Membrane Potential Assay
2.6. Quantification of Reactive Oxygen Species
2.7. Chromatin-Dispersion Test
2.8. Extracellular Concentration of Magnesium
2.9. Extracellular Concentration of Calcium
2.10. Statistical Analysis
3. Results
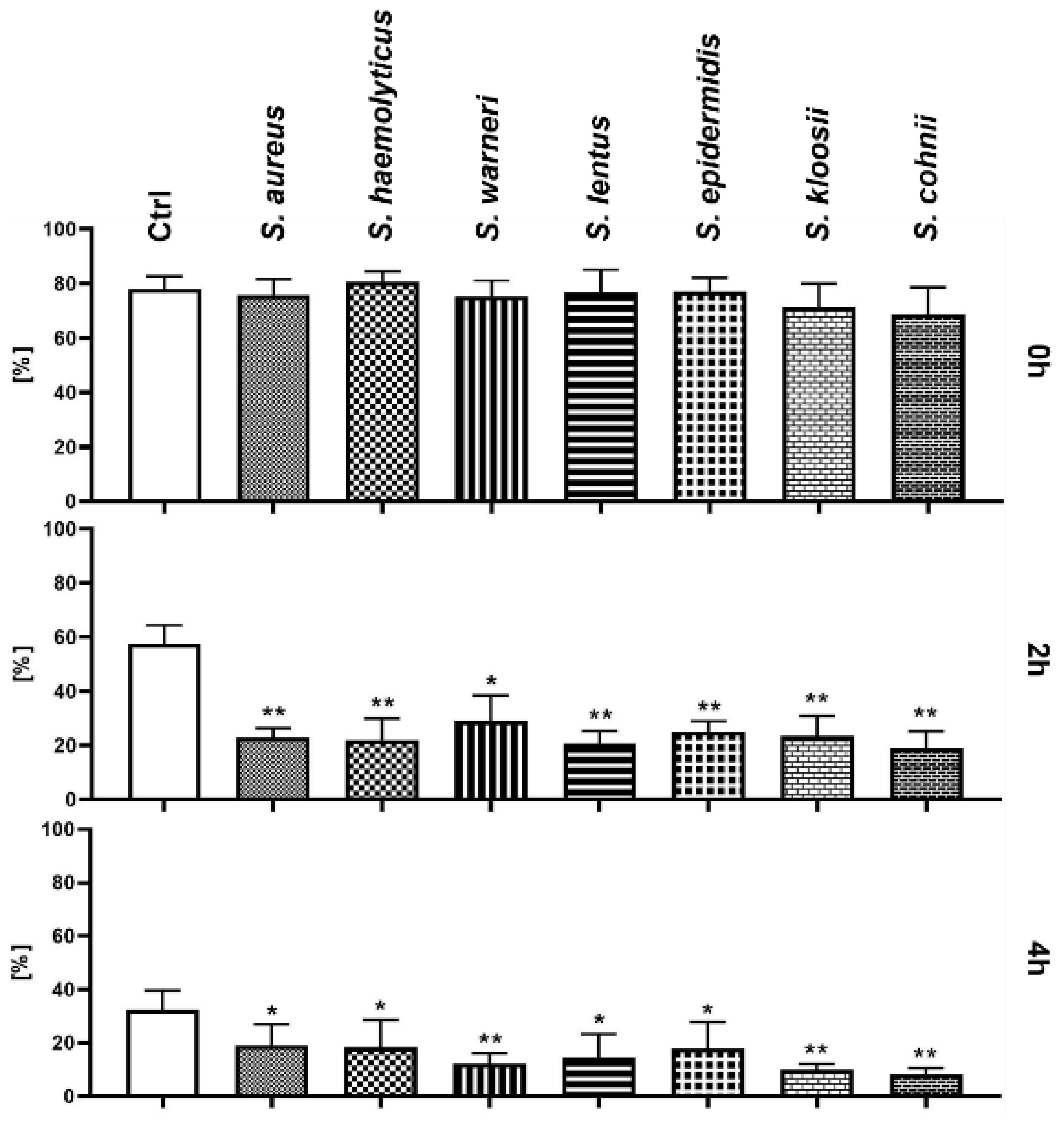
3.1. Effect of Staphylococcus-Induced Bacteriospermia on Total Sperm Motility
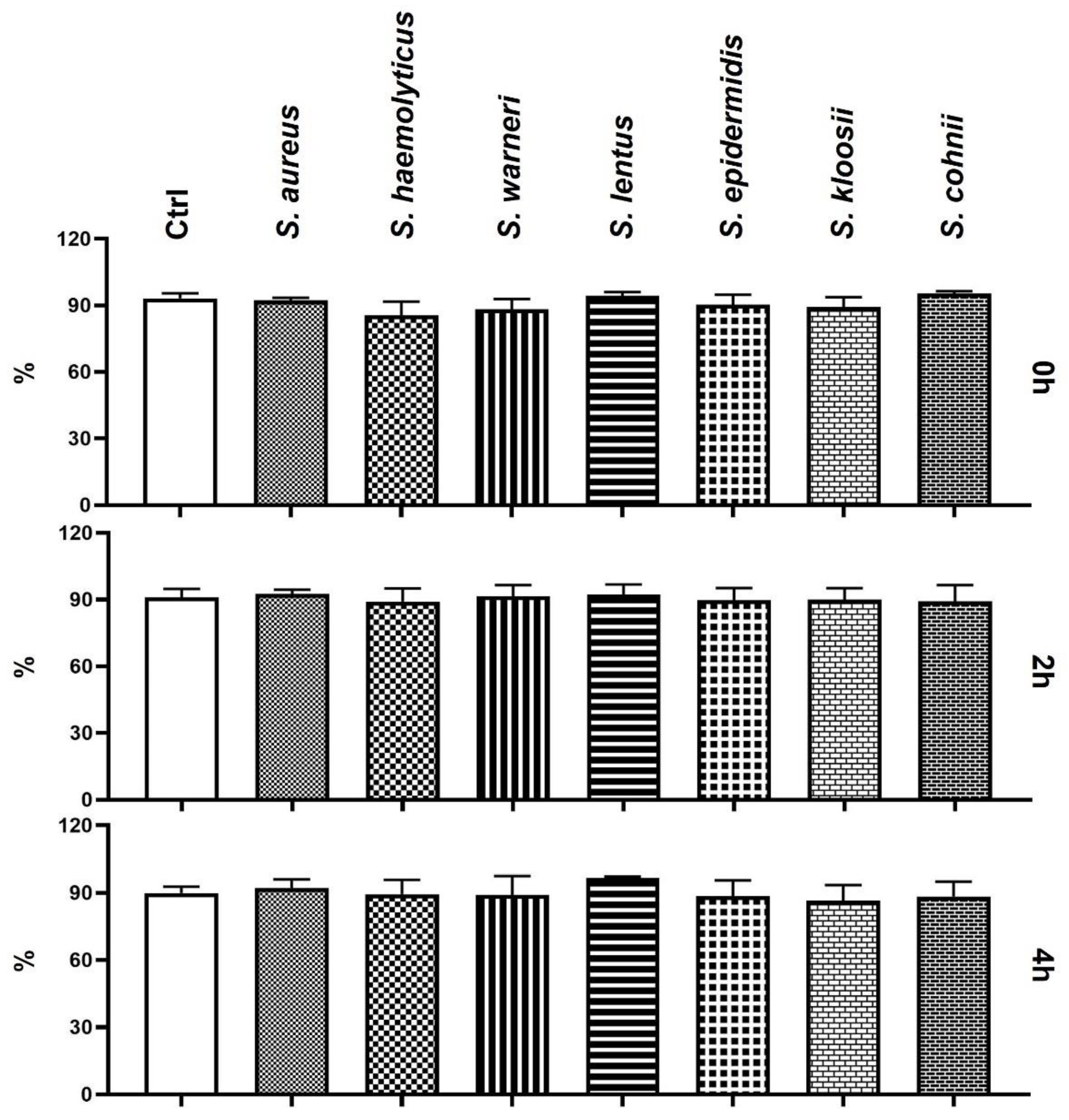
3.2. Effect of Staphylococcus-Induced Bacteriospermia on Plasma Membrane Integrity
3.3. Effect of Staphylococcus-Induced Bacteriospermia on Sperm Mitochondrial Membrane Potential
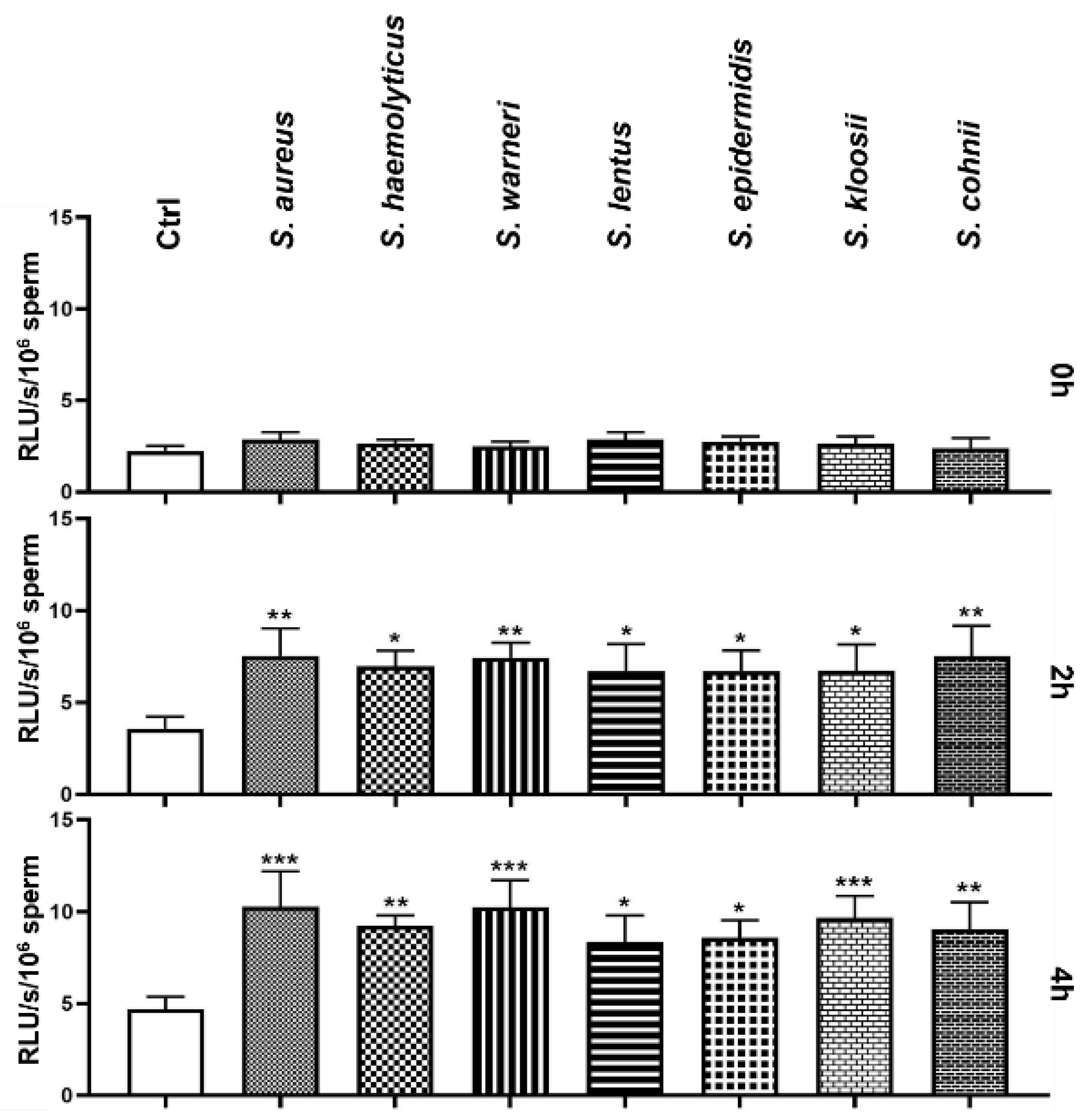
3.4. Effect of Staphylococcus-Induced Bacteriospermia on Global Reactive Oxygen Species Production
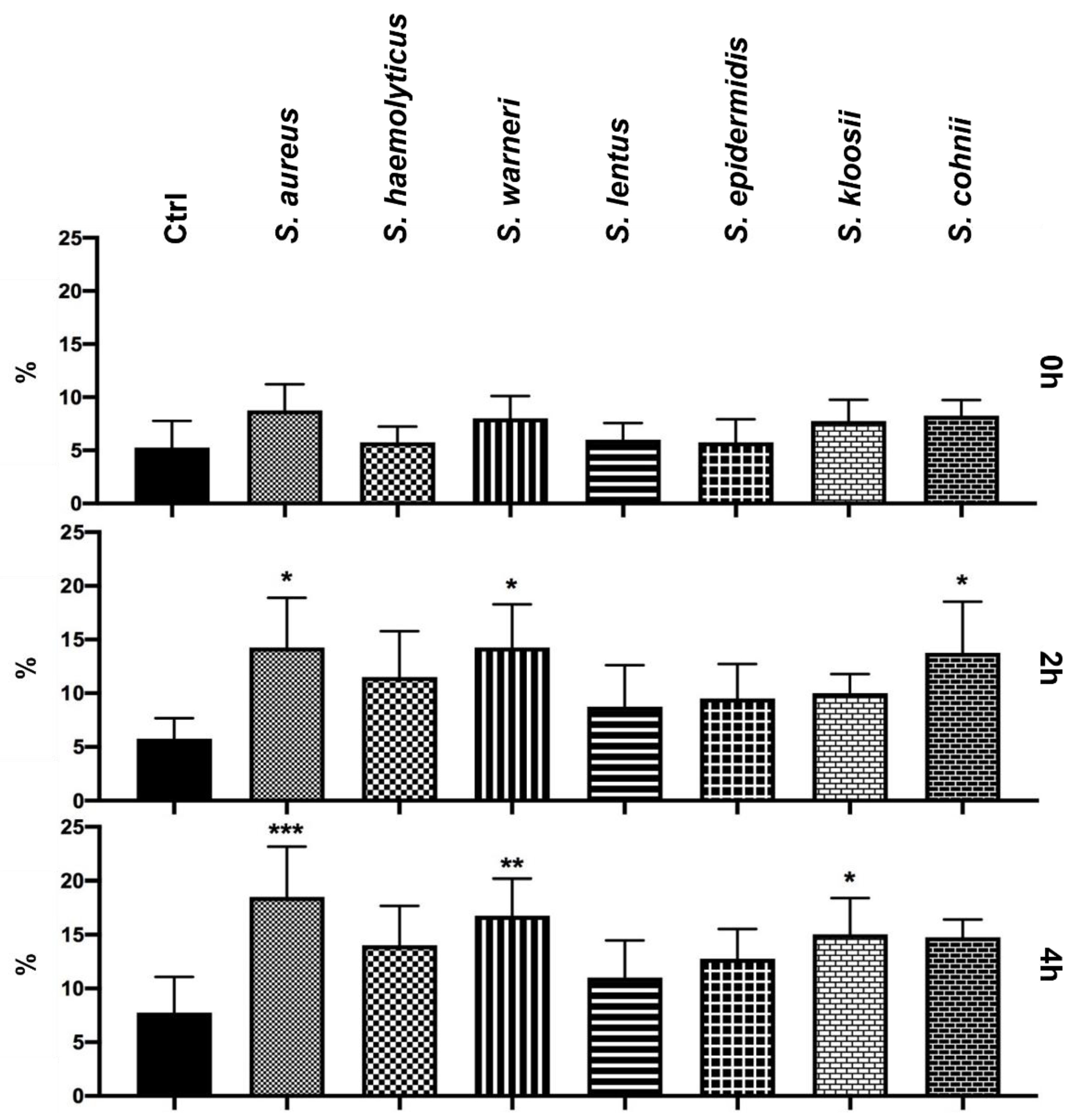
3.5. Effect of Staphylococcus-Induced Bacteriospermia on the DNA Fragmentation in Spermatozoa
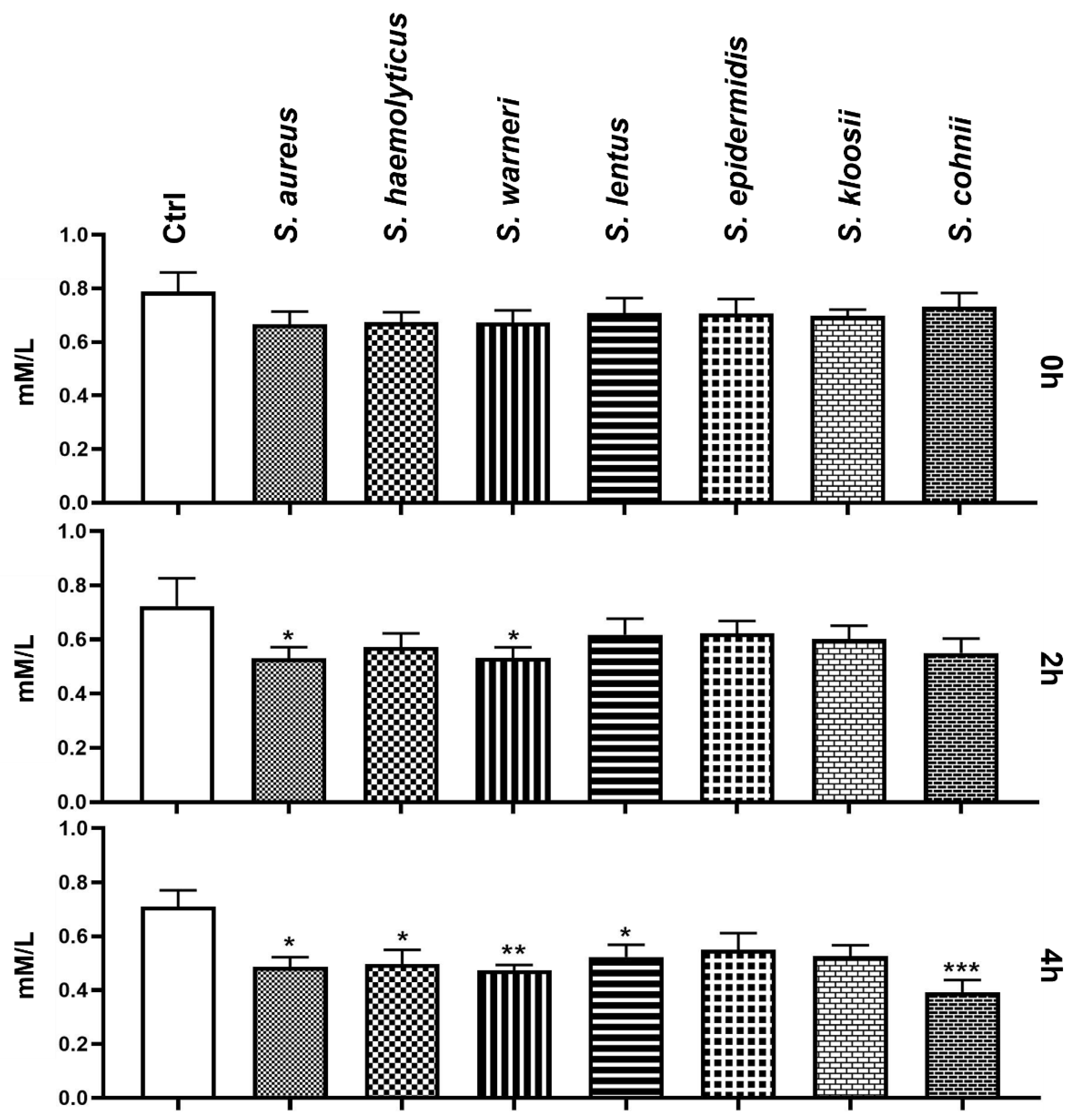
3.6. Effect of Staphylococcus-Induced Bacteriospermia on Extracellular Concentration of Mg
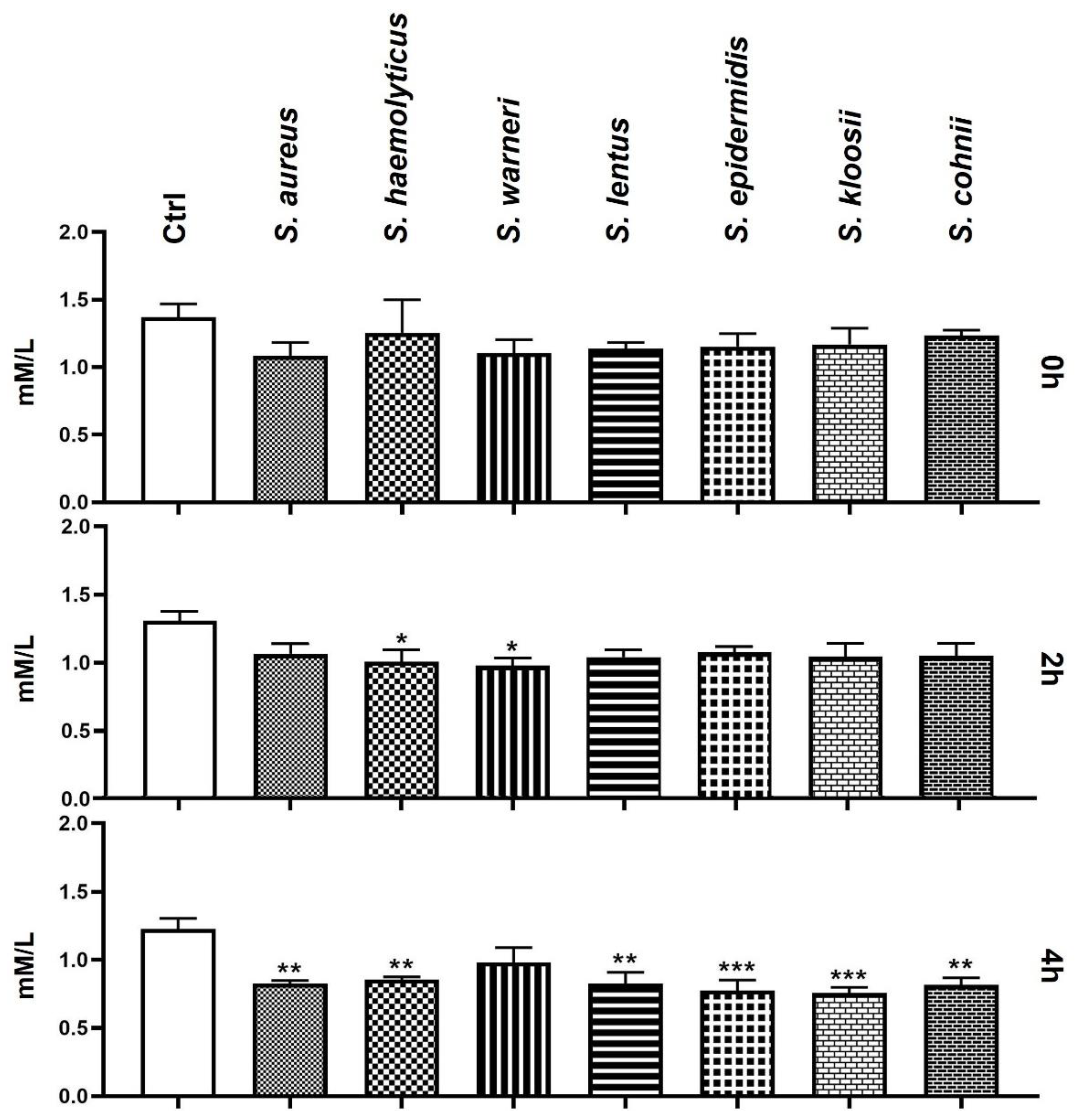
3.7. Effect of Staphylococcus-Induced Bacteriospermia on Extracellular Concentration of Ca
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tvrdá, E.; Belić, L.; Ďuračka, M.; Kováčik, A.; Kačániová, M.; Lukáč, N. The presence of bacterial species in bovine semen and their impact on the sperm quality. Anim. Reprod. Sci. 2018, 194, e3. [Google Scholar] [CrossRef]
- Ďuračka, M.; Belić, L.; Tokárová, K.; Žiarovská, J.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Bacterial communities in bovine ejaculates and their impact on the semen quality. Syst. Biol. Reprod. Med. 2021, 67, 438–449. [Google Scholar] [CrossRef]
- Rossi, C.C.; Pereira, M.F.; Giambiagi-deMarval, M. Underrated Staphylococcus species and their role in antimicrobial resistance spreading. Genet. Mol. Biol. 2020, 43, 1–10. [Google Scholar] [CrossRef] [Green Version]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 30 September 2021).
- McGowan, M.P.; Burger, H.G.; Baker, H.W.G.; De Kretser, D.M.; Kovacs, G. The incidence of non-specific infection in the semen in fertile and sub-fertile males. Int. J. Androl. 1981, 4, 657–662. [Google Scholar] [CrossRef]
- Perumal, P.; Chamuah, J.K.; Srivastava, N.; Kezhavituo, V.; Srivastava, S.K. Infectious Causes of Infertility in Buffalo Bull (Bubalus bubalis). Int. J. Bio-Resour. Stress Manag. 2013, 4, 84–90. [Google Scholar]
- Andrabi, S.M.H.; Khan, L.A.; Shabab, M. Isolation of bacteria in semen and evaluation of antibiotics in extender for cryopreservation of buffalo (Bubalus bubalis) bull spermatozoa. Andrologia 2016, 48, 1166–1174. [Google Scholar] [CrossRef]
- Sannat, C.; Ajit, N.; Sahu, S.B.; Sahasrabudhe, S.A.; Nidhi, R.; Rajesh, K. Effect of Season on Bacterial Load in Semen of Different Breeds of Cattle. J. Anim. Res. 2016, 6, 651–656. [Google Scholar] [CrossRef]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. BioMed. 2016, 14, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Hryhorowicz, M.; Gill, K.; Zarzycka, M.; Gaczarzewicz, D.; Jedrzejczak, P.; Bilinska, B.; Piasecka, M.; Kurpisz, M. The effect of bacteriospermia and leukocytospermia on conventional and nonconventional semen parameters in healthy young normozoospermic males. J. Reprod. Immunol. 2016, 118, 18–27. [Google Scholar] [CrossRef]
- Ďuračka, M.; Kováčik, A.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Bacteria may deteriorate progressive motility of bovine spermatozoa and biochemical parameters of seminal plasma. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 844–847. [Google Scholar] [CrossRef]
- Liang, H.; Miao, M.; Chen, J.; Chen, K.; Wu, B.; Dai, Q.; Wang, J.; Sun, F.; Shi, H.; Yuan, W. The Association between Calcium, Magnesium, and Ratio of Calcium/Magnesium in Seminal Plasma and Sperm Quality. Biol. Trace Elem. Res. 2016, 174, 1–7. [Google Scholar] [CrossRef]
- Martins, A., Jr.; da Cruz, T.E.; Marqui, F.N.; de Souza, D.G.; Berton, T.I.U. Evaluation of Percoll PLUS as a cushion solution during single layer centrifugation of fresh bull semen: Effects of frozen/thawed spermatozoa motility. Anim. Reprod. Sci. 2018, 194, e26. [Google Scholar] [CrossRef] [Green Version]
- Parrish, J.J.; Susko-Parrish, J.; Winer, M.A.; First, N.L. Capacitation of Bovine Sperm by Heparin. Biol. Reprod. 1988, 38, 1171–1180. [Google Scholar] [CrossRef]
- Duracka, M.; Halenar, M.; Tvrda, E. The effect of curcumin on in vitro induced bacterial contamination of rabbit ejaculates by Enterococcus faecalis. MendelNet 2017, 24, 680–684. [Google Scholar]
- Ďuračka, M.; Galovičová, L.; Slávik, M.; Árvay, J.; Tvrdá, E. The in vitro effect of the Origanum vulgare extract on semen. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1089–1092. [Google Scholar] [CrossRef] [Green Version]
- Morrell, J.M.; Wallgren, M. Removal of bacteria from boar ejaculates by Single Layer Centrifugation can reduce the use of antibiotics in semen extenders. Anim. Reprod. Sci. 2011, 123, 64–69. [Google Scholar] [CrossRef]
- Morrell, J.M.; Rodriguez-Martinez, H. Colloid Centrifugation of Semen: Applications in Assisted Reproduction. Am. J. Anal. Chem. 2016, 7, 597–610. [Google Scholar] [CrossRef] [Green Version]
- Fraczek, M.; Wiland, E.; Piasecka, M.; Boksa, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kolanowski, T.; Beutin, L.; Kurpisz, M. Fertilizing potential of ejaculated human spermatozoa during in vitro semen bacterial infection. Fert. Ster. 2014, 102, 711–719. [Google Scholar] [CrossRef]
- Fraczek, M.; Szumala-Kakol, A.; Jedrzejczak, P.; Kamieniczna, M.; Kurpisz, M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fert. Ster. 2007, 88, 1076–1085. [Google Scholar] [CrossRef]
- Huwe, P.; Diemer, T.; Ludwig, M.; Liu, J.; Schiefer, H.G.; Weidner, W. Influence of different uropathogenic microorganisms on human sperm motility parameters in an in vitro. Andrologia 1988, 30, 55–59. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, H.Y.; Cao, Z.G.; Duan, Y.F.; Li, Y.; Ye, Z.Q. Influence of several uropathogenic microorganisms on human sperm motility parameters in vitro. Asian J. Androl. 2002, 4, 179–182. [Google Scholar] [PubMed]
- Fujita, K.; Yokota, T.; Oguri, T.; Fujime, M.; Kitigawa, R. In Vitro adherence of Staphylococcus saprophyticus, Staphylocccus epidermidis, Staphylococcus haemolyticus, and Staphylococcus aureus to human ureter. Urol. Res. 1992, 20, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.; Walev, I.; Berger, T.; Liebetrau, M.; Palmer, M.; Bhakdi, S. Novel path to apoptosis: Small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect. Immun. 1994, 62, 1304–1312. [Google Scholar] [CrossRef] [Green Version]
- Prabha, V.; Gupta, T.; Kaur, S.; Kaur, N.; Kala, S.; Singh, A. Isolation of a spermatozoal immobilization factor from Staphylococcus aureus filtrates. Can. J. Microbiol. 2009, 55, 874–878. [Google Scholar] [CrossRef]
- Gupta, S.; Prabha, V. Human Sperm Interaction with Staphylococcus aureus: A Molecular Approach. J. Pathog. 2012, 2012, 816536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, N.C.; Singh, R.; Gupta, V.; Chauhan, A.; Mavuduru, R.; Prabha, V.; Sharma, P. Contraceptive efficacy of sperm agglutinating factor from Staphylococcus warneri isolated from the cervix of a woman with inexplicable infertility. Reprod. Biol. Endocrinol. 2019, 17, 85. [Google Scholar] [CrossRef] [Green Version]
- Alamo, A.; De Luca, C.; Mongioi, L.M.; Barbagallo, F.; Cannarella, R.; La Vignera, S.; Calogero, A.E.; Condorelli, R.A. Mitochondrial Membrane Potential Predicts 4-h Sperm Motility. Biomedicines 2020, 8, 196. [Google Scholar] [CrossRef]
- Groisman, E.A.; Hollands, K.; Kriner, M.A.; Lee, E.-J.; Park, S.-Y.; Pontes, M.H. Bacterial Mg2+ Homeostasis, Transport, and Virulence. Annu. Rev. Genet. 2013, 47, 625–646. [Google Scholar] [CrossRef] [Green Version]
- Aikawa, J.K. Biochemistry and Physiology of Magnesium. World Rev. Nutr. Diet. 1978, 28, 112–142. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, D.; Miyata, H.; Funato, Y.; Fujihara, Y.; Ikawa, M.; Miki, H. The Mg2+ transporter CNNM4 regulates sperm Ca2+ homeostasis and is essential for reproduction. J. Cell Sci. 2016, 129, 1940–1949. [Google Scholar] [CrossRef] [Green Version]
- Logoglu, G.; Kendirci, A.; Ozgunen, T. The role of seminal calcium in male infertility. J. Isla. Acad. Sci. 1997, 10, 25–27. [Google Scholar]
- Goularte, K.L.; Voloski, F.L.S.; Redú, J.F.M.; Ferreira, C.E.R.; Vieira, A.D.; Duval, E.H.; Mondadori, R.G.; Lucia, T., Jr. Antibiotic resistance in microorganisms isolated in a bull semen stud. Reprod. Domest. Anim. 2020, 55, 318–324. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ďuračka, M.; Husarčíková, K.; Jančov, M.; Galovičová, L.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Staphylococcus-Induced Bacteriospermia In Vitro: Consequences on the Bovine Spermatozoa Quality, Extracellular Calcium and Magnesium Content. Animals 2021, 11, 3309. https://doi.org/10.3390/ani11113309
Ďuračka M, Husarčíková K, Jančov M, Galovičová L, Kačániová M, Lukáč N, Tvrdá E. Staphylococcus-Induced Bacteriospermia In Vitro: Consequences on the Bovine Spermatozoa Quality, Extracellular Calcium and Magnesium Content. Animals. 2021; 11(11):3309. https://doi.org/10.3390/ani11113309
Chicago/Turabian StyleĎuračka, Michal, Kamila Husarčíková, Mikuláš Jančov, Lucia Galovičová, Miroslava Kačániová, Norbert Lukáč, and Eva Tvrdá. 2021. "Staphylococcus-Induced Bacteriospermia In Vitro: Consequences on the Bovine Spermatozoa Quality, Extracellular Calcium and Magnesium Content" Animals 11, no. 11: 3309. https://doi.org/10.3390/ani11113309
APA StyleĎuračka, M., Husarčíková, K., Jančov, M., Galovičová, L., Kačániová, M., Lukáč, N., & Tvrdá, E. (2021). Staphylococcus-Induced Bacteriospermia In Vitro: Consequences on the Bovine Spermatozoa Quality, Extracellular Calcium and Magnesium Content. Animals, 11(11), 3309. https://doi.org/10.3390/ani11113309







