Antioxidative and Oxidative Profiles in Plasma and Saliva in Dairy Cows during Pregnancy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Total Antioxidant Capacity
2.2. The Content of Hydroperoxides
2.3. The Content of Sulfhydryl Groups
2.4. The Content of Bityrosine Bridges
2.5. The Content of Formylokinurenine
2.6. Protein Content
2.7. Statistical Analysis
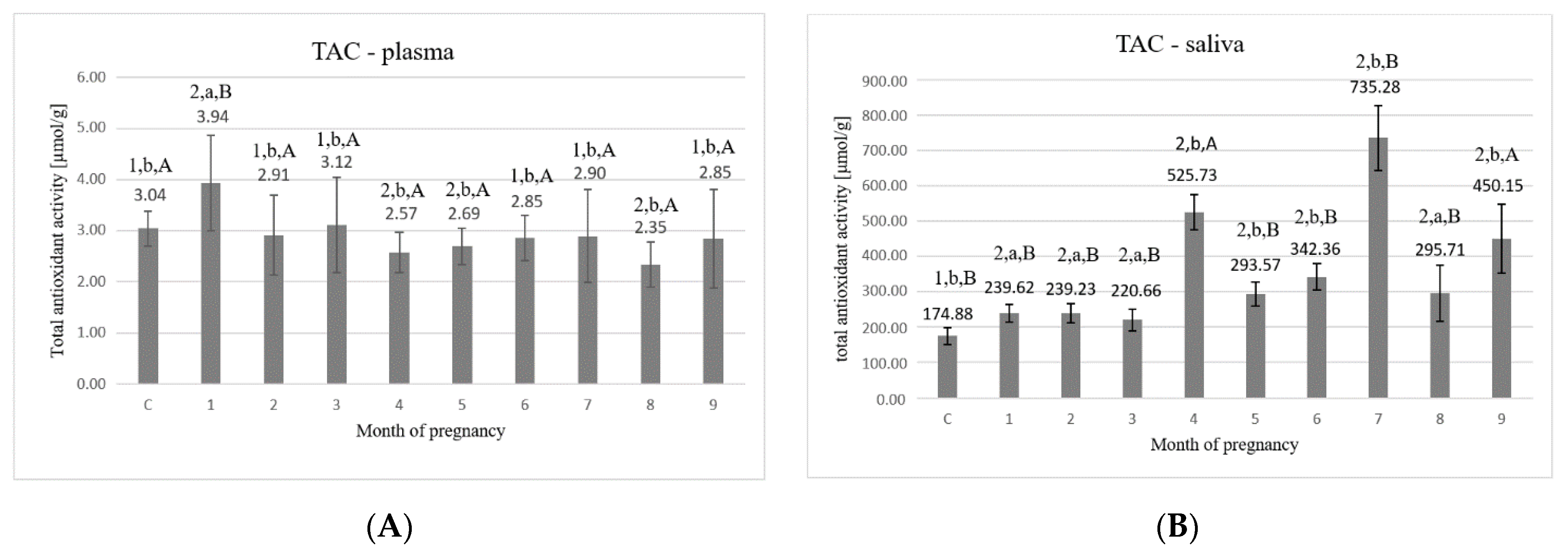
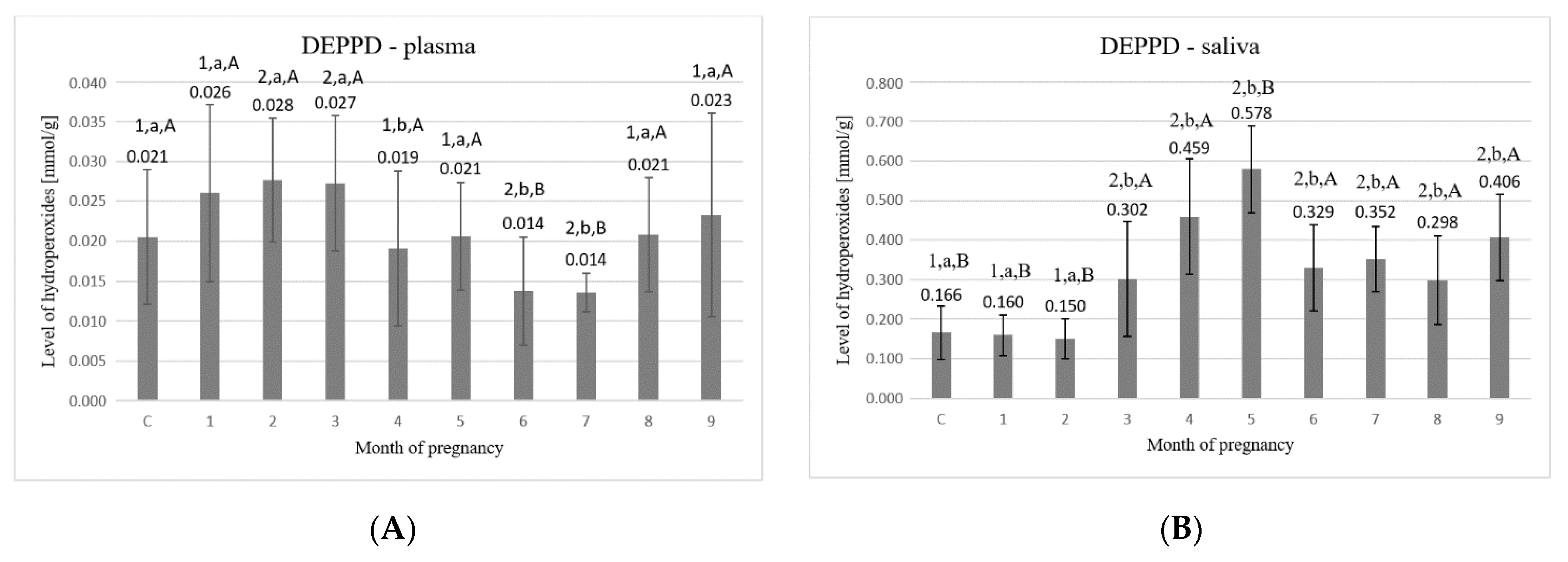
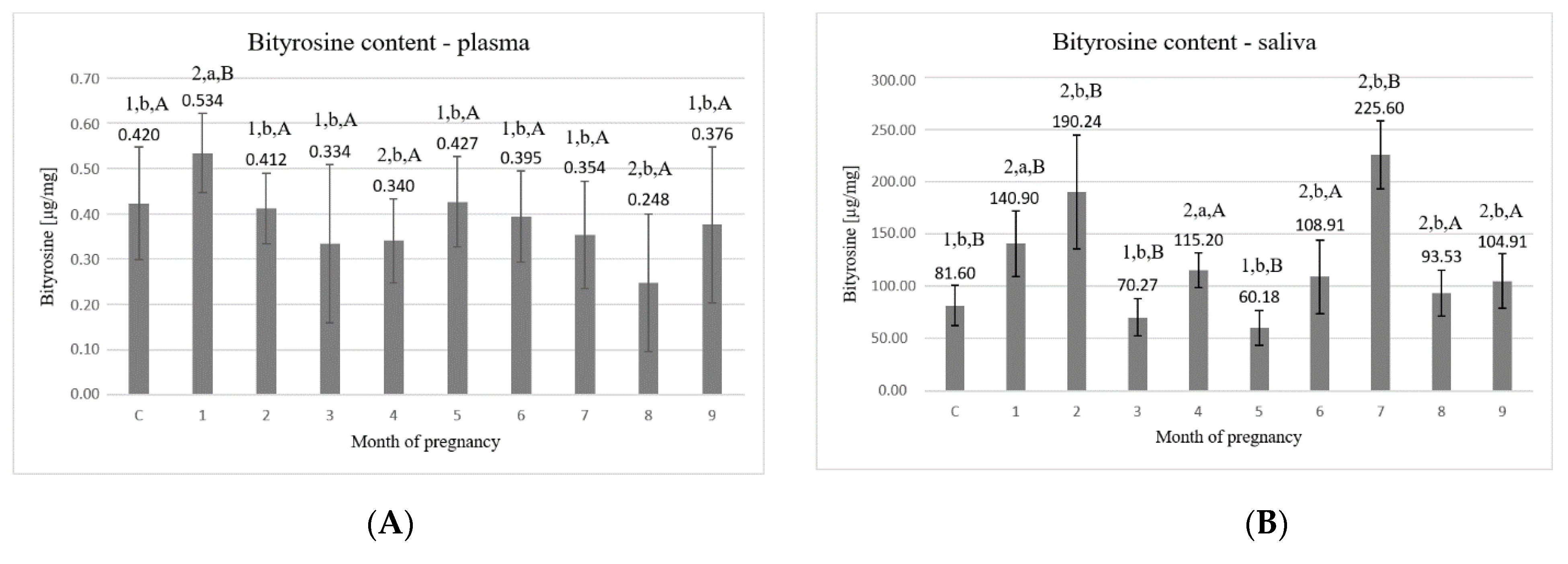
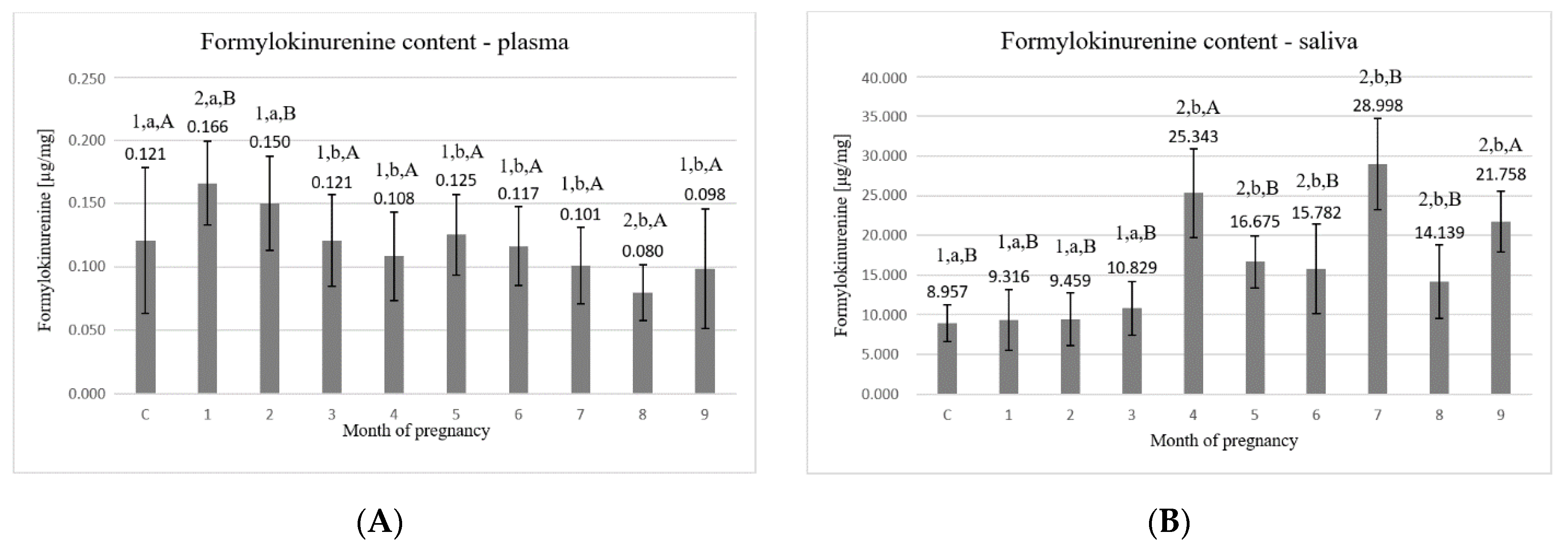
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartosz, G. The second face of oxygen. In Free Radicals in Nature (Original Title: Druga Twarz Tlenu. Wolne Rodniki w Przyrodzie), 1st ed.; PWN: Warsaw, Poland, 2003. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Garrel, C.; Faure, P.; Sugino, N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod. Biomed. Online 2012, 25, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Castillo, C.; Hernández, J.; López-Alonso, M.; Miranda, M.; Luís, J. Values of plasma lipid hydroperoxides and total antioxidant status in healthy dairy cows: Preliminary observations. Arch. Anim. Breed. 2003, 46, 227–233. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta —Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [Green Version]
- Žaja, I.Ž.; Samardžija, M.; Vince, S.; Majić-Balić, I.; Đuričić, D.; Milinković-Tur, S. Influence of different periods of the year and age on the parameters of antioxidative status and oxidative stress in the blood serum of breeding bulls. Reprod. Biol. 2016, 16, 157–164. [Google Scholar] [CrossRef]
- Kankofer, M.; Albera, E.; Feldman, M.; Gundling, N.; Hoedemaker, M. Comparison of antioxidative/oxidative profiles in blood plasma of cows with and without retained fetal placental membranes. Theriogenology 2010, 74, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Turk, R.; Koledić, M.; Macesic, N.; Benić, M.; Dobranić, V.; Đuričić, D.; Cvetnić, L.; Samardžija, M. The role of oxidative stress and inflammatory response in the pathogenesis of mastitis in dairy cows. Mljekarstvo 2017, 67, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Gundelach, Y.; Streuff, B.; Franczyk, M.; Kankofer, M.; Hoedemaker, M. Influence of two different feeding strategies in the dry period on dry matter intake and plasma protein peroxidative and antioxidative profile during dry period and early lactation. BMC Vet. Res. 2020, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Kaushal, N. Introduction to Oxidative Stress Mechanism and Their Modulation; Springer: New Delhi, India, 2014; Volume 366, p. 2014. ISBN 9788132220312. [Google Scholar]
- Wawrzykowski, J.; Franczyk, M.; Ner-Kluza, J.; Silberring, J.; Kankofer, M. 2D Electrophoretic pattern of bovine placental proteins during early-mid pregnancy. J. Mass Spectrom. 2020, 55, e4483. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Hempstock, J.; Jauniaux, E.; Greenwold, N.; Burton, G.J. The contribution of placental oxidative stress to early pregnancy failure. Hum. Pathol. 2003, 34, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Lin, S.W.; Pacifici, R.E. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J. Biol. Chem. 1987, 262, 9914–9920. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lóopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.P.; Diniz, D.G.; Moimaz, S.A.S.; Sumida, D.H.; Okamoto, A.C. Saliva: Reflection of the body. Int. J. Infect. Dis. 2010, 14, e184–e188. [Google Scholar] [CrossRef] [Green Version]
- Schulz, B.; Cooper-White, J.; Punyadeera, C.K. Saliva proteome research: Current status and future outlook. Crit. Rev. Biotechnol. 2013, 33, 246–259. [Google Scholar] [CrossRef]
- Iris, F.F.; Benzie, J.J.S. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay Iris. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Alberti, A.; Bolognini, L.; Macciantelli, D.; Caratelli, M. The radical cation of N,N-diethyl-para-phenylendiamine: A possible indicator of oxidative stress in biological samples. Res. Chem. Intermed. 2000, 26, 253–267. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Diplock, A.T.; Symons, M.C.R. Techniques in Free Radical Research; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Gornal, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret method reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shapiro, A.S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Stanford University Press: Stanford, CA, USA, 1960. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci. Rep. 2015, 35, e00261. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidants and human disease: Some new concepts 1. FASEB J. 1987, 1, 358–364. [Google Scholar] [CrossRef]
- Miller, J.; Brzezinska-Slebodzinska, E.; Madsen, F. Oxidative Stress, Antioxidants, and Animal Function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Bardow, A.; Jensen, S.B.; Nauntofte, B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef]
- Lamy, E.; Mau, M. Saliva proteomics as an emerging, non-invasive tool to study livestock physiology, nutrition and diseases. J. Proteom. 2012, 75, 4251–4258. [Google Scholar] [CrossRef]
- Porter, N.A. Mechanisms for the autoxidation of polyunsaturated lipids. Acc. Chem. Res. 1986, 19, 262–268. [Google Scholar] [CrossRef]
- Gaál, T.; Ribiczeyné-Szabó, P.; Stadler, K.; Jakus, J.; Reiczigel, J.; Kövér, P.; Mézes, M.; Sümeghy, L. Free radicals, lipid peroxidation and the antioxidant system in the blood of cows and newborn calves around calving. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 143, 391–396. [Google Scholar] [CrossRef]
- Colak, E. New Markers of Oxidative Damage to Macromolecules. J. Med. Biochem. 2008, 27, 1–16. [Google Scholar] [CrossRef]
- Kankofer, M. Protein Peroxidation Processes in Bovine Retained and Not-retained Placenta. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2001, 48, 207–212. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.; Lin, Y. Oxidative Stress in Placenta: Health and Diseases. Biomed Res. Int. 2015, 2015, 293271. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, S.; Czapski, G.; Cohen, H.; Meyerstein, D. Free radicals induced peptide damage in the presence of transition metal ions: A plausible pathway for biological deleterious processes. Free Radic. Biol. Med. 1994, 17, 11–18. [Google Scholar] [CrossRef]
- Wawrzykowski, J.; Jamioł, M.; Mojsym, W.; Kankofer, M. The comparison of pro- and antioxidative parameters in plasma and placental tissues during early phase of placental development in cows. Mol. Biol. Rep. 2021, 48, 1291–1297. [Google Scholar] [CrossRef]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sánchez-Illana, Á.; Nuñez-Ramiro, A.; Kuligowski, J.; Cháfer-Pericás, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef]
- Goff, J.; Horst, R.L. Physiological Changes at Parturition and Their Relationship to Metabolic Disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Cortijo, D.R.; Herrera, T.; Rodríguez, P.R.; De Pablo Ángel, L.L.; De La Calle, M.; López-Giménez, M.R.; Mora-Urda, A.I.; Gutiérrez-Arzapalo, P.Y.; Gómez-Rioja, R.; Aguilera, Y.; et al. Maternal plasma antioxidant status in the first trimester of pregnancy and development of obstetric complications. Placenta 2016, 47, 37–45. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.-P.; Skepper, J.N.; Burton, G.J. Onset of Maternal Arterial Blood Flow and Placental Oxidative Stress: A Possible Factor in Human Early Pregnancy Failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Öztürk, L.K.; Akyüz, S.; Yarat, A.; Koç, S.; Gül, N.; Doğan, B. Salivary lipid peroxidation and total sialic acid levels during healthy gestation and postpartum: A longitudinal study. Clin. Biochem. 2010, 43, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Hanschke, N.; Kankofer, M.; Ruda, L.; Höltershinken, M.; Meyer, U.; Frank, J.; Dänicke, S.; Rehage, J. The effect of conjugated linoleic acid supplements on oxidative and antioxidative status of dairy cows. J. Dairy Sci. 2016, 99, 8090–8102. [Google Scholar] [CrossRef]
- Albera, E.; Kankofer, M. The Comparison of Antioxidative/Oxidative Profile in Colostrum, Milk and Blood of Early Post-Partum Cows During their First and Second Lactation. Reprod. Domest. Anim. 2010, 45, e417–e425. [Google Scholar] [CrossRef]
- Puzio, W.; Chrobak, L.; Rutkowski, M.; Franczyk, M.; Kankofer, M. Antioxidative and oxidative profiles in plasma and saliva of cows in different ages and hormonal status. Vet. Ital. 2019, 55, 319–326. [Google Scholar] [PubMed]
- Szczubiał, M.; Kankofer, M.; Dabrowski, R.; Bochniarz, M.; Urban-Chmiel, R. Assessment of lipid and protein peroxidation markers in non-pregnant and pregnant female dogs. Pol. J. Vet. Sci. 2015, 18, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Szczubial, M.; Kankofer, M.; Bochniarz, M.; Dąbrowski, R. Effects of Ovariohysterectomy on Oxidative Stress Markers in Female Dogs. Reprod. Domest. Anim. 2015, 50, 393–399. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielecka, A.; Jamioł, M.; Kankofer, M. Antioxidative and Oxidative Profiles in Plasma and Saliva in Dairy Cows during Pregnancy. Animals 2021, 11, 3204. https://doi.org/10.3390/ani11113204
Bielecka A, Jamioł M, Kankofer M. Antioxidative and Oxidative Profiles in Plasma and Saliva in Dairy Cows during Pregnancy. Animals. 2021; 11(11):3204. https://doi.org/10.3390/ani11113204
Chicago/Turabian StyleBielecka, Arletta, Monika Jamioł, and Marta Kankofer. 2021. "Antioxidative and Oxidative Profiles in Plasma and Saliva in Dairy Cows during Pregnancy" Animals 11, no. 11: 3204. https://doi.org/10.3390/ani11113204
APA StyleBielecka, A., Jamioł, M., & Kankofer, M. (2021). Antioxidative and Oxidative Profiles in Plasma and Saliva in Dairy Cows during Pregnancy. Animals, 11(11), 3204. https://doi.org/10.3390/ani11113204





