The Apelinergic System Immuno-Detection in the Abomasum and Duodenum of Sheep Grazing on Semi-Natural Pasture
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Recruiting and Sample Collection
2.2. Morphological Staining and Immunohistochemistry
2.3. Double-Label Immunohistochemistry
2.4. Statistical Analysis
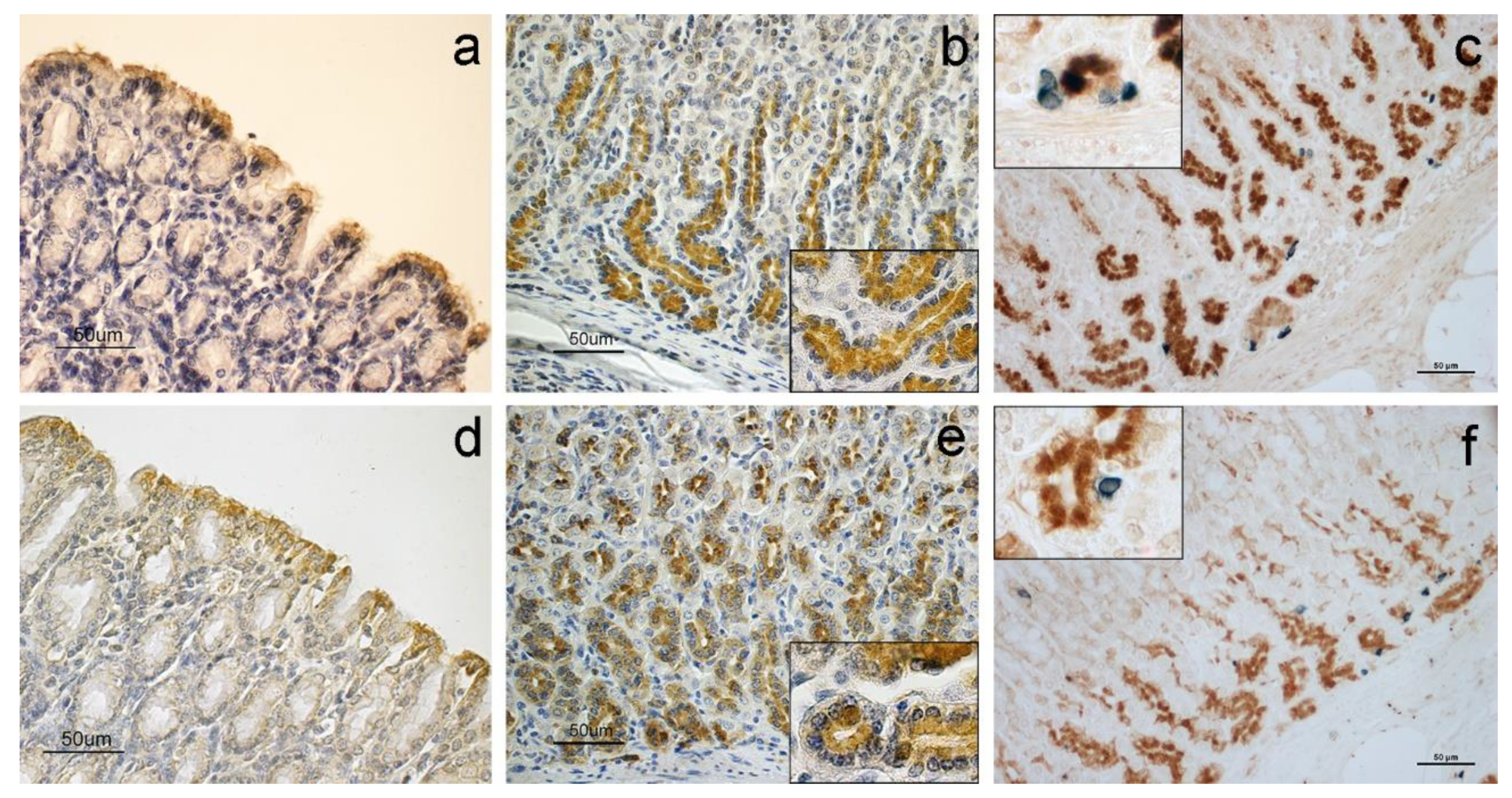
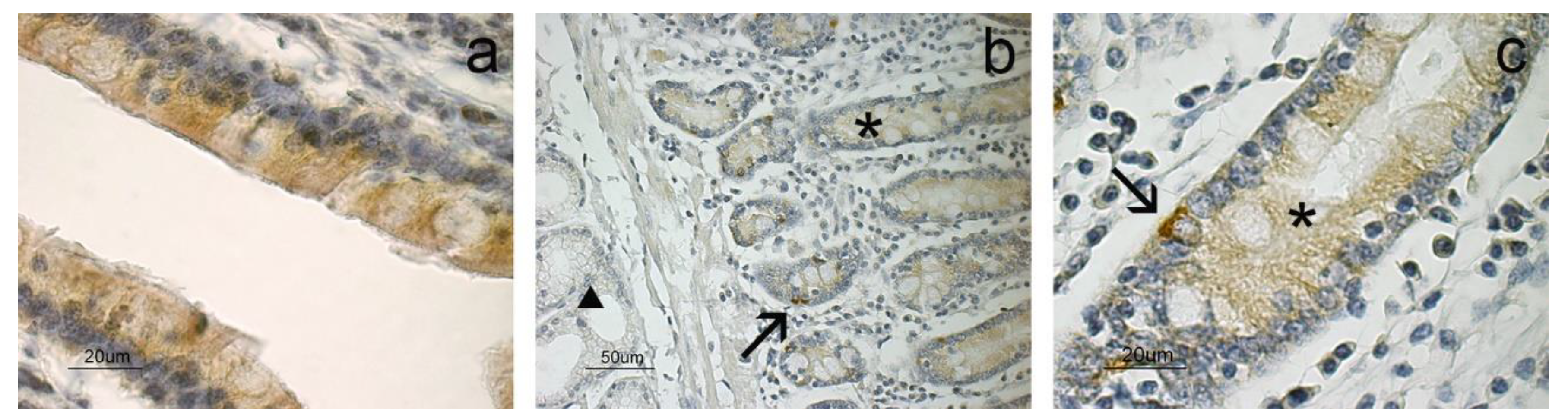
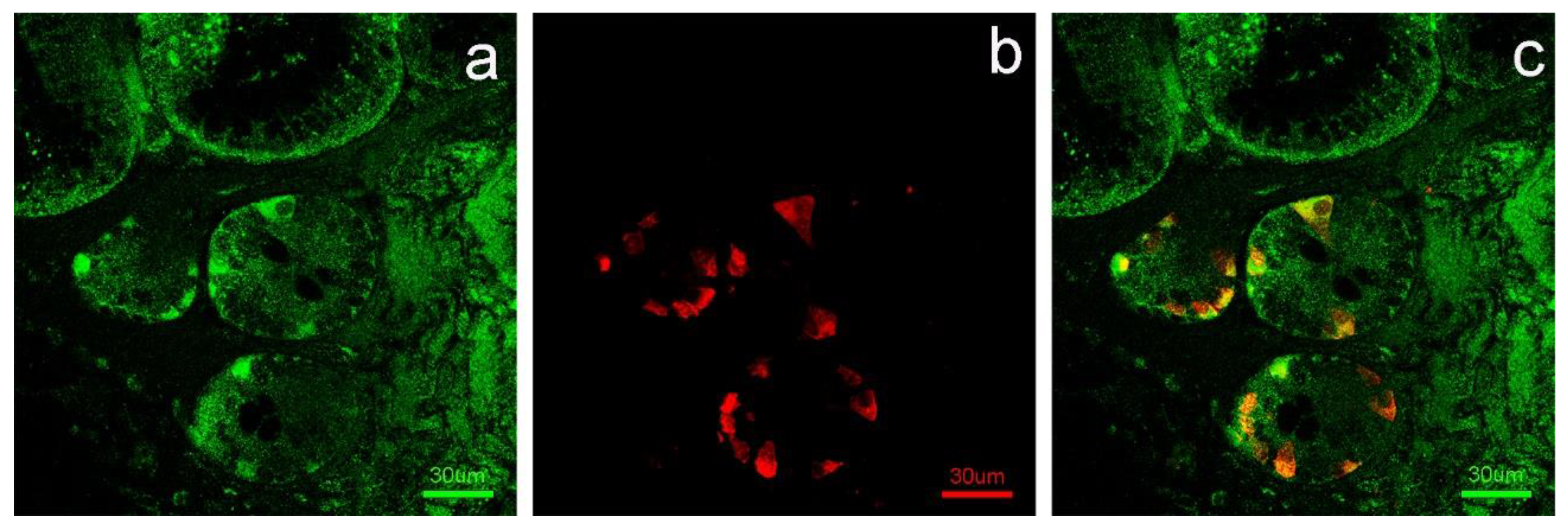
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trayhurn, P.; Bing, C.; Wood, I.S. Adipose Tissue and Adipokines—Energy Regulation from the Human Perspective. J. Nutr. 2006, 136, 1935–1939. [Google Scholar] [CrossRef]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476. [Google Scholar] [CrossRef]
- Kurowska, P.; Barbe, A.; Rózycka, M.; Chmielińska, J.; Dupont, J.; Rak, A. Apelin in reproductive physiology and pathology of different species: A critical review. Int. J. Endocrinol. 2018, 6, 9170480. [Google Scholar] [CrossRef] [PubMed]
- Kleinz, M.J.; Davenport, A.P. Emerging roles of apelin in biology and medicine. Pharmacol. Ther. 2005, 107, 198–211. [Google Scholar] [CrossRef]
- Kapica, M.; Jankowska, A.; Antushevich, H.; Pietrzak, P.; Bierla, J.B.; Dembinski, A.; Zabielski, R. The effect of exogenous apelin on the secretion of pancreatic juice in anaesthetized rats. J. Physiol. Pharmacol. 2012, 63, 53–60. [Google Scholar] [PubMed]
- O’Carroll, A.M.; Lolait, S.J.; Harris, L.E.; Pope, G.R. The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J. Endocrinol. 2013, 219, R13-35. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.R.; Bharati, J.; Bharti, M.K.; Kar, D.; Sahoo, P.R. Adipokines as metabolic modulators of ovarian functions in livestock: A mini-review. J. Adv. Vet. Anim. Res. 2016, 3, 206–213. [Google Scholar] [CrossRef]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigné, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M.V.; Purhonen, A.K.; Miettinen, P.; Pääkkönen, M.; Pirinen, E.; Alhava, E.; Akerman, K.; Herzig, K.H. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul. Pept. 2005, 130, 7–13. [Google Scholar] [CrossRef]
- Castan-Laurell, I.; Dray, C.; Attané, C.; Duparc, T.; Knauf, C.; Valet, P. Apelin, diabetes, and obesity. Endocrine 2011, 40, 1. [Google Scholar] [CrossRef]
- Pitkin, S.L.; Maguire, J.J.; Bonner, T.I.; Davenport, A.P. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol. Rev. 2010, 62, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Mercati, F.; Maranesi, M.; Dall’aglio, C.; Petrucci, L.; Pasquariello, R.; Tardella, F.M.; De Felice, E.; Scocco, P. Apelin system in mammary gland of sheep reared in semi-natural pastures of the central apennines. Animals 2018, 8, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercati, F.; Scocco, P.; Maranesi, M.; Acuti, G.; Petrucci, L.; Cocci, P.; Renzi, A.; De Felice, E.; Dall’Aglio, C. Apelin system detection in the reproductive apparatus of ewes grazing on semi-natural pasture. Theriogenology 2019, 139, 156–166. [Google Scholar] [CrossRef]
- Dall’Aglio, C.; Mercati, F.; De Felice, E.; Tardella, F.M.; Kamphues, J.; Cappai, M.G.; Scocco, P. Influence of Different Feed Physical Forms on Mandibular Gland in Growing Pigs. Animals 2020, 10, 910. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, X.; Liu, M.; Chen, L. Function and regulation of apelin/APJ system in digestive physiology and pathology. J. Cell Physiol. 2019, 234, 7796–7810. [Google Scholar] [CrossRef]
- Lv, S.Y.; Yang, Y.J.; Chen, Q. Regulation of feeding behavior, gastrointestinal function and fluid homeostasis by apelin. Peptides 2013, 44, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Susaki, E.; Wang, G.; Cao, G.; Wang, H.Q.; Englander, E.W.; Greeley, G.H. Apelin cells in the rat stomach. Regul. Pept. 2005, 129, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Anini, Y.; Wei, W.; Qi, X.; O’Carroll, A.M.; Mochizuki, T.; Wang, H.Q.; Hellmich, M.R.; Englander, E.W.; Greeley, G.H. Apelin, a New Enteric Peptide: Localization in the Gastrointestinal Tract, Ontogeny, and Stimulation of Gastric Cell Proliferation and of Cholecystokinin Secretion. Endocrinology 2004, 145, 1342–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Kundu, R.; Han, S.; Qi, X.; Englander, E.W.; Quertermous, T.; Greeley, G.H. Ontogeny of apelin and its receptor in the rodent gastrointestinal tract. Regul. Pept. 2009, 158, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Taheri, S.; Murphy, K.; Cohen, M.; Sujkovic, E.; Kennedy, A.; Dhillo, W.; Dakin, C.; Sajedi, A.; Ghatei, M.; Bloom, S. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem. Biophys. Res. Commun. 2002, 291, 1208–1212. [Google Scholar] [CrossRef]
- O’Shea, M.; Hansen, M.J.; Tatemoto, K.; Morris, M.J. Inhibitory effect of apelin-12 on nocturnal food intake in the rat. Nutr. Neurosci. 2003, 6, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Sunter, D.; Hewson, A.K.; Dickson, S.L. Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neurosci. Lett. 2003, 353, 1–4. [Google Scholar] [CrossRef]
- Gordon, I.J. Browsing and grazing ruminants: Are they different beasts? For. Ecol. Manag. 2003, 181, 13–21. [Google Scholar] [CrossRef]
- Aryal, A.; Coogan, S.C.P.; Ji, W.; Rothman, J.M.; Raubenheimer, D. Foods, macronutrients and fibre in the diet of blue sheep (Psuedoisnayaur) in the Annapurna Conservation Area of Nepal. Ecol. Evol. 2015, 5, 4006–4017. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci.Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Catorci, A.; Cesaretti, S.; Gatti, R. Effect of long-term abandonment and spring grazing on floristic and functional composition of dry grasslands in a central Apennine farmland. Pol. J. Ecol. 2013, 61, 505–518. [Google Scholar]
- Scocco, P.; Piermarteri, K.; Malfatti, A.; Tardella, F.M.; Catorci, A. Effects of summer rainfall variations on sheep body state and farming sustainability in sub-mediterranean pastoral system. Span. J. Agric. Res. 2016, 14, 8. [Google Scholar] [CrossRef] [Green Version]
- Scocco, P.; Mercati, F.; Tardella, F.M.; Catorci, A. Increase of forage dryness induces differentiated anatomical response in the sheep rumen compartments. Microsc. Res. Tech. 2016, 79, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Eurell, J.A.; Frappier, B.L. Dellmann’s Textbook of Veterinary Histology; Wiley-Blackwell: Hoboken, NJ, USA, 2013; p. 416. [Google Scholar]
- Mercati, F.; Dall’aglio, C.; Timperi, L.; Scocco, P.; De Felice, E.; Maranesi, M. Epithelial expression of the hormone leptin by bovine skin. Eur. J. Histochem. 2019, 63, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Maranesi, M.; Di Loria, A.; Dall’Aglio, C.; Piantedosi, D.; Lepri, E.; Ciaramella, P.; Mercati, F. Leptin System in Obese Dog Skin: A Pilot Study. Animals 2020, 10, 2338. [Google Scholar] [CrossRef]
- Dall’Aglio, C.; Pedini, V.; Scocco, P.; Boiti, C.; Ceccarelli, P. Immunohistochemical evidence of Orexin-A in the pancreatic beta cells of domestic animals. Res. Vet. Sci. 2010, 89, 147–149. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Lambrecht, N.W.G.; Yakubov, I.; Zer, C.; Sachs, G. Transcriptomes of purified gastric ECL and parietal cells: Identification of a novel pathway regulating acid secretion. Physiol. Genom. 2006, 25, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, N.; Tanimoto, A.; Hondo, E.; Andrén, A.; Cottrell, D.F.; Sasaki, M.; Yamada, J. Immunohistochemical study of the ontogeny of prochymosin- and pepsinogen-producing cells in the abomasum of sheep. Anat. Histol. Embryol. 2001, 30, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Simoens, P. AnatomiaComparata Dei MammiferiDomestici; Edagricole-New Business Media: Milano, Italy, 2012; Volume 7. [Google Scholar]
- Oberg, K. Gastric neuroendocrine cells and secretory products. Yale J. Biol. Med. 1998, 71, 149–154. [Google Scholar] [PubMed]
- Cinti, S.; Matteis, R.D.; Picó, C.; Ceresi, E.; Obrador, A.; Maffeis, C.; Oliver, J.; Palou, A. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 789–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habata, Y.; Fujii, R.; Hosoya, M.; Fukusumi, S.; Kawamata, Y.; Hinuma, S.; Kitada, C.; Nishizawa, N.; Murosaki, S.; Kurokawa, T.; et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta Mol. Cell Res. 1999, 1452, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Wang, G.; Qiu, S.; De la Motte, C.; Wang, H.Q.; Gomez, G.; Englander, E.W.; Greeley Jr, G.H. Increased colonic apelin production in rodents with experimental colitis and in humans with IBD. Regul. Pept. 2007, 142, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Wattez, J.S.; Ravallec, R.; Cudennec, B.; Knauf, C.; Dhulster, P.; Valet, P.; Breton, C.; Vieau, D.; Lesage, J. Apelin stimulates both cholecystokinin and glucagon-like peptide 1 secretions in vitro and in vivo in rodents. Peptides 2013, 48, 134–136. [Google Scholar] [CrossRef]
- Scocco, P.; Rivaroli, S.; Mercati, F.; Tardella, F.M.; Malfatti, A.; De Felice, E.; Catorci, A. Anatomy for economy: Starting from the rumen keratinization degree to enhance the farm income. Econ. Agro-Aliment. 2018, 20, 261–272. [Google Scholar] [CrossRef]
- Barbato, O.; De Felice, E.; Todini, L.; Menchetti, L.; Malfatti, A.; Scocco, P. Effects of Feed Supplementation on Nesfatin-1, Insulin, Glucagon, Leptin, T3, Cortisol, and BCS in Milking Ewes Grazing on Semi-Natural Pastures. Animals 2021, 11, 682. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Salvador, M.T.; Rodriguez-Yoldi, M.C.; Alcalde, A.I.; Rodriguez-Yoldi, M.J. 5-HT receptor subtypes involved in the serotonin-induced inhibition of L-leucine absorption in rabbit jejunum. Life Sci. 1997, 61, 309–318. [Google Scholar] [CrossRef]
- Arruebo, M.P.; Mesonero, J.E.; Murillo, M.D.; Alcalde, A.I. Effect of serotonin on D-galactose transport across the rabbit jejunum. Reprod. Nutr. Dev. 1989, 29, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, M.B.; Witte, A.B. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol. 2008, 193, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Saksena, S.; Tyagi, S.; Alrefai, W.A.; Malakooti, J.; Sarwar, Z.; Turner, J.R.; Ramaswamy, K.; Dudeja, P.K. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology 2005, 128, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Saksena, S.; Gill, R.K.; Tyagi, S.; Alrefai, W.A.; Sarwar, Z.; Ramaswamy, K.; Dudeja, P.K. Involvement of c-Src and protein kinase C delta in the inhibition of Cl(−)/OH− exchange activity in Caco-2 cells by serotonin. J. Biol. Chem. 2005, 280, 11859–11868. [Google Scholar] [CrossRef] [Green Version]
- Flemström, G.; Mäkelä, K.; Purhonen, A.K.; Sjöblom, M.; Jedstedt, G.; Walkowiak, J.; Herzig, K.H. Apelin stimulation of duodenal bicarbonate secretion: Feeding-dependent and mediated via apelin-induced release of enteric cholecystokinin. Acta Physiol. 2011, 201, 141–150. [Google Scholar] [CrossRef]
| Organs | Searched Molecules | Immunoreactive Structures | M × F | E × p | M × D |
|---|---|---|---|---|---|
| Abomasum | APLN | Lining epithelium | 1.15 | 1.35 | 1 |
| Fundic glands | 1.98 | 2.13 | 1.18 | ||
| APLNR | Lining epithelium | 0.85 | 0.87 | 0.48 | |
| Fundic glands | 1.18 | 1.2 | 0.31 | ||
| Duodenum | APLNR | Lining epithelium | 1.87 | 1.79 | 1.03 |
| Intestinal crypts | 1.5 | 1.66 | 0.84 | ||
| Neuroendocrine cells | 1.7 | 2.33 | 2.7 |
| Organs | Searched Molecules | Immunoreactive Structures | Kruskal–WallisTest | Wilcoxon Rank Sum Test | ||
|---|---|---|---|---|---|---|
| M × F vs. E × p | M × F vs. M × D | E × p vs. M × D | ||||
| Abomasum | APLN | Lining epithelium | 0.1567 | 0.41980 | 0.41980 | 0.22803 |
| Fundic glands | 1.439 × 10−7 | 0.0702 | 7.92 × 10−6 | 7.92 × 10−6 | ||
| APLNR | Lining epithelium | 0.000352 | 0.8329 | 0.00171 | 0.00171 | |
| Fundic glands | 3.129 × 10−7 | 0.7981 | 8.277 × 10−6 | 8.277 × 10−6 | ||
| Duodenum | APLNR | Lining epithelium | 2.827 × 10−7 | 0.4076 | 8.517 × 10−6 | 8.517 × 10−6 |
| Intestinal crypts | 1.716 × 10−6 | 0.2282 | 1.453 × 10−4 | 8.829 × 10−6 | ||
| Neuroendocrine cells | 0.002526 | 0.0601 | 0.0080 | 0.0601 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmioli, E.; Dall’Aglio, C.; Bellesi, M.; Tardella, F.M.; Moscatelli, S.; Scocco, P.; Mercati, F. The Apelinergic System Immuno-Detection in the Abomasum and Duodenum of Sheep Grazing on Semi-Natural Pasture. Animals 2021, 11, 3173. https://doi.org/10.3390/ani11113173
Palmioli E, Dall’Aglio C, Bellesi M, Tardella FM, Moscatelli S, Scocco P, Mercati F. The Apelinergic System Immuno-Detection in the Abomasum and Duodenum of Sheep Grazing on Semi-Natural Pasture. Animals. 2021; 11(11):3173. https://doi.org/10.3390/ani11113173
Chicago/Turabian StylePalmioli, Elisa, Cecilia Dall’Aglio, Michele Bellesi, Federico Maria Tardella, Sara Moscatelli, Paola Scocco, and Francesca Mercati. 2021. "The Apelinergic System Immuno-Detection in the Abomasum and Duodenum of Sheep Grazing on Semi-Natural Pasture" Animals 11, no. 11: 3173. https://doi.org/10.3390/ani11113173
APA StylePalmioli, E., Dall’Aglio, C., Bellesi, M., Tardella, F. M., Moscatelli, S., Scocco, P., & Mercati, F. (2021). The Apelinergic System Immuno-Detection in the Abomasum and Duodenum of Sheep Grazing on Semi-Natural Pasture. Animals, 11(11), 3173. https://doi.org/10.3390/ani11113173








