Hatching Success Rather Than Temperature-Dependent Sex Determination as the Main Driver of Olive Ridley (Lepidochelys olivacea) Nesting Activity in the Pacific Coast of Central America
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
2.2. Beach Color
2.3. Standardizing Nesting Activity
- Constant number of nests: Ti = T; one parameter, T;
- Exponential model: Ti = T0 er.i, where the two parameters T0 and r are the number of nests at time 0 and the growth rate, respectively;
- Year-specific number of nests: Ti; Y parameters, T1 to TY.
2.4. Relationship between Sand Color and Nesting Activity
2.5. Thermal Reaction Norm for Hatching Success and Sex Ratio
3. Results
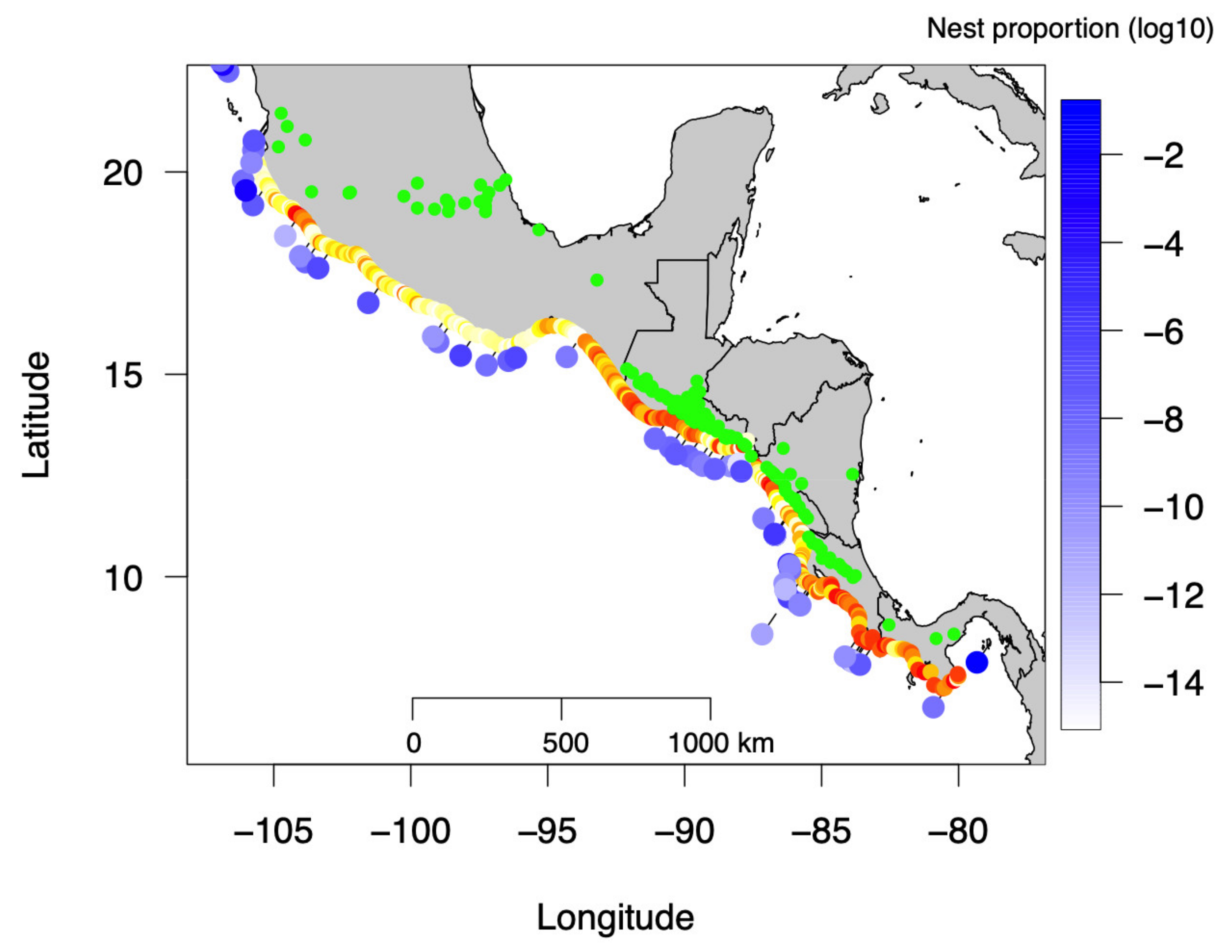
3.1. Beach Albedo from Satellite Images
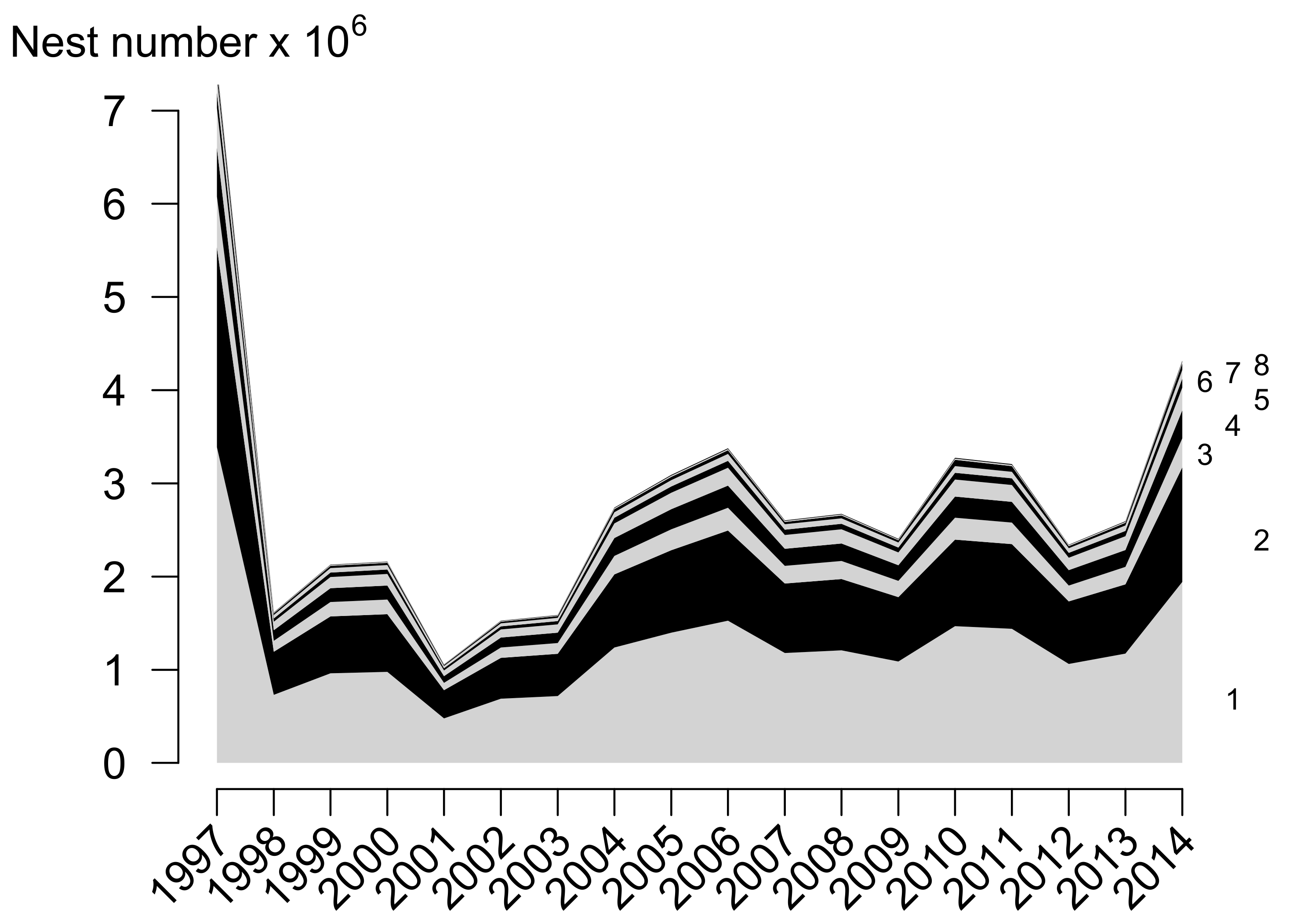
3.2. Temporal Olive Ridley Nest Abundance in Pacific Central America
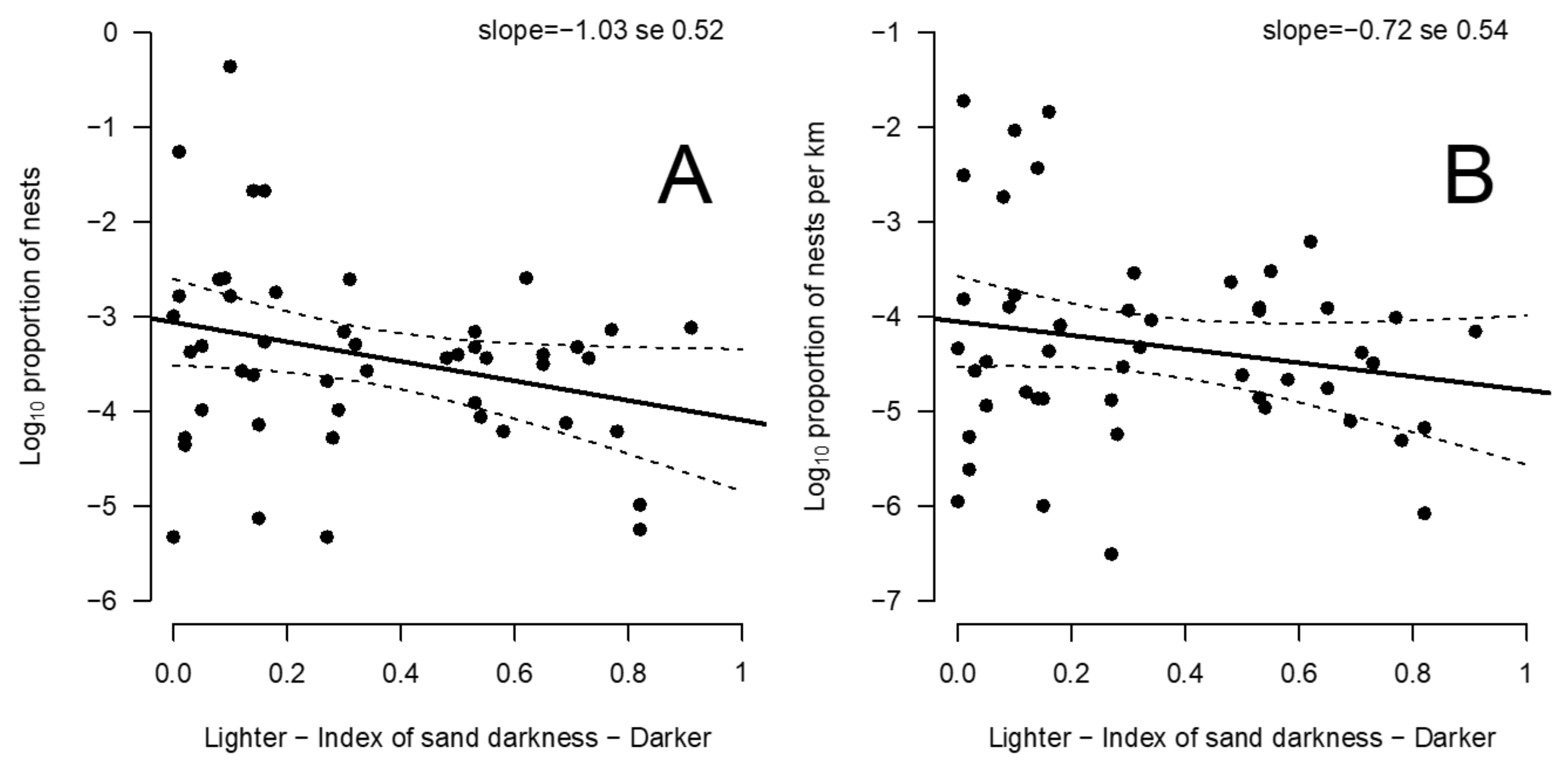
3.3. Relationship between Beach Albedo and Olive Ridley Nest Number and Density
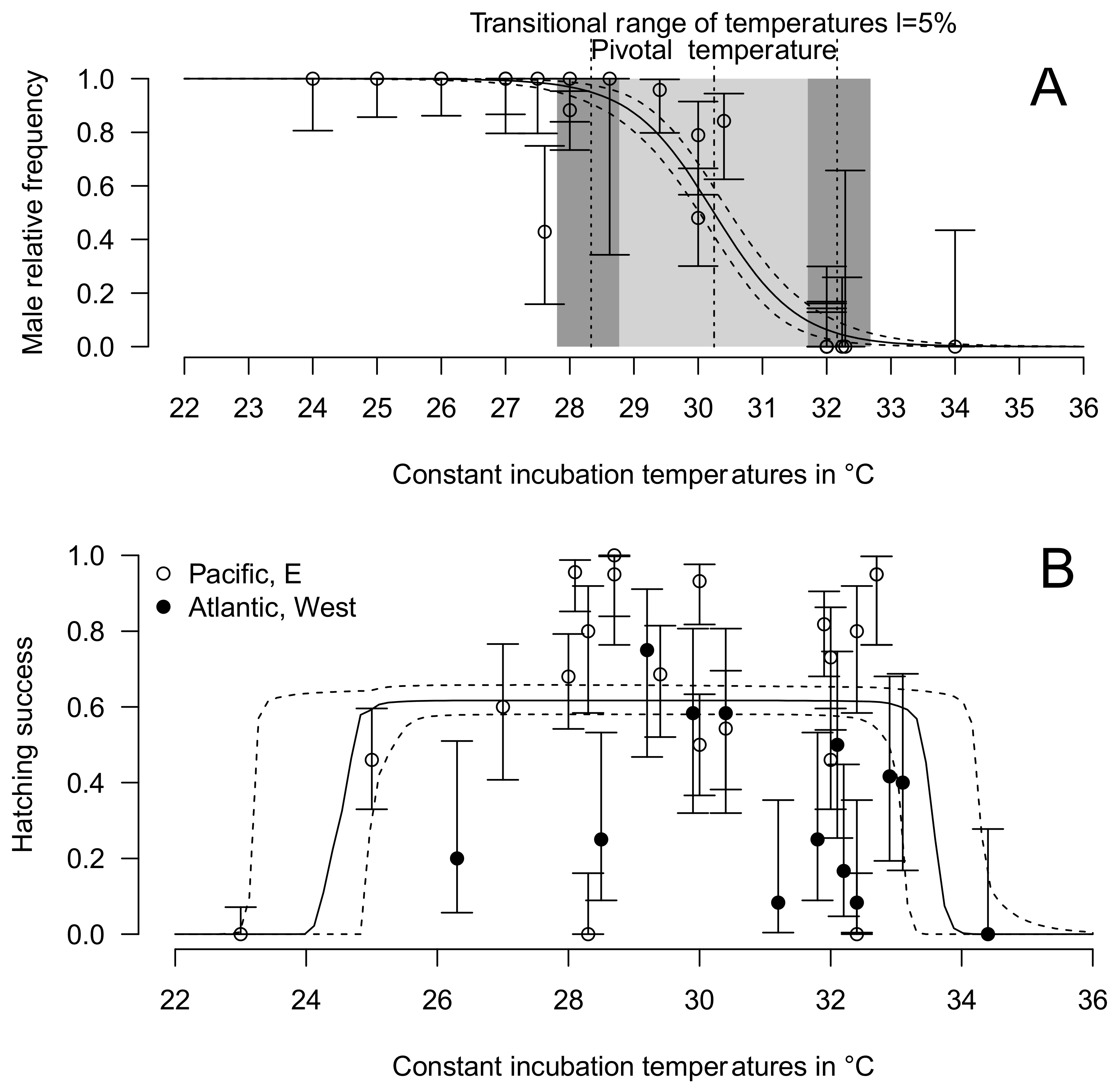
3.4. Thermal Reaction Norm for Sex Ratio and Hatching Success
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamrowski, R.L.; Limpus, C.; Jones, R.; Anderson, S.; Hamann, M. Temporal changes in artificial light exposure of marine turtle nesting areas. Glob. Chang. Biol. 2014, 20, 2437–2449. [Google Scholar] [CrossRef]
- Girondot, M.; Godfrey, M.H.; Ponge, L.; Rivalan, P. Modeling approaches to quantify leatherback nesting trends in French Guiana and Suriname. Chelonian Conserv. Biol. 2007, 6, 37–46. [Google Scholar] [CrossRef]
- Freedberg, S.; Wade, M.J. Cultural inheritance as a mechanism for population sex-ratio bias in reptiles. Evolution 2001, 55, 1049–1055. [Google Scholar] [CrossRef]
- Escalona, T.; Valenzuela, N.; Adams, D.C. Do local environmental factors and lunar cycle influence timing and synchrony of oviposition of a turtle with strict nocturnal nesting? Diversity 2019, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.L.M.; Luschi, P.; Hays, G.C. Detecting female precise natal philopatry in green turtles using assignment methods. Mol. Ecol. 2007, 16, 61–74. [Google Scholar]
- Lawrence, E. (Ed.) Henderson’s Dictionary of Biological Terms, 12th ed.; Prentice Hall: New York, NY, USA, 2000. [Google Scholar]
- Meylan, A.B.; Bowen, B.W.; Avise, J.C. A genetic test of the natal homing versus social facilitation models for green turtle migration. Science 1990, 248, 724–727. [Google Scholar]
- Hays, G.C.; Mazaris, A.D.; Schofield, G.; Laloë, J.O. Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination. Proc. Biol. Sci. 2017, 284, 20162576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, R.A. The Nest Environment and the Embryonic Development of Sea Turtles. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: New York, NY, USA, 1997; pp. 83–106. [Google Scholar]
- Kamel, S.J. Vegetation cover predicts temperature in nests of the hawksbill sea turtle: Implications for beach management and offspring sex ratios. Endanger. Species Res. 2013, 20, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Girondot, M.; Kaska, Y. Nest temperatures in a loggerhead-nesting beach in Turkey is more determined by sea surface temperature than air temperature. J. Therm. Biol. 2015, 47, 13–18. [Google Scholar] [CrossRef]
- Hays, G.C.; Ashworth, J.S.; Barnsley, M.J.; Broderick, A.C.; Emery, D.R.; Godley, B.J.; Henwood, A.; Jones, E.L. The importance of sand albedo for the thermal conditions on sea turtle nesting beaches. Oikos 2001, 93, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Fontes, A.F. Soil Albedo in Relation to Soil Color, Moisture and Roughness. Ph.D Thesis, The University of Arizona, Tucson, AZ, USA, 1996. [Google Scholar]
- Post, D.F.; Fimbres, A.; Matthias, A.D.; Sano, E.E.; Accioly, L.; Batchily, A.K.; Ferreira, L.G. Predicting soil albedo from soil color and spectral reflectance data. Soil Sci. Soc. Am. J. 2000, 64, 1027–1034. [Google Scholar] [CrossRef]
- Coates, A.G.; Jackson, J.B.C.; Collins, L.S.; Cronin, T.M.; Dowsett, H.J.; Bybell, L.M.; Jung, P.; Obando, J.A. Closure of the Isthmus of Panama: The near-shore marine record of Costa Rica and western Panama. Geol. Soc. Am. Bull. 1992, 104, 814–828. [Google Scholar] [CrossRef]
- Shanker, K.; Abreu-Grobois, A.; Bezy, V.; Briseño, R.; Colman, L.; Girard, A.; Girondot, M.; Jensen, M.; Manoharakrishnan, M.; Rguez-Baron, J.M.; et al. Olive ridleys. The Quirky Turtles that Conquered the World. SWOT 2021, 16, 24–31. [Google Scholar]
- Siebert, L.; Simkin, T. Volcanoes of the World: An Illustrated Catalog of Holocene Volcanoes and Their Eruptions. Available online: http://www.volcano.si.edu (accessed on 1 June 2019).
- DiMatteo, A.; Fujioka, E.; Wallace, B.; Hutchinson, B.; Cleary, J.; Halpin, P. SWOT Database Online. Available online: http://seamap.env.duke.edu/swot (accessed on 10 July 2011).
- Girondot, M. Embryogrowth: Tools to Analyze the Thermal Reaction Norm of Embryo Growth, 8.1. The Comprehensive R Archive Network. 2021. Available online: https://cran.r-project.org/package=embryogrowth (accessed on 20 September 2011).
- Castheloge, V.D.; de D. dos Santos, M.R.; de Castilhos, J.C.; de J. Filho, P.R.; de C. Gomes, L.; Clemente-Carvalho, R.B.G.; Ferreira, P.D. Pivotal temperature and hatchling sex ratio of olive ridley sea turtles Lepidochelys olivacea from the South Atlantic coast of Brazil. Herpetol. Conserv. Biol. 2018, 13, 488–496. [Google Scholar]
- McCoy, C.J.; Vogt, R.C.; Censky, E.J. Temperature-controlled sex determination in the sea turtle Lepidochelys olivacea. J. Herpetol. 1983, 17, 404–406. [Google Scholar] [CrossRef]
- Wibbels, T.; Rostal, D.C.; Byles, R. High pivotal temperature in the sex determination of the olive ridley sea turtle, Lepidochelys olivacea, from Playa Nancite, Costa Rica. Copeia 1998, 1998, 1086–1088. [Google Scholar]
- Michel-Morfin, J.E.; Gómez Muñoz, V.M.; Navarro Rodríguez, C. Morphometric model for sex assessment in hatchling olive ridley sea turtles. Chelonian Conserv. Biol. 2001, 40, 53–58. [Google Scholar]
- Navarro Sánchez, E.J. Efecto de la Temperatura de Incubación y la Diferenciación Sexual Sobre la Morfología de Crías de Tortuga Marina Lepidochelys olivacea. Master Thesis, Universidad Nacional Autónoma de México, México City, México, 2015. [Google Scholar]
- Ruiz García, N.A. Efectos de la Temperatura Sobre el Desarrollo Embrionario y el Desempeño de Crías de la Tortuga Golfina, Lepidochelys olivacea. Master Thesis, Universidad Nacional Autónoma de México, México City, México, 2014. [Google Scholar]
- Merchant-Larios, H.; Díaz-Hernández, V.; Marmolejo-Valencia, A. Gonadal morphogenesis and gene expression in reptiles with temperature-dependent sex determination. Sex. Dev. 2010, 4, 50–61. [Google Scholar] [CrossRef]
- Merchant-Larios, H.; Ruiz-Ramirez, S.; Moreno-Mendoza, N.; Marmolejo-Valencia, A. Correlation among thermosensitive period, estradiol response, and gonad differentiation in the sea turtle Lepidochelys olivacea. Gen. Comp. Endocrinol. 1997, 107, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Merchant-Larios, H.; Villalpando-Fierro, I.; Centeno-Urruiza, B. Gonadal morphogenesis under controlled temperature in the sea turtle Lepidochelys olivacea. Herpetol. Monogr. 1989, 3, 43–61. [Google Scholar]
- Sandoval Espinoza, S. Proporción Sexual en Crías de Tortuga Lepidochelys olivacea en Corrales de Incubación del Pacífico Mexicano. Ph.D. Thesis, Instituto Politécnico Nacional, La Paz, México, 2012. [Google Scholar]
- López Correa, J.Y. Diferenciación Gonádica en Crias de Lepidochelys olivacea (Eschscholtz, 1829) (Testudinata: Cheloniidae). Ph.D. Thesis, Instituto Politécnico Nacional, Centro Interdisciplinaria de Ciencias Marinas, La Paz, México, 2010. [Google Scholar]
- Wallace, B.P.; DiMatteo, A.D.; Hurley, B.J.; Finkbeiner, E.M.; Bolten, A.B.; Chaloupka, M.Y.; Hutchinson, B.J.; Abreu-Grobois, F.A.; Amorocho, D.; Bjorndal, K.A.; et al. Regional management units for marine turtles: A novel framework for prioritizing conservation and research across multiple scales. PLoS ONE 2010, 5, e15465. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M.; Bjorndal, K.A.; Bolten, A.B.; Bolker, B.M. Intraspecific application of the mid-domain effect model: Spatial and temporal nest distributions of green turtles, Chelonia mydas, at Tortuguero, Costa Rica. Ecol. Lett. 2005, 8, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, 2.5–6, The Comprehensive R Archive Network: 2019.
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Girondot, M. Phenology: Tools to Manage a Parametric Function that Describes Phenology, 7.8. The Comprehensive R Archive Network. 2021. Available online: https://cran.r-project.org/package=phenology (accessed on 20 September 2011).
- Abreu-Grobois, F.A.; Morales-Mérida, B.A.; Hart, C.E.; Guillon, J.-M.; Godfrey, M.H.; Navarro, E.; Girondot, M. Recent advances on the estimation of the thermal reaction norm for sex ratios. PeerJ 2020, 8, e8451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulin, V.; Delmas, V.; Girondot, M.; Godfrey, M.H.; Guillon, J.-M. Temperature-dependent sex determination and global change: Are some species at greater risk? Oecologia 2009, 160, 493–506. [Google Scholar] [CrossRef]
- Yu, L.; Gong, P. Google Earth as a virtual globe tool for Earth science applications at the global scale: Progress and perspectives. Int. J. Remote. Sens. 2012, 33, 3966–3986. [Google Scholar] [CrossRef]
- Valverde, R.A.; Wingard, S.; Gómez, F.; Tordoir, M.T.; Orrego, C.M. Field lethal incubation temperature of olive ridley sea turtle Lepidochelys olivacea embryos at a mass nesting rookery. Endanger. Species Res. 2010, 12, 77–86. [Google Scholar] [CrossRef]
- Morales-Merida, B.A.; Bustamante, D.M.; Monsinjon, J.; Girondot, M. Reaction norm of embryo growth rate dependent on incubation temperature in the Olive Ridley sea turtle, Lepidochelys olivacea, from Pacific Central America. J. Embryol. 2018, 1, 12–24. [Google Scholar]
- Morales-Mérida, B.A.; Contreras-Mérida, M.R.; Girondot, M. Pipping dynamics in marine turtle Lepidochelys olivacea nests. Trends Dev. Biol. 2019, 12, 23–30. [Google Scholar]
- Binhammer, M.R.; Beange, M.; Arauz, R. Sand temperature, sex ratios, and nest success in olive ridley sea turtles. Mar. Turt. Newsl. 2019, 159, 5–9. [Google Scholar]
- Dornfeld, T.C.; Robinson, N.J.; Tomillo, P.S.; Paladino, F.V. Ecology of solitary nesting olive ridley sea turtles at Playa Grande, Costa Rica. Mar. Biol. 2014, 162, 123–139. [Google Scholar] [CrossRef]
- Santidrián Tomillo, P.; Oro, D.; Paladino, F.V.; Piedra, R.; Sieg, A.E.; Spotila, J.R. High beach temperatures increased female-biased primary sex ratios but reduced output of female hatchlings in the leatherback turtle. Biol. Conserv. 2014, 176, 71–79. [Google Scholar] [CrossRef]
- Valenzuela, N.; Literman, R.; Neuwald, J.L.; Mizoguchi, B.; Iverson, J.B.; Riley, J.L.; Litzgus, J.D. Extreme thermal fluctuations from climate change unexpectedly accelerate demographic collapse of vertebrates with temperature-dependent sex determination. Sci. Rep. 2019, 9, 4254. [Google Scholar] [CrossRef] [Green Version]
- Tilley, D.; Ball, S.; Ellick, J.; Godley, B.J.; Weber, N.; Weber, S.B.; Broderick, A.C. No evidence of fine scale thermal adaptation in green turtles. J. Exp. Mar. Biol. Ecol. 2019, 514–515, 110–117. [Google Scholar] [CrossRef]
- Monsinjon, J.R.; Wyneken, J.; Rusenko, K.; López-Mendilaharsu, M.; Lara, P.; Santos, A.; dei Marcovaldi, M.A.G.; Fuentes, M.M.P.B.; Kaska, Y.; Tucek, J.; et al. The climatic debt of loggerhead sea turtle populations in a warming world. Ecol. Indic. 2019, 107, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Patrício, A.R.; Hawkes, L.A.; Monsinjon, J.R.; Godley, B.J.; Fuentes, M. Climate change and marine turtles: Recent advances and future directions. Endanger. Species Res. 2021, 44, 363–395. [Google Scholar] [CrossRef]
- Pieau, C. Sur la proportion sexuelle chez les embryons de deux Chéloniens (Testudo graeca L. et Emys orbicularis L.) issus d’oeufs incubés artificiellement. Comptes Rendus De L’académie Des Sci. Paris Série D 1971, 272, 3071–3074. [Google Scholar]
- Pieau, C. Effets de la température sur le développement des glandes génitales chez les embryons de deux Chéloniens, Emys orbicularis L. et Testudo graeca L. Comptes Rendus De L’académie Des Sci. Paris Série D 1972, 274, 719–722. [Google Scholar]
| Temporal Model | AIC | ΔAIC | Akaike Weight |
|---|---|---|---|
| Constant | 2254.397 | 12.53 | 0.002 |
| Exponential | 2247.399 | 5.53 | 0.06 |
| Year-specific | 2241.867 | 0 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales Mérida, A.; Helier, A.; Cortés-Gómez, A.A.; Girondot, M. Hatching Success Rather Than Temperature-Dependent Sex Determination as the Main Driver of Olive Ridley (Lepidochelys olivacea) Nesting Activity in the Pacific Coast of Central America. Animals 2021, 11, 3168. https://doi.org/10.3390/ani11113168
Morales Mérida A, Helier A, Cortés-Gómez AA, Girondot M. Hatching Success Rather Than Temperature-Dependent Sex Determination as the Main Driver of Olive Ridley (Lepidochelys olivacea) Nesting Activity in the Pacific Coast of Central America. Animals. 2021; 11(11):3168. https://doi.org/10.3390/ani11113168
Chicago/Turabian StyleMorales Mérida, Alejandra, Aude Helier, Adriana A. Cortés-Gómez, and Marc Girondot. 2021. "Hatching Success Rather Than Temperature-Dependent Sex Determination as the Main Driver of Olive Ridley (Lepidochelys olivacea) Nesting Activity in the Pacific Coast of Central America" Animals 11, no. 11: 3168. https://doi.org/10.3390/ani11113168
APA StyleMorales Mérida, A., Helier, A., Cortés-Gómez, A. A., & Girondot, M. (2021). Hatching Success Rather Than Temperature-Dependent Sex Determination as the Main Driver of Olive Ridley (Lepidochelys olivacea) Nesting Activity in the Pacific Coast of Central America. Animals, 11(11), 3168. https://doi.org/10.3390/ani11113168








