Long-Distance Movements of Feral Cats in Semi-Arid South Australia and Implications for Conservation Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Essl, F.; Bacher, S.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Katsanevakis, S.; Kowarik, I.; Kühn, I.; Pyšek, P.; Rabitsch, W.; et al. Which taxa are alien? Criteria, applications, and uncertainties. BioScience 2018, 68, 496–509. [Google Scholar] [CrossRef]
- Doherty, T.S.; Dickman, C.R.; Johnson, C.N.; Legge, S.M.; Ritchie, E.G.; Woinarski, J.C.Z. Impacts and management of feral cats Felis catus in Australia. Mammal. Rev. 2017, 47, 83–97. [Google Scholar] [CrossRef]
- Walton, Z.; Samelius, G.; Odden, M.; Willebrand, T. Long-distance dispersal in red foxes Vulpes vulpes revealed by GPS tracking. Eur. J. Wildl. Res. 2018, 64, 64. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.H.; Sargeant, A.B. Dispersal patterns of red foxes relative to population density. J. Wildl. Manag. 1993, 57, 526–533. [Google Scholar] [CrossRef]
- Powell, R.A.; Mitchell, M.S. What is a home range? J. Mammal. 2012, 93, 948–958. [Google Scholar] [CrossRef] [Green Version]
- Bonte, D.; Van Dyck, H.; Bullock, J.M.; Coulon, A.; Delgado, M.; Gibbs, M.; Lehouck, V.; Matthysen, E.; Mustin, K.; Saastamoinen, M.; et al. Costs of dispersal. Biol. Rev. 2012, 87, 290–312. [Google Scholar] [CrossRef]
- Mueller, T.; Fagan, W.F.; Grimm, V. Integrating individual search and navigation behaviors in mechanistic movement models. Theor. Ecol. 2011, 4, 341–355. [Google Scholar] [CrossRef]
- Teitelbaum, C.S.; Mueller, T. Beyond migration: Causes and consequences of nomadic animal movements. Trends Ecol. Evol. 2019, 34, 569–581. [Google Scholar] [CrossRef]
- Burt, W.H. Territoriality and home range concepts as applied to mammals. J. Mammal. 1943, 24, 346–352. [Google Scholar] [CrossRef]
- Harestad, A.S.; Bunnel, F.L. Home range and body weight--A reevaluation. Ecology 1979, 60, 389–402. [Google Scholar] [CrossRef]
- Moseby, K.E.; Stott, J.; Crisp, H. Movement patterns of feral predators in an arid environment implications for control through poison baiting. Wildl. Res. 2009, 36, 422–435. [Google Scholar] [CrossRef]
- Recio, M.R.; Mathieu, R.; Maloney, R.; Seddon, P.J. First results of feral cats (Felis catus) monitored with GPS collars in New Zealand. N. Zealand J. Ecol. 2010, 34, 288–296. [Google Scholar]
- Yamane, A.; Ono, Y.; Doi, T. Home range size and spacing pattern of a feral cat population on a small island. J. Mammal. Soc. Jpn. 1994, 19, 9–20. [Google Scholar] [CrossRef]
- Roshier, D.A.; Carter, A. Space use and interactions of two introduced mesopredators, European red fox and feral cat, in an arid landscape. Ecosphere 2021, 12, e03628. [Google Scholar] [CrossRef]
- McGregor, H.; Legge, S.; Potts, J.; Jones, M.; Johnson, C. Density and home range of feral cats in north-western Australia. Wildl. Res. 2015, 42, 223–231. [Google Scholar] [CrossRef]
- Konecny, M.J. Home range and activity patterns of feral house cats in the Galápagos Islands. Oikos 1987, 50, 17–23. [Google Scholar] [CrossRef]
- Bengsen, A.J.; Algar, D.; Ballard, G.; Buckmaster, T.; Comer, S.; Fleming, P.J.S.; Friend, J.A.; Johnston, M.; McGregor, H.; Moseby, K.; et al. Feral cat home-range size varies predictably with landscape productivity and population density. J. Zool. 2016, 298, 112–120. [Google Scholar] [CrossRef]
- Aguilar, G.D.; Farnworth, M.J.; Winder, L. Mapping the stray domestic cat (Felis catus) population in New Zealand: Species distribution modelling with a climate change scenario and implications for protected areas. Appl. Geogr. 2015, 63, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, T.C.; Wiskirchen, K.H.; Ditchkoff, S.S. A novel method for detecting extra-home range movements (EHRMs) by animals and recommendations for future EHRM studies. PLoS ONE 2020, 15, e0242328. [Google Scholar] [CrossRef]
- Stark, D.J.; Vaughan, I.P.; Ramirez Saldivar, D.A.; Nathan, S.K.S.S.; Goossens, B. Evaluating methods for estimating home ranges using GPS collars: A comparison using proboscis monkeys (Nasalis larvatus). PLoS ONE 2017, 12, e0174891. [Google Scholar] [CrossRef]
- Peris, A.; Closa, F.; Marco, I.; Acevedo, P.; Barasona, J.A.; Casas-Díaz, E. Towards the comparison of home range estimators obtained from contrasting tracking regimes: The wild boar as a case study. Eur. J. Wildl. Res. 2020, 66, 32. [Google Scholar] [CrossRef]
- Silva, I.; Crane, M.; Marshall, B.M.; Strine, C.T. Reptiles on the wrong track? Moving beyond traditional estimators with dynamic Brownian Bridge Movement Models. Mov. Ecol. 2020, 8, 43. [Google Scholar] [CrossRef]
- De Preu, N.; Pearce, D. Bounceback Progress Report. Dep. Environ. Herit. Adel 2006. Unpublished report. [Google Scholar]
- Brandle, R.; Mooney, T.; De Preu, N.; Garnett, S.; Latch, P.; Lindenmayer, D.; Woinarski, J. Broadscale feral predator and herbivore control for yellow-footed rock-wallabies Petrogale xanthopus ssp. xanthopus: Improved resilience for plants and animals= Bounceback. In Recovering Australian Threatened Species: A Book of Hope; Garnett, S., Latch, P., Lindenmayer, D., Woinarski, J., Eds.; CSIRO Publishing: Collingwood, Australia, 2018; pp. 135–146. [Google Scholar]
- Moseby, K.; Hodgens, P.; Bannister, H.; Mooney, P.; Brandle, R.; Lynch, C.; Young, C.; Jansen, J.; Jensen, M. The ecological costs and benefits of a feral cat poison-baiting programme for protection of reintroduced populations of the western quoll and brushtail possum. Austral. Ecol. 2021. [Google Scholar] [CrossRef]
- Moseby, K.E.; Brandle, R.; Hodgens, P.; Bannister, H.L. Can reintroductions to degraded habitat succeed? A test using the common brushtail possum. Austral. Ecol. 2020, 45, 675–690. [Google Scholar] [CrossRef]
- Moseby, K.E.; Hodgens, P.; Peacock, D.; Mooney, P.; Brandle, R.; Lynch, C.; West, R.; Young, C.M.; Bannister, H.; Copley, P.; et al. Intensive monitoring, the key to identifying cat predation as a major threat to native carnivore (Dasyurus geoffroii) reintroduction. Biodivers. Conserv. 2021, 30, 1547–1571. [Google Scholar] [CrossRef]
- Newsome, A. Feral cats: An overview. In The Impact of Cats on Native Wildlife; Potter, C., Ed.; Australian National Parks and Wildlife Service: Canberra, Australia, 1991; pp. 7–13. [Google Scholar]
- Edwards, G.P.; De Preu, N.; Shakeshaft, B.J.; Crealy, I.V.; Paltridge, R.M. Home range and movements of male feral cats (Felis catus) in a semiarid woodland environment in central Australia. Austral. Ecol. 2001, 26, 93–101. [Google Scholar] [CrossRef]
- Palmer, R.; Anderson, H.; Richards, B. Predator Control Baiting and Monitoring Program, Yarraloola and Red Hill, Pilbara Region, Western Australia; Department of Biodiversity, Conservation and Attractions, Government of Western Australia: Perth, Australia, 2020; Annual and Final Report–Year 5. Available online: https://library.dbca.wa.gov.au/static/FullTextFiles/072463.pdf (accessed on 20 September 2021).
- Hebblewhite, M.; Haydon, D.T. Distinguishing technology from biology: A critical review of the use of GPS telemetry data in ecology. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2303–2312. [Google Scholar] [CrossRef]
- Matthews, A.; Ruykys, L.; Ellis, B.; FitzGibbon, S.; Lunney, D.; Crowther, M.S.; Glen, A.S.; Purcell, B.; Moseby, K.; Stott, J.; et al. The success of GPS collar deployments on mammals in Australia. Aust. Mammal. 2013, 35, 65–83. [Google Scholar] [CrossRef]
- Seddon, P.J.; Maloney, R. Tracking Wildlife Radio-Tag Signals by Light Fixed-Wing Aircraft; Department of Conservation: Wellington, New Zealand, 2004. [Google Scholar]
- Molsher, R.; Newsome, A.; Dickman, C. Feeding ecology and population dynamics of the feral cat (Felis catus) in relation to the availability of prey in central-eastern New South Wales. Wildl. Res. 1999, 26, 593–607. [Google Scholar] [CrossRef]
- McGregor, H.W.; Cliff, H.B.; Kanowski, J. Habitat preference for fire scars by feral cats in Cape York Peninsula, Australia. Wildl. Res. 2016, 43, 623–633. [Google Scholar] [CrossRef]
- BOM. Australian Government Bureau of Meteorology: Climate Statistics for Australian Locations. Available online: www.bom.gov.au (accessed on 25 March 2021).
- QGIS Development Team. QGIS, Version 3.6, QGIS Geographic Information System. 2020. Available online: https://www.qgis.org (accessed on 21 March 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 8 October 2021).
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com (accessed on 20 September 2021).
- Bjørneraas, K.; Van Moorter, B.; Rolandsen, C.M.; Herfindal, I. Screening global positioning system location data for errors using animal movement characteristics. J. Wildl. Manag. 2010, 74, 1361–1366. [Google Scholar] [CrossRef]
- Clausen, L.; Cowen, S.; Pinder, J.; Danks, A.; Thomas, A.; Bell, L.; Speldewinde, P.; Comer, S.; Algar, D. Fortescue Marsh Feral Cat Baiting Program (Christmas Creek Water Management Scheme) Year 5 Annual Report; Department of Parks and Wildlife: Perth, Australia, 2016. [Google Scholar]
- Pettigrew, J. A burst of feral cats in the Diamantina: A lesson for the management of pest species. In Cat Management Workshop Proceedings; Queensland Department of Environment and Heritage: Brisbane, Australia, 1993; Volume 25, pp. 25–32. [Google Scholar]
- Paton, D.C. Developing a community-based feral cat control program for Kangaroo Island. In Kangaroo Island Rotary Club Seminar; 2003; Unpublished. [Google Scholar]
- McGregor, H.W.; Legge, S.; Jones, M.E.; Johnson, C.N. Extraterritorial hunting expeditions to intense fire scars by feral cats. Sci. Rep. 2016, 6, 22559. [Google Scholar] [CrossRef] [Green Version]
- Seaman, D.E.; Powell, R.A. An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 1996, 77, 2075–2085. [Google Scholar] [CrossRef] [Green Version]
- Kie, J.G.; Matthiopoulos, J.; Fieberg, J.; Powell, R.A.; Cagnacci, F.; Mitchell, M.S.; Gaillard, J.-M.; Moorcroft, P.R. The home-range concept: Are traditional estimators still relevant with modern telemetry technology? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2221–2231. [Google Scholar] [CrossRef] [Green Version]
- Wilson, G.; Gray, R.J.; Sofyan, H. Identifying the variation in utilization density estimators and home ranges of elephant clans in Aceh, Sumatra, Indonesia. Eur. J. Wildl. Res. 2020, 66, 88. [Google Scholar] [CrossRef]
- Calenge, C. The package adehabitat for the R software: Tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Jansen, J.; Jansen, J.; Jones, M.E.; Brandle, R.; Peacock, D.E. Adaption of an ocean floor transect survey model to an open landscape connecting rabbit warrens to geology and landform. Manuscript in preparation. 2021. [Google Scholar]
- Burrows, N.D.; Algar, D.; Robinson, A.D.; Sinagra, J.; Ward, B.; Liddelow, G. Controlling introduced predators in the Gibson Desert of Western Australia. J. Arid Environ. 2003, 55, 691–713. [Google Scholar] [CrossRef]
- Moseby, K.E.; Hill, B.M. The use of poison baits to control feral cats and red foxes in arid South Australia I. Aerial baiting trials. Wildl. Res. 2011, 38, 338–349. [Google Scholar] [CrossRef]
- Lazenby, B.T.; Mooney, N.J.; Dickman, C.R. Effects of low-level culling of feral cats in open populations: A case study from the forests of southern Tasmania. Wildl. Res. 2014, 41, 407–420. [Google Scholar] [CrossRef]
- Stobo-Wilson, A.M.; Brandle, R.; Johnson, C.N.; Jones, M.E. Management of invasive mesopredators in the Flinders Ranges, South Australia: Effectiveness and implications. Wildl. Res. 2020, 47, 720–730. [Google Scholar] [CrossRef]
- Lieury, N.; Ruette, S.; Devillard, S.; Albaret, M.; Drouyer, F.; Baudoux, B.; Millon, A. Compensatory immigration challenges predator control: An experimental evidence-based approach improves management. J. Wildl. Manag. 2015, 79, 425–434. [Google Scholar] [CrossRef]
- Minnie, L.; Gaylard, A.; Kerley, G.I. Compensatory life-history responses of a mesopredator may undermine carnivore management efforts. J. Appl. Ecol. 2016, 53, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Morin, D.J.; Kelly, M.J. The dynamic nature of territoriality, transience and biding in an exploited coyote population. Wildl. Biol. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Lohr, C.A.; Algar, D. Managing feral cats through an adaptive framework in an arid landscape. Sci. Total Environ. 2020, 720, 137631. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Woolmore, C.; Latham, M.C.; Latham, A.D.M.; Pech, R.P.; Anderson, D.P. Seasonal and individual variation in selection by feral cats for areas with widespread primary prey and localised alternative prey. Wildl. Res. 2015, 41, 650–661. [Google Scholar] [CrossRef]
- Doherty, T.S.; Davis, R.A.; van Etten, E.J.B.; Algar, D.; Collier, N.; Dickman, C.R.; Edwards, G.; Masters, P.; Palmer, R.; Robinson, S. A continental-scale analysis of feral cat diet in Australia. J. Biogeogr. 2015, 42, 964–975. [Google Scholar] [CrossRef]
- Fagan, W.F.; Lewis, M.A.; Auger-Méthé, M.; Avgar, T.; Benhamou, S.; Breed, G.; LaDage, L.; Schlägel, U.E.; Tang, W.-w.; Papastamatiou, Y.P.; et al. Spatial memory and animal movement. Ecol. Lett. 2013, 16, 1316–1329. [Google Scholar] [CrossRef]
- Myers, K.; Parker, B. Effect of Severe Drought on Rabbit Numbers and Distribution in a Refuge Area in Semiarid North-western New South Wales. Wildl. Res. 1975, 2, 103–120. [Google Scholar] [CrossRef]
- Wells, K.; O’Hara, R.B.; Cooke, B.D.; Mutze, G.J.; Prowse, T.A.A.; Fordham, D.A. Environmental effects and individual body condition drive seasonal fecundity of rabbits: Identifying acute and lagged processes. Oecologia 2016, 181, 853–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, J.; Bowen, Z. Population dynamics, diet and aspects of the biology of feral cats and foxes in arid South Australia. Wildl. Res. 2001, 28, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Holden, C.; Mutze, G. Impact of rabbit haemorrhagic disease on introduced predators in the Flinders Ranges, South Australia. Wildl. Res. 2002, 29, 615–626. [Google Scholar] [CrossRef]
- McGregor, H.; Moseby, K.; Johnson, C.N.; Legge, S. The short-term response of feral cats to rabbit population decline: Are alternative native prey more at risk? Biol. Invasions 2020, 22, 799–811. [Google Scholar] [CrossRef]
- Williams, C.K.; Parer, I.; Coman, B.; Burley, J.; Braysher, M. Managing Vertebrate Pests: Rabbits; Australian Government Publishing Service: Canberra, Australia, 1995. [Google Scholar]
- Moseby, K.E.; McGregor, H.; Read, J.L. The lethal 23%: Predator demography influences predation risk for threatened prey. Anim. Conserv. 2021, 24, 217–229. [Google Scholar] [CrossRef]
- Christensen, P.E.; Ward, B.G.; Sims, C. Predicting bait uptake by feral cats, Felis catus, in semi-arid environments. Ecol. Manag. Restor. 2012, 14, 47–53. [Google Scholar] [CrossRef]
- Devillard, S.; Say, E.J.; Pontier, D. Molecular and behavioural analyses reveal male-biased dispersal between social groups of domestic cats. Écoscience 2004, 11, 175–180. [Google Scholar] [CrossRef]
- Nathan, R.; Getz, W.M.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, P.E. A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonewald-Cox, C.M.; Bayless, J.W. The boundary model: A geographical analysis of design and conservation of nature reserves. Biol. Conserv. 1986, 38, 305–322. [Google Scholar] [CrossRef]
- Landres, P.B.; Knight, R.L.; Pickett, S.T.; Cadenasso, M. Ecological effects of administrative boundaries. Steward. Across Boundaries 1998, 39, 39–64. [Google Scholar]
- Martins, T.; Brooke, M.d.L.; Hilton, G.; Farnsworth, S.; Gould, J.; Pain, D. Costing eradications of alien mammals from islands. Anim. Conserv. 2006, 9, 439–444. [Google Scholar] [CrossRef]
- Lurgi, M.; Ritchie, E.G.; Fordham, D.A. Eradicating abundant invasive prey could cause unexpected and varied biodiversity outcomes: The importance of multispecies interactions. J. Appl. Ecol. 2018, 55, 2396–2407. [Google Scholar] [CrossRef] [Green Version]
- Jansen, J.; Dean, A.T.; Johnson, C.N.; Axford, G.; Lynch, C.; Moseby, K.E.; Brandle, R.; Peacock, D.E.; Jones, M.E. Effect of rabbit control on cat activity in an open landscape. Manuscript in preparation. 2021. [Google Scholar]
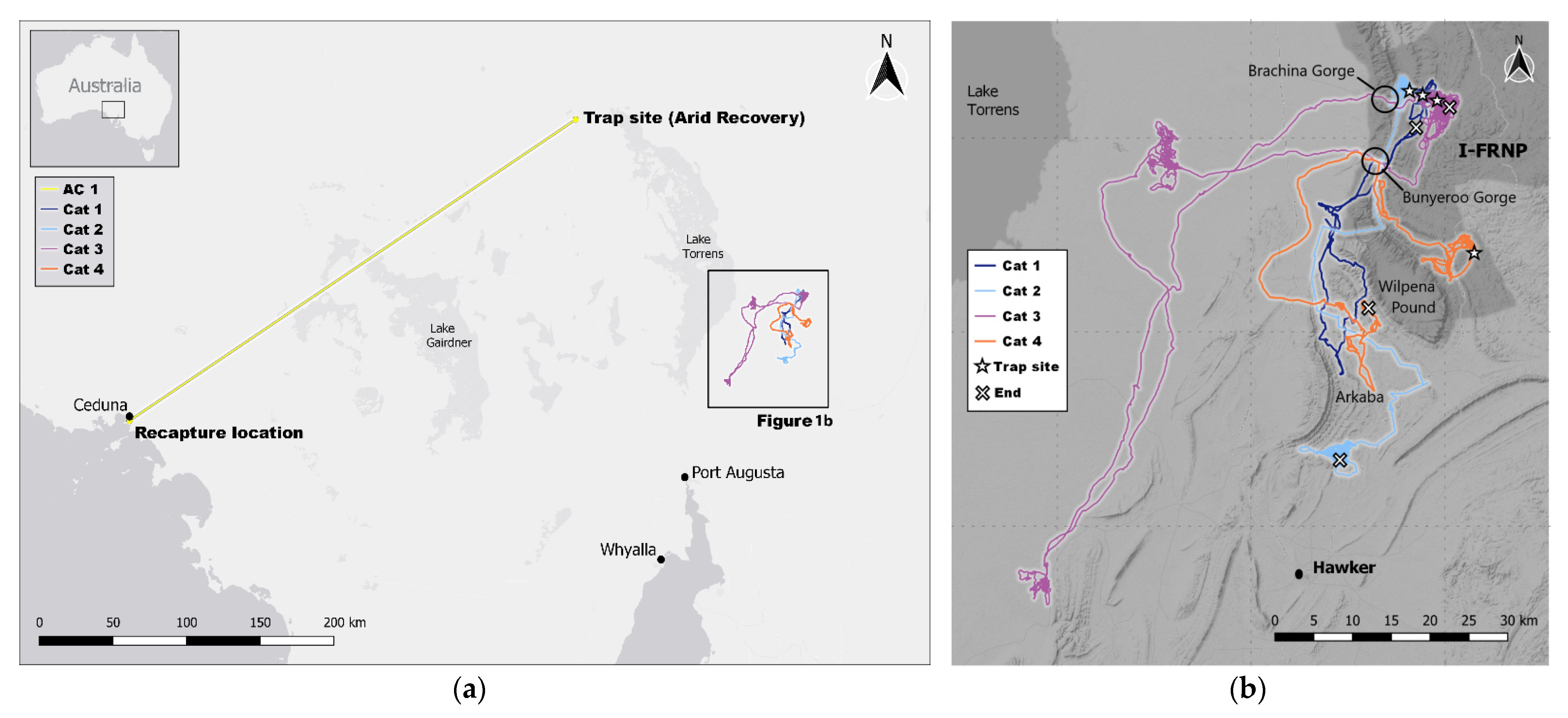
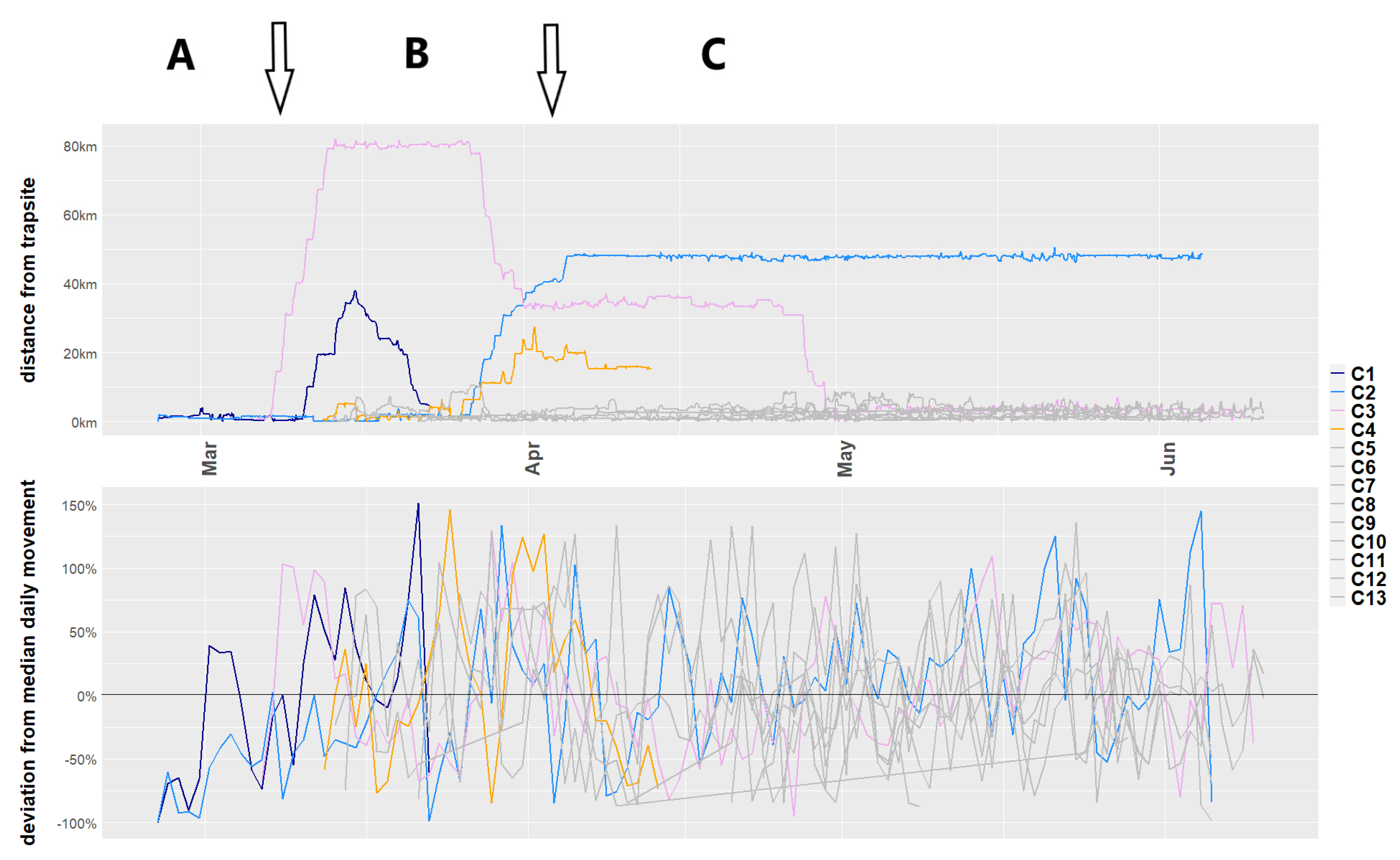
| Cat 1 | Cat 2 | Cat 3 | Cat 4 | AC1 | |
| Sex | M | M | M | F | M |
| Trap date | 25 February 2020 | 25 February 2020 | 6 March 2020 | 12 March 2020 | 19 February 2017 |
| Trap site | IFRNP Brachina | IFRNP Brachina | IFRNP Brachina | IFRNP Upalinna | Arid Recovery |
| Trap site Easting | 271,800 | 270,929 | 272,157 | 278,284 | 685,223 |
| Trap site Northing | 6,531,098 | 6,531,166 | 6,530,531 | 6,510,908 | 6,652,470 |
| Zone | 54 J | 54 J | 54 J | 54 J | 53 |
| Weight (kg) | 2.1 | 2.7 | 4.1 | 2.4 | 2.6 |
| Collar weight (g) | 80 | 89 | 90 | 83 | 65 |
| Collar % of bodyweight | 3.81 | 3.27 | 2.22 | 3.42 | 2.5 |
| Colour | grey tabby | bright grey tabby | grey tabby | grey tabby | grey tabby |
| Fate | Natural death | Eradicat® intake | Euthanised | Natural death | Euthanised |
| Last GPS movement | 20 March 2020 | 5 June 2020 | 9 June 2020 | 10 April 2020 | 11 July 2019 |
| Last location | Yanyanna track | Arkaba South | Brachina East | Arkaba North | Ceduna |
| Last location Easting | 271,318 | 260,719 | 273,138 | 264,922 | 370,358 |
| Last location Northing | 6,526,582 | 6,483,663 | 6,530,200 | 6,503,602 | 6,442,562 |
| Days collared | 39 | 103 | 128 | 42 | 872 |
| Final weight (kg) | - | 2.8 | 4.3 | - | 5.0 |
| Weight loss/gain (g) | - | 30 | 235 | - | 2400 |
| Median distance/day (m) | 7350 | 6525 | 9375 | 7220 | - |
| Mean dist./day ± SD (m) | 6920 ± 4480 | 6702 ± 3632 | 9938 ± 4664 | 7808 ± 4593 | |
| Total distance (km) | 193.8 | 683.6 | 954.1 | 257.7 | - |
| Longest diameter distance (km) | 40.5 | 52.7 | 85.6 | 31.2 | 369 |
| 95% MCP (ha) | 23,341 | 56,804 | 157,834 | 42,743 | - |
| 95% KDE href (ha) | 49,841 | 47,348 | 207,727 | 43,514 | - |
| 95% KDE lscv (ha) | 6683 | 1634 | 19,171 | 5095 | - |
| 95% BRB (ha) | 2815 | 4764 | 6332 | 6575 | - |
| 50% BRB (ha) | 130 | 100 | 314 | 88 | - |
| Locality | Distance Moved | Home Range Size | Duration Used for Calculation | Method of Calculating | Reference |
|---|---|---|---|---|---|
| Flinders Ranges (IFRNP) and Roxby Downs (AR), SA | 85 km and returned (IFRNP) and 370 km (AR) | max. male (IFRNP) 157,834 ha max. female (FR) 42,743 ha | 3 months (IFRNP) and 2 years (AR) | 95% MCP and other | This study |
| Yathong Nature Reserve, NSW | 8–48 and over 200 km | max. male 990 ha max. female 270 ha | 3 years | MCP | [28] |
| Scotia Wildlife Reserve, NSW | male: 164 km and returned, female: 150 km and returned | max. male 331,351 ha max. female 196,566 ha | seasonal over 3 years | 95% MCP and other | [14] |
| Fortescue River (Pilbara), WA | male: 130 km and returned | - | in 19 days | - | [41] |
| Diamantina, QLD | female cat 110 km and all 7 males ≥ 20 km | - | in 10 days | - | [42] |
| Kangaroo Island, SA | Aprox. 50 km | max. male 567 ha max. female 467 ha | 2 years | MCP | [43] |
| Arid Recovery Reserve, SA | 45 km in 2 days, 26 km in 3 days 35 km in 8 months and returned | max. male 13,198 ha max. female 3565 ha | 2 months | 95% MCP | [11] |
| Hamilton Downs station, NT | 34 km | mean male 2210.5 ha | 15–24 months | 100% MCP | [29] |
| Kimberley, WA | 30 km and returned | max. male 2006 ha | 3 years | 95% kernel-based | [44] |
| Pilbara, WA | - | max. male 20,897 ha max. female 22,904 ha | 2 years | 95% MCP | [30] |
| global | - | median male 510 ha max. female 2320 ha | - | MCP, kernel-based and other | [17] |
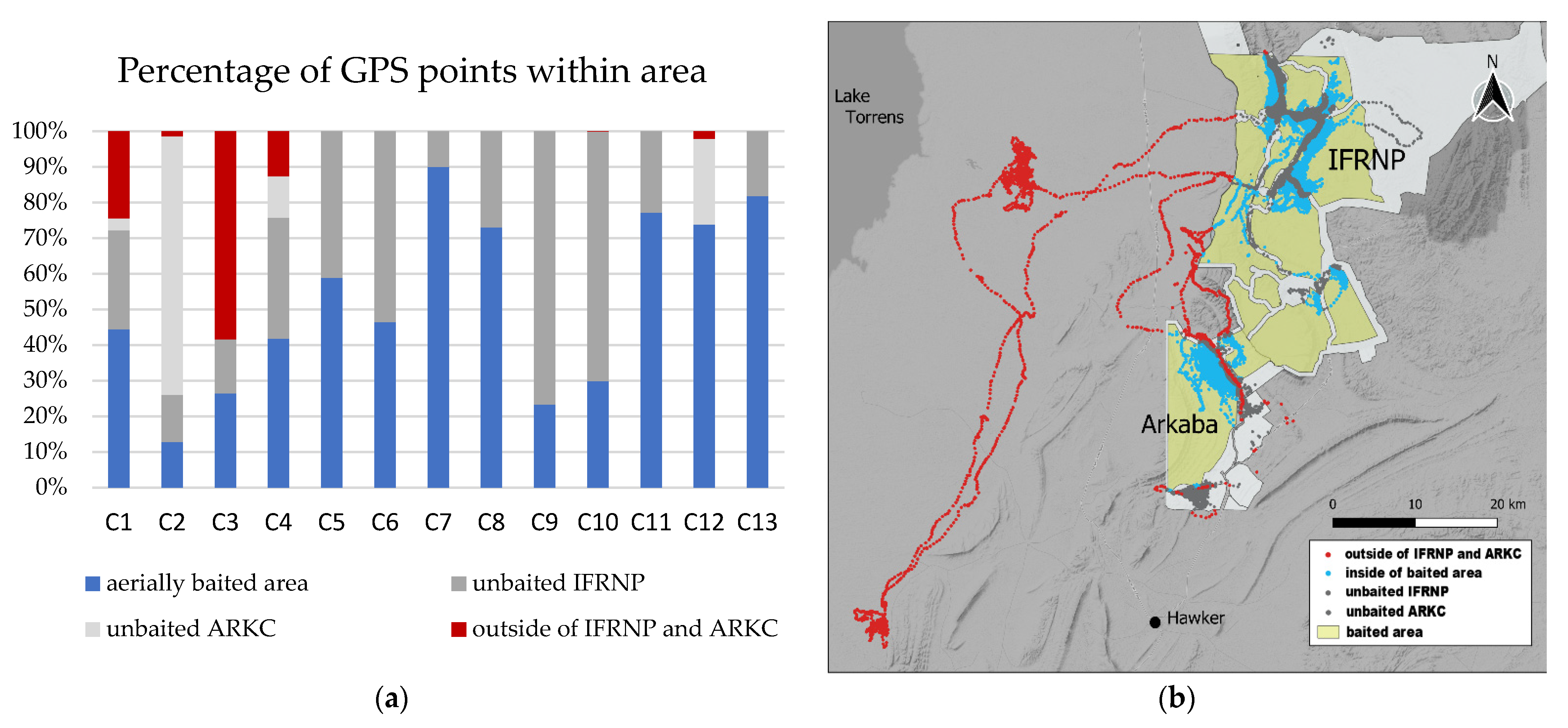
| ID | Number of GPS Fixes | Number of GPS Fixes Within | Percentage of GPS Fixes Within | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baited Area | Unbaited IFRNP | Unbaited ARKC | Outside | Baited Area | Unbaited IFRNP | Unbaited ARKC | Outside | ||
| C1 | 1884 | 836 | 524 | 64 | 460 | 44.4 | 27.8 | 3.4 | 24.4 |
| C2 | 6014 | 766 | 797 | 4369 | 82 | 12.7 | 13.3 | 72.7 | 1.4 |
| C3 | 7677 | 2031 | 1163 | 0 | 4483 | 26.5 | 15.2 | 0 | 58.4 |
| C4 | 2222 | 929 | 753 | 260 | 280 | 41.8 | 33.9 | 11.7 | 12.6 |
| C5 | 4704 | 2769 | 1935 | 0 | 0 | 58.9 | 41.1 | 0 | 0 |
| C6 | 5029 | 2337 | 2692 | 0 | 0 | 46.5 | 53.5 | 0 | 0 |
| C7 | 4266 | 3840 | 426 | 0 | 0 | 90.0 | 10.0 | 0 | 0 |
| C8 | 2079 | 1519 | 560 | 0 | 0 | 73.1 | 26.9 | 0 | 0 |
| C9 | 1526 | 356 | 1170 | 0 | 0 | 23.3 | 76.7 | 0 | 0 |
| C10 | 3653 | 1090 | 2561 | 0 | 2 | 29.8 | 70.1 | 0 | 0.1 |
| C11 | 2330 | 1798 | 532 | 0 | 0 | 77.2 | 22.8 | 0 | 0 |
| C12 | 7092 | 5233 | 0 | 1707 | 152 | 73.8 | 0 | 24.1 | 2.1 |
| C13 | 4614 | 3777 | 837 | 0 | 0 | 81.9 | 18.1 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, J.; McGregor, H.; Axford, G.; Dean, A.T.; Comte, S.; Johnson, C.N.; Moseby, K.E.; Brandle, R.; Peacock, D.E.; Jones, M.E. Long-Distance Movements of Feral Cats in Semi-Arid South Australia and Implications for Conservation Management. Animals 2021, 11, 3125. https://doi.org/10.3390/ani11113125
Jansen J, McGregor H, Axford G, Dean AT, Comte S, Johnson CN, Moseby KE, Brandle R, Peacock DE, Jones ME. Long-Distance Movements of Feral Cats in Semi-Arid South Australia and Implications for Conservation Management. Animals. 2021; 11(11):3125. https://doi.org/10.3390/ani11113125
Chicago/Turabian StyleJansen, Jeroen, Hugh McGregor, Geoff Axford, Abbey T. Dean, Sebastien Comte, Chris N. Johnson, Katherine E. Moseby, Robert Brandle, David E. Peacock, and Menna E. Jones. 2021. "Long-Distance Movements of Feral Cats in Semi-Arid South Australia and Implications for Conservation Management" Animals 11, no. 11: 3125. https://doi.org/10.3390/ani11113125
APA StyleJansen, J., McGregor, H., Axford, G., Dean, A. T., Comte, S., Johnson, C. N., Moseby, K. E., Brandle, R., Peacock, D. E., & Jones, M. E. (2021). Long-Distance Movements of Feral Cats in Semi-Arid South Australia and Implications for Conservation Management. Animals, 11(11), 3125. https://doi.org/10.3390/ani11113125






