Developing a Reliable Welfare Assessment Tool for Captive Hibernatory Bear Species
Abstract
:Simple Summary
Abstract
1. Introduction
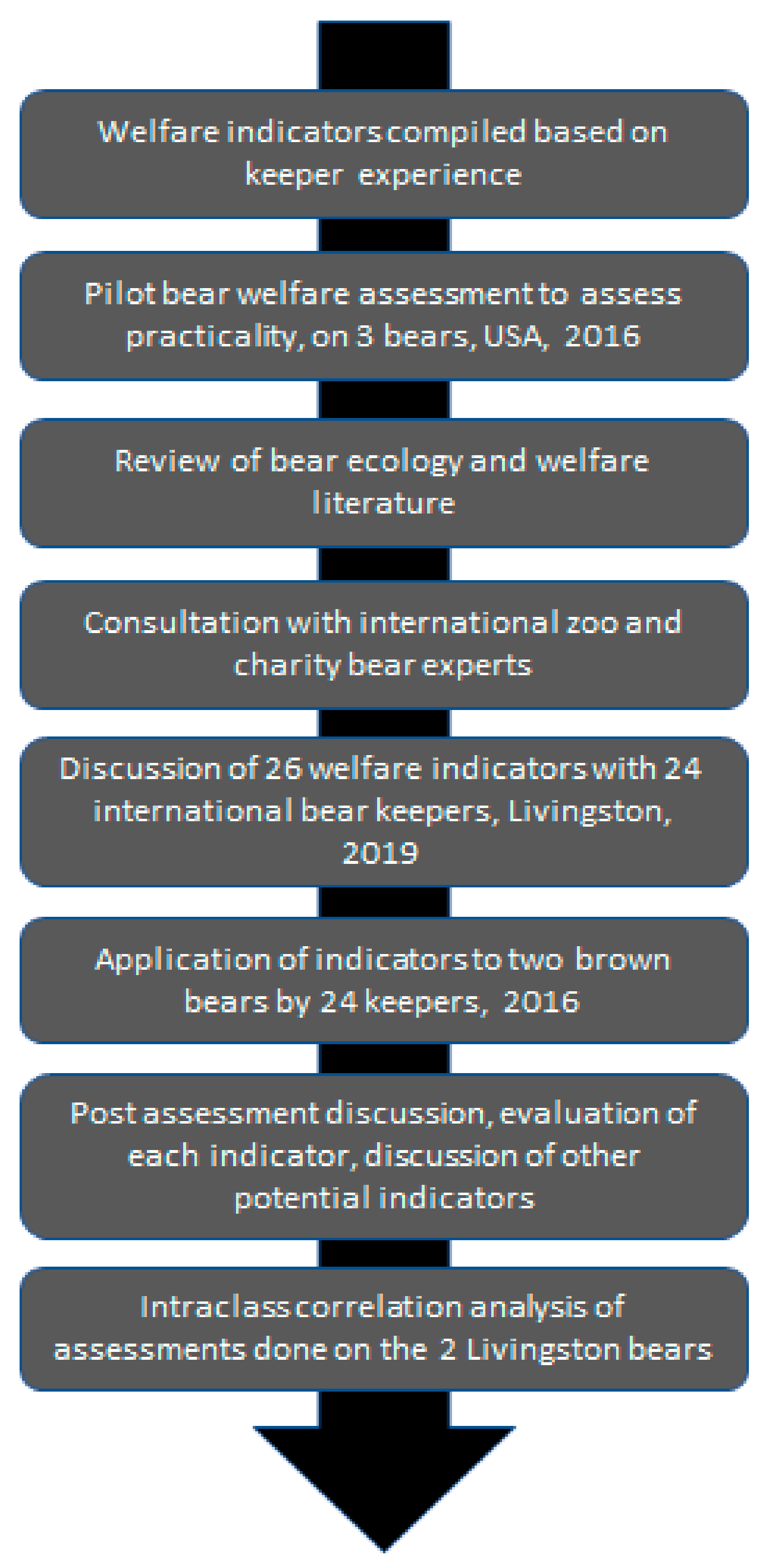
2. Materials and Methods
2.1. Welfare Assessment Tool
2.2. Training Guide
2.3. Recruitment and Data Collection
2.4. Data Analysis
3. Results
3.1. Assessment Tool
3.2. Subjects and Study Sites
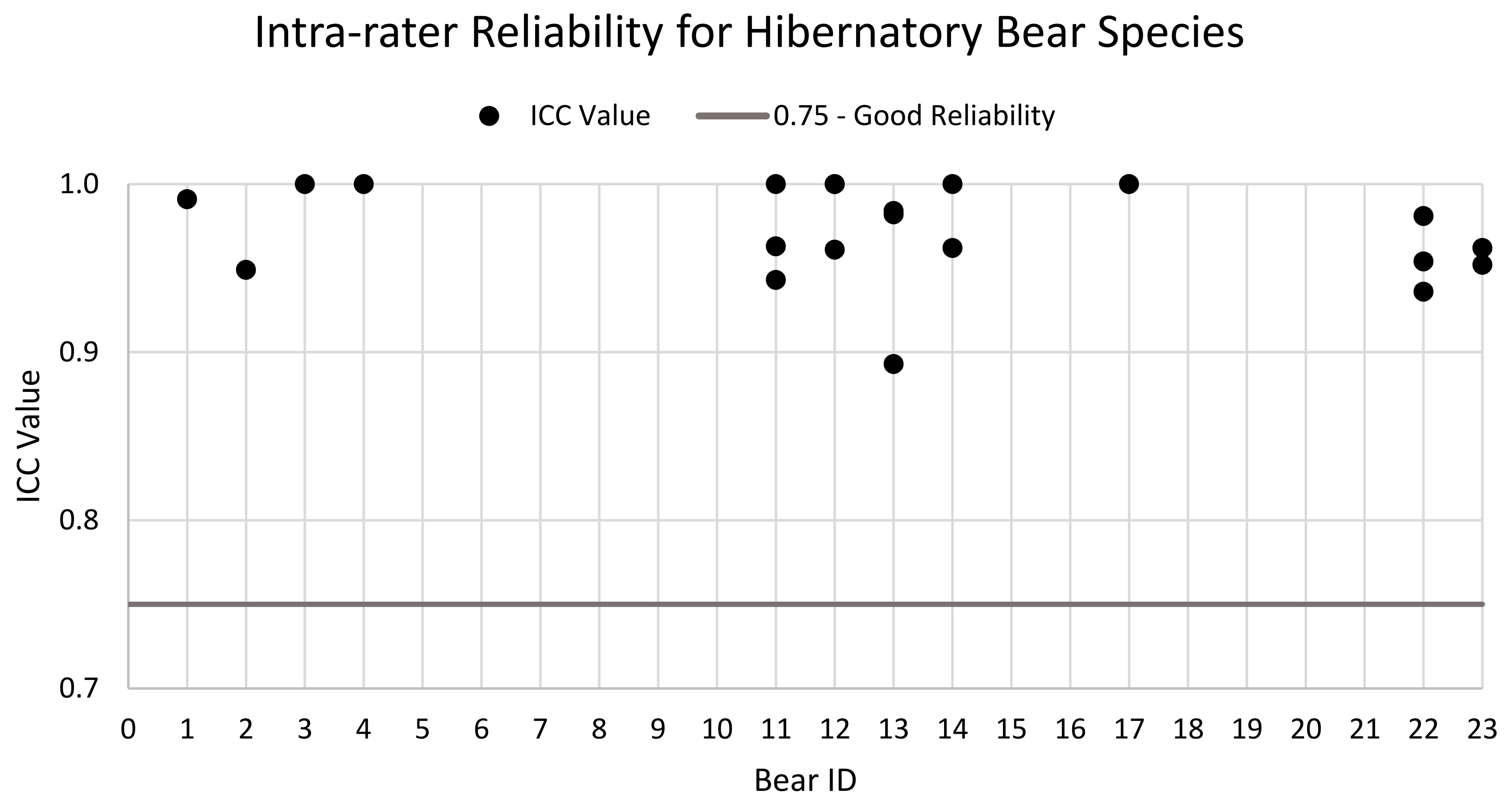
3.3. Reliability Analysis
3.4. Item Reliability
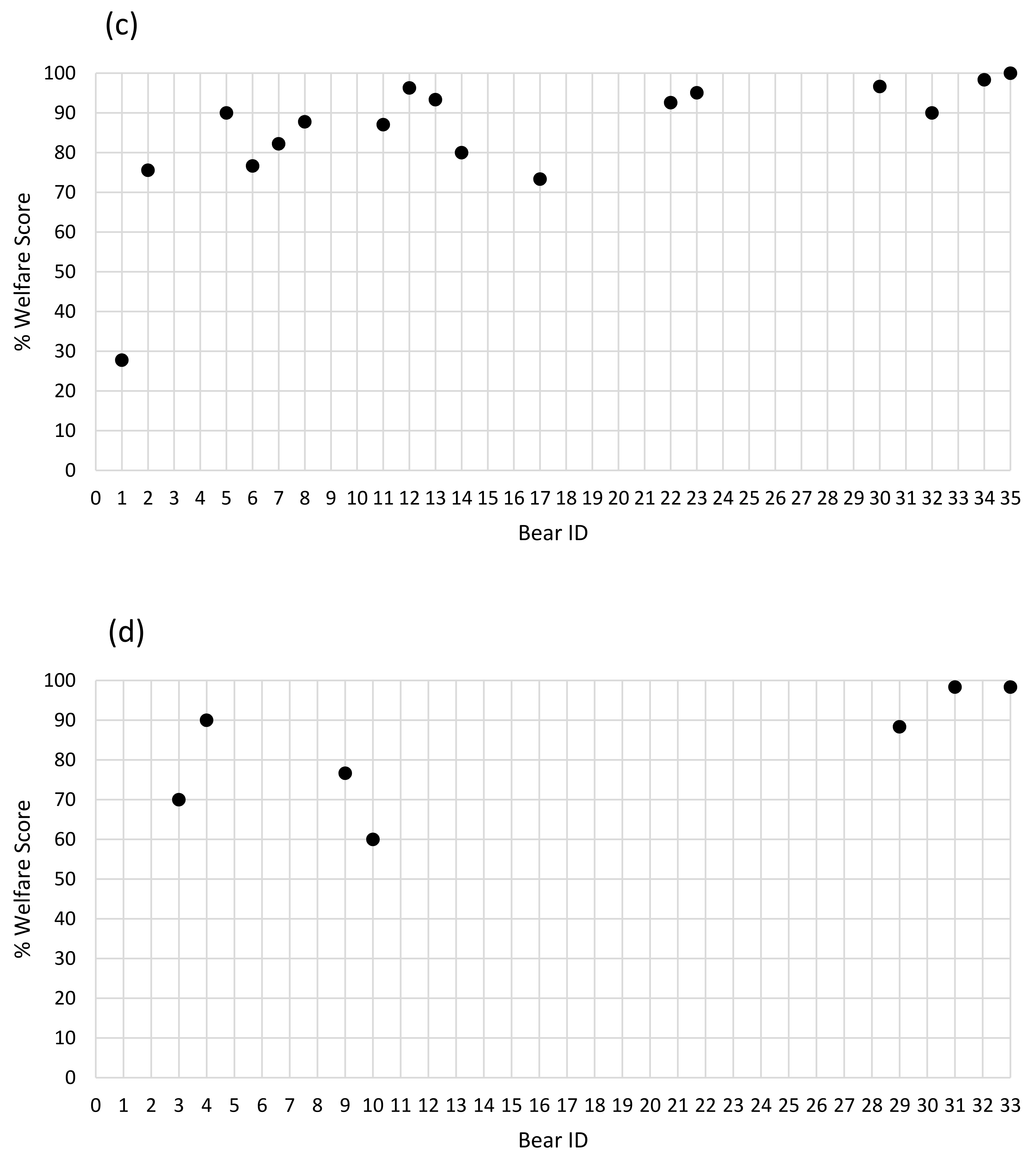
3.5. Composite Assessment Scores
4. Discussion
4.1. Assessment Tool
4.2. Inter- and Intra-Rater Reliability
4.3. Item Reliability
4.4. Welfare Scores
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellor, D.J.; Hunt, S.; Gusset, M. Caring for Wildlife: The World Zoo and Aquarium Animal Welfare Strategy; WAZA Executive Office: Gland, Switzerland, 2015. [Google Scholar]
- Sherwen, S.; Hemsworth, L.M.; Beausoleil, N.J.; Embury, A.; Mellor, D.J. An Animal Welfare Risk Assessment Process for Zoos. Animals 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Blackett, T.A.; McKenna, C.; Kavanagh, L.; Morgan, D.R. The welfare of wild animals in zoological institutions: Are we meeting our duty of care? Int. Zoo Yearb. 2017, 51, 187–202. [Google Scholar] [CrossRef]
- Maślak, R.; Sergiel, A.; Bowles, D.; Paśko, L. The Welfare of Bears in Zoos: A Case Study of Poland. J. Appl. Anim. Welf. Sci. 2016, 19, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Clubb, R.; Mason, G. Captivity effects on wide-ranging carnivores. Nature 2003, 425, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Kroshko, J.; Clubb, R.; Harper, L.; Mellor, E.; Moehrenschlager, A.; Mason, G. Stereotypic route tracing in captive Carnivora is predicted by species-typical home range sizes and hunting styles. Anim. Behav. 2016, 117, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Species360 Zoological Information Management System (ZIMS). 2020. Available online: https://www.species360.org/about-us/contact-us/ (accessed on 30 June 2020).
- Garshelis, D.L. Chapter 4: Variation in Ursid Life Histories, Is There an Outlier? In Giant Pandas: Biology and Conservation; Lindburg, D., Baragona, K., Eds.; University of California Press: California, CA, USA, 2004; pp. 53–73. [Google Scholar]
- Barber, J.C. Programmatic approaches to assessing and improving animal welfare in zoos and aquariums. Zoo Biol. 2009, 28, 519–530. [Google Scholar] [CrossRef]
- Department for Environment, Food, and Rural Affairs (DEFRA). Chapter 4: Animal welfare and its assessment in zoos. In DEFRA, Zoos Expert Committee Handbook; DEFRA: Bristol, UK, 2012; pp. 104–156. Available online: https://www.gov.uk/government/publications/zoos-expert-committee-handbook (accessed on 28 April 2020).
- Yon, L.; Williams, E.; Harvey, N.D.; Asher, L. Development of a behavioural welfare assessment tool for routine use with captive elephants. PLoS ONE 2019, 14, e0210783. [Google Scholar] [CrossRef] [Green Version]
- De Graaf, S.; Ampe, B.; Tuyttens, F. Assessing dairy cow welfare at the beginning and end of the indoor period using the Welfare Quality® protocol. Anim. Welf. 2017, 26, 213–221. [Google Scholar] [CrossRef]
- Temple, D.; Dalmau, A.; de la Torre, J.L.R.; Manteca, X.; Velarde, A. Application of the Welfare Quality® protocol to assess growing pigs kept under intensive conditions in Spain. J. Vet. Behav. 2011, 6, 138–149. [Google Scholar] [CrossRef]
- Buijs, S.; Ampe, B.; Tuyttens, F.A.M. Sensitivity of the Welfare Quality® broiler chicken protocol to differences between intensively reared indoor flocks: Which factors explain overall classification? Animal 2017, 11, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Clegg, I.; Borger-Turner, J.; Eskelinen, H. C-Well: The development of a welfare assessment index for captive bottlenose dolphins (Tursiops truncatus). Anim. Welf. 2015, 24, 267–282. [Google Scholar] [CrossRef]
- Kuhar, C.W.; Stoinski, T.S.; Lukas, K.E.; Maple, T. Gorilla Behavior Index revisited: Age, housing and behavior. Appl. Anim. Behav. Sci. 2006, 96, 315–326. [Google Scholar] [CrossRef]
- Melfi, V.A. There are big gaps in our knowledge, and thus approach, to zoo animal welfare: A case for evidence-based zoo animal management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef]
- Main, D.C.J.; Whay, H.R.; Leeb, C.; Webster, A.J.F. Formal Animal-Based Welfare Assessment in UK Certification Schemes. Anim. Welf. 2007, 16, 233–236. [Google Scholar]
- Main, D.C.J. Application of Welfare Assessment to Commercial Livestock Production. J. Appl. Anim. Welf. Sci. 2009, 12, 97–104. [Google Scholar] [CrossRef]
- Farm Animal Welfare Council (FAWC). Five Freedoms. 2009. Available online: https://webarchive.nationalarchives.gov.uk/20121010012427/http://www.fawc.org.uk/freedoms.htm (accessed on 20 April 2020).
- Whitham, J.C.; Wielebnowski, N. Animal-based welfare monitoring: Using keeper ratings as an assessment tool. Zoo Biol. 2009, 28, 545–560. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, D.; Yeon, S. Hibernation behaviour and ethogram of captive Asiatic black bear (Ursus thibetanus). Veterinární Med. 2020, 65, 1–7. [Google Scholar] [CrossRef]
- Robbins, C.T.; Lopez-Alfaro, C.; Rode, K.; Tøien, Ø.; Nelson, O.L. Hibernation and seasonal fasting in bears: The energetic costs and consequences for polar bears. J. Mammal. 2012, 93, 1493–1503. [Google Scholar] [CrossRef]
- Forthman, D.L.; Elder, S.D.; Bakeman, R.; Kurkowski, T.W.; Noble, C.C.; Winslow, S.W. Effects of feeding enrichment on behavior of three species of captive bears. Zoo Biol. 1992, 11, 187–195. [Google Scholar] [CrossRef]
- Law, G.; Reid, A. Enriching the lives of bears in zoos. Int. Zoo Yearb. 2010, 44, 65–74. [Google Scholar] [CrossRef]
- Ross, S.R. Issues of choice and control in the behaviour of a pair of captive polar bears (Ursus maritimus). Behav. Process. 2006, 73, 117–120. [Google Scholar] [CrossRef]
- Owen, M.A.; Swaisgood, R.R.; Czekala, N.M.; Lindburg, D.G. Enclosure choice and well-being in giant pandas: Is it all about control? Zoo Biol. 2005, 24, 475–481. [Google Scholar] [CrossRef]
- Sergiel, A.; Maślak, R. Part II: The Welfare of Zoo Animals, Chapter 4: The welfare of captive bears. In The Welfare of Animals in Zoos and EU Legal Standards; Gardocka, T., Gruszczyńska, A., Maślak, R., Sergiel, A., Eds.; ELIPSA: Warsaw, Poland, 2014; pp. 129–139. [Google Scholar]
- Clubb, R.; Mason, G.J. Natural behavioural biology as a risk factor in carnivore welfare: How analysing species differences could help zoos improve enclosures. Appl. Anim. Behav. Sci. 2007, 102, 303–328. [Google Scholar] [CrossRef] [Green Version]
- Hosey, G.R. How does the zoo environment affect the behaviour of captive primates? Appl. Anim. Behav. Sci. 2005, 90, 107–129. [Google Scholar] [CrossRef]
- Ross, S.R.; Schapiro, S.J.; Hau, J.; Lukas, K.E. Space use as an indicator of enclosure appropriateness: A novel measure of captive animal welfare. Appl. Anim. Behav. Sci. 2009, 121, 42–50. [Google Scholar] [CrossRef]
- AZA Bear Taxon Advisory Group. Polar Bear (Ursus maritimus) Care Manual; Association of Zoos and Aquariums: Montgomery County, MD, USA, 2009; Available online: https://www.aza.org/animal-care-manuals (accessed on 4 June 2020).
- Carlstead, K.; Seidensticker, J.; Baldwin, R. Environmental enrichment for zoo bears. Zoo Biol. 1991, 10, 3–16. [Google Scholar] [CrossRef]
- Shepherdson, D.; Lewis, K.D.; Carlstead, K.; Bauman, J.; Perrin, N. Individual and environmental factors associated with stereotypic behavior and fecal glucocorticoid metabolite levels in zoo housed polar bears. Appl. Anim. Behav. Sci. 2013, 147, 268–277. [Google Scholar] [CrossRef]
- Salas, M.; Manteca, X.; Abáigar, T.; Delclaux, M.; Enseñat, C.; Martínez-Nevado, E.; Quevedo, M.; Fernández-Bellon, H. Using Farm Animal Welfare Protocols as a Base to Assess the Welfare of Wild Animals in Captivity—Case Study: Dorcas Gazelles (Gazella dorcas). Animals 2018, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Morfeld, K.A.; Lehnhardt, J.; Alligood, C.; Bolling, J.; Brown, J.L. Development of a Body Condition Scoring Index for Female African Elephants Validated by Ultrasound Measurements of Subcutaneous Fat. PLoS ONE 2014, 9, e93802. [Google Scholar] [CrossRef]
- AZA Bear Taxon Advisory Group. Sun and Sloth Bear Care Manual; Association of Zoos and Aquariums: Montgomery County, MD, USA, 2019; Available online: https://www.aza.org/animal-care-manuals (accessed on 4 June 2020).
- Mononen, J.; Møller, S.H.; Hansen, S.; Hovland, A.; Koistinen, T.; Lidfors, L.; Malmkvist, J.; Vinke, C.; Ahola, L. The development of on-farm welfare assessment protocols for foxes and mink: The WelFur project. Anim. Welf. 2012, 21, 363–371. [Google Scholar] [CrossRef]
- Bourne, D.C.; Cracknell, J.M.; Bacon, H.J. Veterinary issues related to bears (Ursidae). Int. Zoo Yearb. 2010, 44, 16–32. [Google Scholar] [CrossRef]
- Henry, J.D.; Herrero, S.M. Social Play in the American Black Bear: Its Similarity to Canid Social Play and an Examination of its Identifying Characteristics. Integra. Comp. Biol. 1974, 14, 371–389. [Google Scholar] [CrossRef]
- Fagan, R.; Fagan, J. Play behavior of brown bears (Ursus arctos) and human presence at Pack Creek, Admiralty Island, Alas-ka. In Bears: Their Biology and Management, International Conference on Bear Research and Management; International Association for Bear Research and Management: Victoria, BC, Canada, 1990; Volume 8, pp. 315–319. [Google Scholar]
- Fagen, R.; Fagan, J. Juvenile survival and benefits of play behaviour in brown bears, Ursus arctos. Evol. Ecol. Res. 2004, 6, 89102. [Google Scholar]
- Evans, A.L.; Singh, N.J.; Friebe, A.; Arnemo, J.M.; Laske, T.G.; Fröbert, O.; Swenson, J.E.; Blanc, S. Drivers of hibernation in the brown bear. Front. Zool. 2016, 13, 17. [Google Scholar] [CrossRef]
- Carlstead, K.; Mellen, J.; Kleiman, D.G. Black Rhinoceros (Diceros bicornis) in U.S. Zoos: I. Individual Behavior Profiles and Their Relationship to Breeding Success. Zoo Biol. 1999, 18, 17–34. [Google Scholar] [CrossRef]
- Wielebnowski, N.C. Behavioral differences as predictors of breeding status in captive cheetahs. Zoo Biol. 1999, 18, 335–349. [Google Scholar] [CrossRef]
- Less, E.H.; Kuhar, C.W.; Dennis, P.M.; Lukas, K.E. Assessing inactivity in zoo gorillas using keeper ratings and behavioral data. Appl. Anim. Behav. Sci. 2012, 137, 74–79. [Google Scholar] [CrossRef]
- Khadpekar, Y.; Whiteman, J.P.; Durrant, B.S.; Owen, M.A.; Prakash, S. Approaches to studying behavior in captive sloth bears through animal keeper feedback. Zoo Biol. 2018, 37, 408–415. [Google Scholar] [CrossRef]
- Rose, P.; Brereton, J.E.; Gardner, L. Developing flamingo husbandry practices through workshop communication. J. Zoo Aqua. Res. 2016, 4, 115–121. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Hall, C.; Bremner-Harrison, S. Exploring the relationship between personality and social interactions in zoo-housed elephants: Incorporation of keeper expertise. Appl. Anim. Behav. Sci. 2019, 221, 104876. [Google Scholar] [CrossRef]
- Gibson, A. Assessing Quality of life in bears. In Proceedings of the Advancing Bear Care, Omaha, NE, USA, 8 October 2016; p. 38. [Google Scholar]
- Bacon, H.; Gibson, A. Bear Welfare Assessment. In Proceedings of the Advancing Bear Care, Livingston, UK, 10 October 2019; pp. 29–31. [Google Scholar]
- Bauer, E.; Babitz, M.; Boedeker, N.; Hellmuth, H. Approaches to understanding and managing pacing in sloth bears in a zo-ological setting. Int. J. Comp. Psychol. 2013, 26, 53–74. [Google Scholar] [CrossRef]
- Tabellario, S.; Babitz, M.A.; Bauer, E.B.; Brown-Palsgrove, M. Picture recognition of food by sloth bears (Melursus ursinus). Anim. Cogn. 2020, 23, 227–231. [Google Scholar] [CrossRef]
- Tan, H.; Ong, S.; Langat, G.; Bahaman, A.; Sharma, R.; Sumita, S. The influence of enclosure design on diurnal activity and stereotypic behaviour in captive Malayan Sun bears (Helarctos malayanus). Res. Vet. Sci. 2013, 94, 228–239. [Google Scholar] [CrossRef]
- McGowan, R.T.S.; Robbins, C.T.; Alldredge, J.R.; Newberry, R.C. Contrafreeloading in grizzly bears: Implications for captive foraging enrichment. Zoo Biol. 2010, 29, 484–502. [Google Scholar] [CrossRef]
- Maślak, R.; Sergiel, A.; Hill, S.P. Some aspects of locomotory stereotypies in spectacled bears (Tremarctos ornatus) and changes in behavior after relocation and dental treatment. J. Vet. Behav. 2013, 8, 335–341. [Google Scholar] [CrossRef]
- Föllmi, J.; Steiger, A.; Walzer, C.; Robert, N.; Geissbühler, U.; Doherr, M.; Wenker, C. A scoring system to evaluate physical condition and quality of life in geriatric zoo mammals. Anim. Welf. 2007, 16, 309–318. [Google Scholar]
- Kitchener, A. The problems of old bears in zoos. Int. Zoo News 2004, 51, 282–293. [Google Scholar]
- Pastorino, G.Q.; Christodoulides, Y.; Curone, G.; Pearce-Kelly, P.; Faustini, M.; Albertini, M.; Preziosi, R.; Mazzola, S.M. Behavioural Profiles of Brown and Sloth Bears in Captivity. Animals 2017, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Spoolder, H.; de Rosa, G.; Hörning, B.; Waiblinger, S.; Wemelsfelder, F. Integrating Parameters to Assess On-farm Welfare. Anim. Welf. 2003, 12, 529–534. [Google Scholar]
- Botreau, R.; Gaborit, M.; Veissier, I. Applying Welfare Quality® Strategy to Design a Welfare Assessment Tool for Foxes and Mink Farms. In Proceedings of the Xth International Scientific Congress in Fur Animal Production, Copenhagen, Denmark, 21–24 August 2012; pp. 460–468. [Google Scholar] [CrossRef]
- Barnard, S.; Pedernera, C.; Candeloro, L.; Ferri, N.; Velarde, A.; Villa, P.F.D. Development of a new welfare assessment protocol for practical application in long-term dog shelters. Vet. Rec. 2016, 178, 18. [Google Scholar] [CrossRef]
- Bonaparte-Saller, M.; Mench, J.A. Assessing the dyadic social relationships of female african (Loxodonta africana) and asian (Elephas maximus) zoo elephants using proximity, tactile contact, and keeper surveys. Appl. Anim. Behav. Sci. 2018, 199, 45–51. [Google Scholar] [CrossRef]
- King, J.E.; Landau, V.I. Can chimpanzee (Pan troglodytes) happiness be estimated by human raters? J. Res. Personal. 2003, 37, 1–15. [Google Scholar] [CrossRef]
- Phillips, C.J.; Tribe, A.; Lisle, A.; Galloway, T.K.; Hansen, K. Keepers’ rating of emotions in captive big cats, and their use in determining responses to different types of enrichment. J. Vet. Behav. 2017, 20, 22–30. [Google Scholar] [CrossRef]
- Gosling, S.D. Personality dimensions in spotted hyenas (Crocuta crocuta). J. Comp. Psychol. 1998, 112, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Skovlund, C.; Kirchner, M.; Moos, L.; Alsted, N.; Manteca, X.; Tallo-Parra, O.; Stelvig, M.; Forkman, B. A critical review of animal-based welfare indicators for polar bears (Ursus maritimus) in zoos: Identification and evidence of validity. Anim. Welf. 2021, 30, 1–18. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yasutake, A. Seasonal changes in body weight of female Asiatic black bears under captivity. Mammal Study 1999, 24, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hilderbrand, G.V.; Jenkins, S.G.; Schwartz, C.C.; Hanley, T.A.; Robbins, C.T. Effect of seasonal differences in dietary meat intake on changes in body mass and composition in wild and captive brown bears. Can. J. Zool. 1999, 77, 1623–1630. [Google Scholar] [CrossRef]
- Soriano, A.I.; Vinyoles, D.; Maté, C. Abnormales Verhalten in Zwei Gefangen Grizzlybär-Weibchen (Ursus arctos linnaeus, 1758): Einzelne Unterschiede Und Saisonale Variationen. Zool. Gart. 2017, 86, 88–101. [Google Scholar] [CrossRef]
- Fernandez, E.J.; Yoakum, E.; Andrews, N. Seasonal and daily activity of two zoo-housed grizzly bears (Ursus arctos horri-bilis). J. Zool. Bot. Gard. 2020, 1, 1–12. [Google Scholar] [CrossRef]
- Itoh, K.; Ide, K.; Kojima, Y.; Terada, M. Hibernation exhibit for Japanese black bear Ursus thibetanus japonicus at Ueno Zoological Gardens. Int. Zoo Yearb. 2010, 44, 55–64. [Google Scholar] [CrossRef]
- Forde, J. The Development and Benefits of Captive Torpor in European Brown Bears. In Proceedings of the Advancing Bear Care, Livingston, UK, 10 October 2019; p. 41. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Main, D.C.J.; Webster, A.J.F.; Green, L.E. Animal Welfare Assessment in Farm Assurance Schemes. Acta Agric. Scand. Sect. A Anim. Sci. 2001, 51, 108–113. [Google Scholar] [CrossRef]
- de Vet, H.C.; Terwee, C.B.; Knol, D.L.; Bouter, L. When to use agreement versus reliability measures. J. Clin. Epidemiol. 2006, 59, 1033–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitham, J.C.; Wielebnowski, N. New directions for zoo animal welfare science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar] [CrossRef]
- Held, S.D.; Špinka, M. Animal play and animal welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Blois-Heulin, C.; Rochais, C.; Camus, S.; Fureix, C.; Lemasson, A.; Lunel, C.; Bezard, E.; Hausberger, M. Animal Welfare: Could Adult Play be a False Friend? Anim. Behav. Cogn. 2015, 2, 156–185. [Google Scholar] [CrossRef]
- Heesen, R.; Genty, E.; Rossano, F.; Zuberbühler, K.; Bangerter, A. Social play as joint action: A framework to study the evolution of shared intentionality as an interactional achievement. Learn. Behav. 2017, 45, 390–405. [Google Scholar] [CrossRef]
- Mason, G.J. Stereotypies: A critical review. Anim. Behav. 1991, 41, 1015–1037. [Google Scholar] [CrossRef] [Green Version]
- Mason, G.J.; Latham, N.R. Can’t Stop, Won’t Stop: Is Stereotypy a Reliable Animal Welfare Indicator? Anim. Welf. 2004, 13, 57–69. [Google Scholar]
- Liu, D.; Wang, Z.; Tian, H.; Yu, C.; Zhang, G.; Wei, R.; Zhang, H. Behavior of giant pandas (Ailuropoda melanoleuca) in captive conditions: Gender differences and enclosure effects. Zoo Biol. 2003, 22, 77–82. [Google Scholar] [CrossRef]
- Hüber, D. Rehabilitation and reintroduction of captive-reared bears: Feasibility and methodology for European brown bears Ursus arctos. Int. Zoo Yearb. 2010, 44, 47–54. [Google Scholar] [CrossRef]
- Fischbacher, M.; Schmid, H. Feeding Enrichment and Stereotypic Behavior in Spectacled Bears. Zoo Biol. 1999, 18, 363–371. [Google Scholar] [CrossRef]
- Carlstead, K.; Paris, S.; Brown, J.L. Good keeper-elephant relationships in North American zoos are mutually beneficial to welfare. Appl. Anim. Behav. Sci. 2019, 211, 103–111. [Google Scholar] [CrossRef]
- Ward, S.J.; Melfi, V. Keeper-Animal Interactions: Differences between the Behaviour of Zoo Animals Affect Stockmanship. PLoS ONE 2015, 10, e0140237. [Google Scholar] [CrossRef]




| Indicator | Description | Score |
|---|---|---|
| Physical Health (n = 12) | ||
| Weight | Normal range for species and season | 3 |
| Outside of normal range | 1 | |
| Body Condition Score 1 | Score of 4, 5 or 6 | 3 |
| Score of 1, 2, 3, 7, 8, or 9 | 1 | |
| Visual Assessment of eyes, nose, teeth, skin, haircoat, claws, footpads, and injuries/wounds | No abnormality present | 3 |
| Any abnormality present | 1 | |
| Mobility Hindlimb Assessment 1 | Mobility score of 1 | 3 |
| Mobility score of 2 | 2 | |
| Mobility score of 3–4 | 1 | |
| Mobility score of 5–6 | 0 | |
| Cincinnati Gait Assessment 1 | 0–10 | 3 |
| 11–40 | 2 | |
| 41–60 | 1 | |
| 61+ | 0 | |
| Behaviour (n = 10) | ||
| Foraging | Bear searches for food throughout the environment | Score 3 if behavior observed daily, score 2 if 2–3× per week, 1 if less than twice a week, 0 if never observed |
| Climbing | Bear uses structures or trees within the enclosure to climb off the ground | |
| Social Play | Bear interacts positively with other bears, including wrestling/swatting/interacting with soft, open mouth | |
| Object Play | Bear interacts positively and non-repetitively with an object | |
| Nest Building | Bear gathers provided materials together into a pile to rest on during the day | |
| Aggression | Bear displays aggressive behaviour including growling, roaring, charging or swatting towards another bear or a human | Score 0 if behaviour is observed daily, 1 if 2–3× per week, 2 if less than twice a week, 3 if never observed |
| Abnormal Behaviour/Stereotypy | Bear displays repetitive head-swaying, pacing, rocking, pouncing, or object rolling/placing behaviour | |
| Feeding | Bear is fed a seasonally variable diet according to behaviours shown | 3 |
| Bear’s diet remains generally stable but may change once or twice per year | 2 | |
| Bear is fed a standard ration through the year or fed to maintain a consistent bodyweight | 1 | |
| Hibernatory/Winter Torpor Behaviours | Bear has displayed seasonal dormancy/torpor and been encouraged to sleep through the winter | 3 |
| Bear has slowed down but only slept for short intermittent periods through the winter | 2 | |
| Bear has slowed down but remained awake through the year | 1 | |
| Bear has been routinely exhibited and not encouraged to sleep | 0 | |
| Environmental Enrichment | Enrichment is provided daily through an enrichment calendar, type and time of presentation is varied, enrichment may be incorporated into normal husbandry or a training programme | 3 |
| Enrichment is provided 2–3× per week, though type and time may only vary occasionally | 2 | |
| Enrichment is provided less than twice a week | 1 | |
| Enrichment is never provided | 0 | |
| Environment (n = 7) | ||
| Access to Indoor and Outdoor areas | 24 h (except enclosure servicing) | 3 |
| Access to both areas > 18 h/day | 2 | |
| Access limited to one area for at least 7 h | 1 | |
| Local Temperature Range/Climate | Species endemic to local area or endemic to similar climatic conditions | 3 |
| Environment is broadly temperate, arctic or tropical in line with species ecology | 2 | |
| Environmental climate is dissimilar to species natural environment | 1 | |
| Water Source for Bathing | Present | 3 |
| Absent | 1 | |
| Surfaces and Substrates | Naturalistic and varied substrates to enable a range of behaviours including digging, nesting, bathing and foraging | 3 |
| Naturalistic substrates are provided but behavioural opportunities are limited | 2 | |
| Enclosure contains a mixture of artificial and natural surfaces (e.g., primarily artificial but with a digging pit and/or log piles) | 1 | |
| Surfaces and substrates cannot be manipulated and provide no behavioural opportunities | 0 | |
| View Out of Enclosure | Enclosure consists of glass windows or fencing which allows the bear to see surrounding area outside enclosure | 3 |
| Enclosure is surrounded by high walls with only ‘open’ area utilised as a visitor viewing area | 1 | |
| Cover and Privacy | Enclosure offers varied topography, visual barriers and limited viewing | 3 |
| Enclosure offers some privacy | 2 | |
| Animal is on view to visitors without cover | 1 | |
| Spatial Complexity | Enclosure offers environmental complexity and a range of behavioural opportunities including climbing, bathing, foraging and exploration over a variety of 3D spaces | 3 |
| Enclosure offers some environmental complexity and some behavioural opportunities but this could be improved | 2 | |
| Enclosure is simple with limited opportunities to display natural behaviours | 1 | |
| Enclosure is barren with no complexity | 0 | |
| Bear ID | Day | ICC Value | 95% Confidence Interval |
|---|---|---|---|
| 5 | 1 | 0.710 | 0.461–0.855 |
| 6 | 1 | 0.790 | 0.610–0.895 |
| 7 | 1 | 0.839 | 0.702–0.919 |
| 8 | 1 | 0.826 | 0.679–0.912 |
| 11 | 1 | 0.943 | 0.894–0.971 |
| 2 | 0.963 | 0.932–0.982 | |
| 3 | 1.0 | 1.0 | |
| 12 | 1 | 0.961 | 0.928–0.980 |
| 2 | 1.0 | 1.0 | |
| 3 | 1.0 | 1.0 | |
| 13 | 1 | 0.964 | 0.929–0.983 |
| 2 | 0.877 | 0.774–0.938 | |
| 3 | 0.965 | 0.935–0.982 | |
| 14 | 1 | 0.872 | 0.728–0.940 |
| 2 | 0.957 | 0.909–0.980 | |
| 22 | 1 | 0.983 | 0.966–0.993 |
| 2 | 1.0 | 1.0 | |
| 23 | 1 | 0.982 | 0.960–0.992 |
| 2 | 1.0 | 1.0 | |
| 29 | 1 | 0.955 | 0.905–0.979 |
| 30 | 1 | 0.903 | 0.794–0.954 |
| 32 | 1 | 0.739 | 0.447–0.877 |
| Assessor ID | Bear ID | ICC Value | 95% Confidence Interval |
|---|---|---|---|
| A1 | 1 | 0.991 | 0.983–0.995 |
| A2 | 2 | 0.949 | 0.906–0.975 |
| A3 | 3 | 1.0 | 1.0 |
| A3 | 4 | 1.0 | 1.0 |
| A8 | 11 | 0.943 | 0.894–0.971 |
| A8 | 12 | 0.961 | 0.928–0.980 |
| A8 | 13 | 0.893 | 0.791–0.950 |
| A9 | 11 | 1.0 | 1.0 |
| A9 | 12 | 1.0 | 1.0 |
| A9 | 13 | 0.982 | 0.968–0.991 |
| A10 | 11 | 0.963 | 0.932–0.982 |
| A10 | 12 | 1.0 | 1.0 |
| A10 | 13 | 0.984 | 0.971–0.992 |
| A11 | 14 | 0.962 | 0.919–0.982 |
| A12 | 14 | 1.0 | 1.0 |
| A14 | 17 | 1.0 | 1.0 |
| A17 | 22 | 0.936 | 0.880–0.968 |
| A17 | 23 | 0.952 | 0.911–0.976 |
| A18 | 22 | 0.954 | 0.898–0.979 |
| A18 | 23 | 0.962 | 0.917–0.983 |
| A19 | 22 | 0.981 | 0.954–0.992 |
| Bear ID | Bear Species | Assessors | Indicators | ICC Value | 95% Confidence Interval |
|---|---|---|---|---|---|
| 11 | Eurasian brown bear | A8, A9 and A10 (3 assessments each) | Social Play | 0.667 | −4.878–0.991 |
| Object Play | 1.0 | * | |||
| 13 | Eurasian brown bear | A8, A9 and A10 (3 assessments each) | Object Play | 1.0 | * |
| Hibernatory Behaviour | 0.857 | −0.045–0.996 | |||
| 14 | Eurasian brown bear | A11 and A12 (2 assessments each) | Object Play | 0.889 | −3.414–1.0 |
| Environmental Enrichment | 1.0 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maher, C.J.; Gibson, A.; Dixon, L.M.; Bacon, H. Developing a Reliable Welfare Assessment Tool for Captive Hibernatory Bear Species. Animals 2021, 11, 3090. https://doi.org/10.3390/ani11113090
Maher CJ, Gibson A, Dixon LM, Bacon H. Developing a Reliable Welfare Assessment Tool for Captive Hibernatory Bear Species. Animals. 2021; 11(11):3090. https://doi.org/10.3390/ani11113090
Chicago/Turabian StyleMaher, Chloe J., Angela Gibson, Laura M. Dixon, and Heather Bacon. 2021. "Developing a Reliable Welfare Assessment Tool for Captive Hibernatory Bear Species" Animals 11, no. 11: 3090. https://doi.org/10.3390/ani11113090
APA StyleMaher, C. J., Gibson, A., Dixon, L. M., & Bacon, H. (2021). Developing a Reliable Welfare Assessment Tool for Captive Hibernatory Bear Species. Animals, 11(11), 3090. https://doi.org/10.3390/ani11113090






