Fibropapillomatosis and Chelonid Alphaherpesvirus 5 Infection in Kemp’s Ridley Sea Turtles (Lepidochelys kempii)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Retrospective Analysis of Rehabilitation Cases and Stranding Data
2.2. Sample Collection—Blood and Tumors
2.3. DNA Extraction and qPCR for ChHV5 F-UL30 with Sequence Confirmation
2.4. Whole-Genome Shotgun Sequencing of Tumor Samples
2.4.1. Viral Phylogenetics
2.4.2. Kemp’s Ridley Mitochondrial Genome Analysis
3. Results
3.1. Retrospective Case Series Analysis
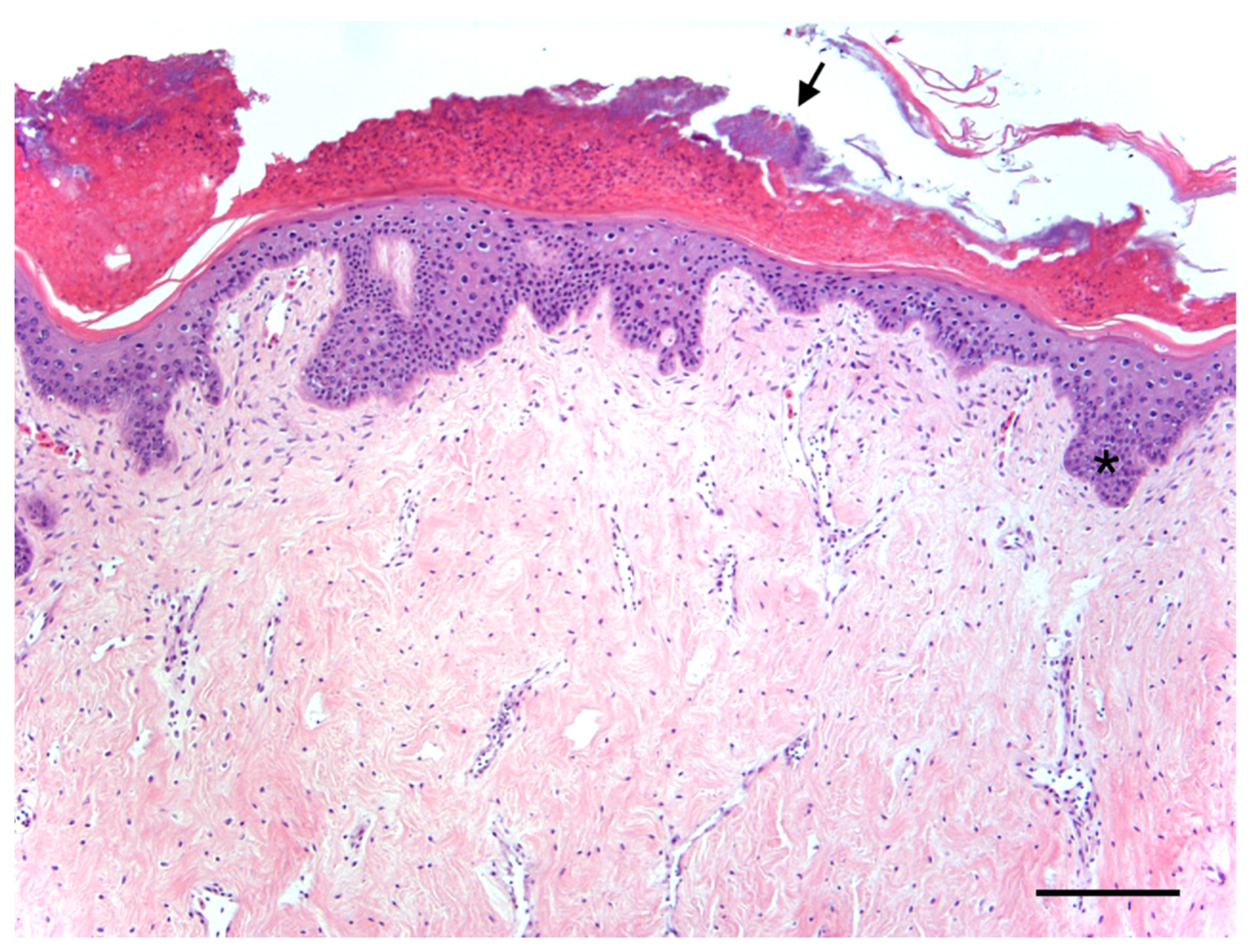
Case Report of Fibropapillomatosis in a Stranded Kemp’s Ridley Turtle in Massachusetts
3.2. qPCR for ChHV5 DNA
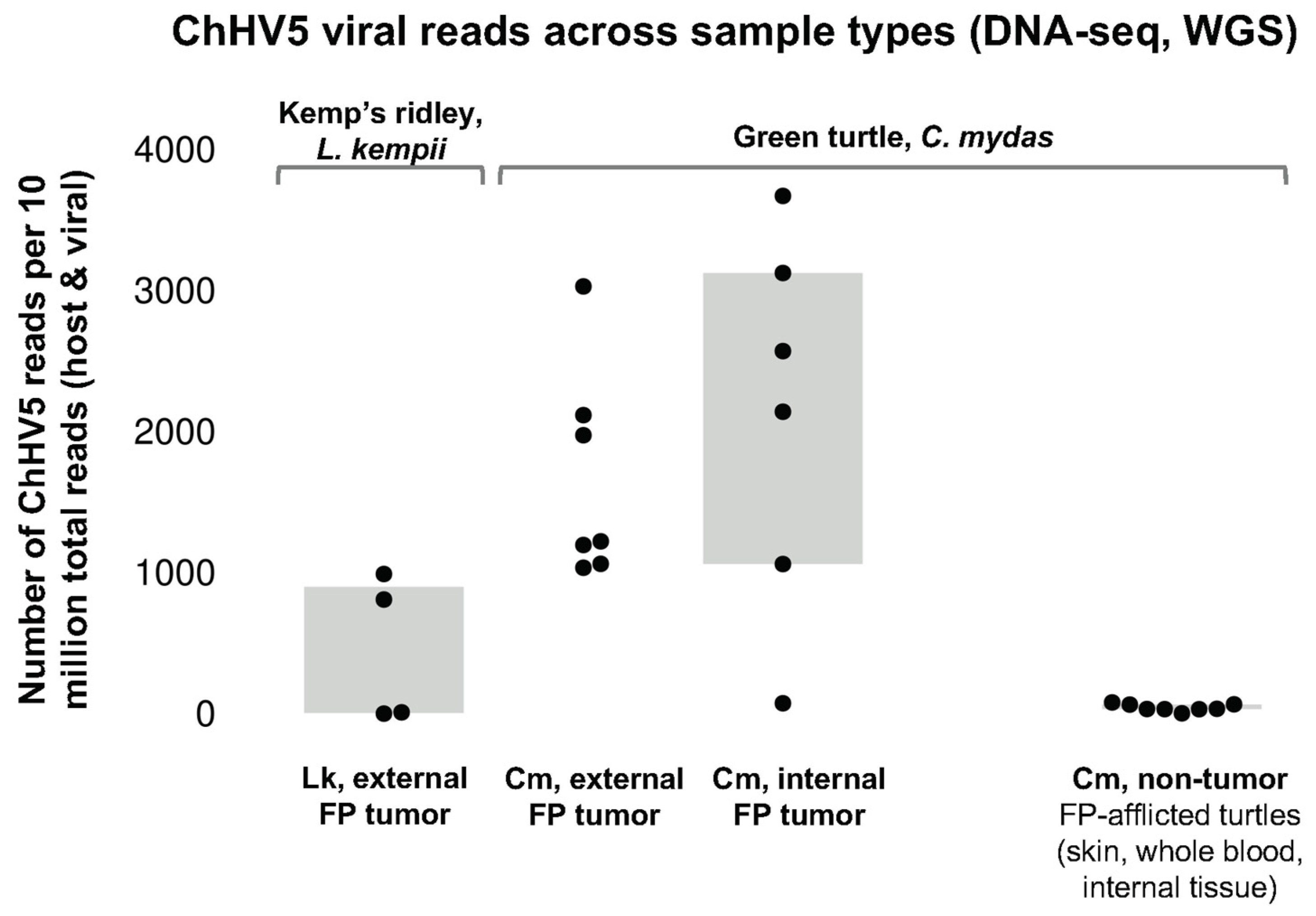
3.3. TPM Normalization
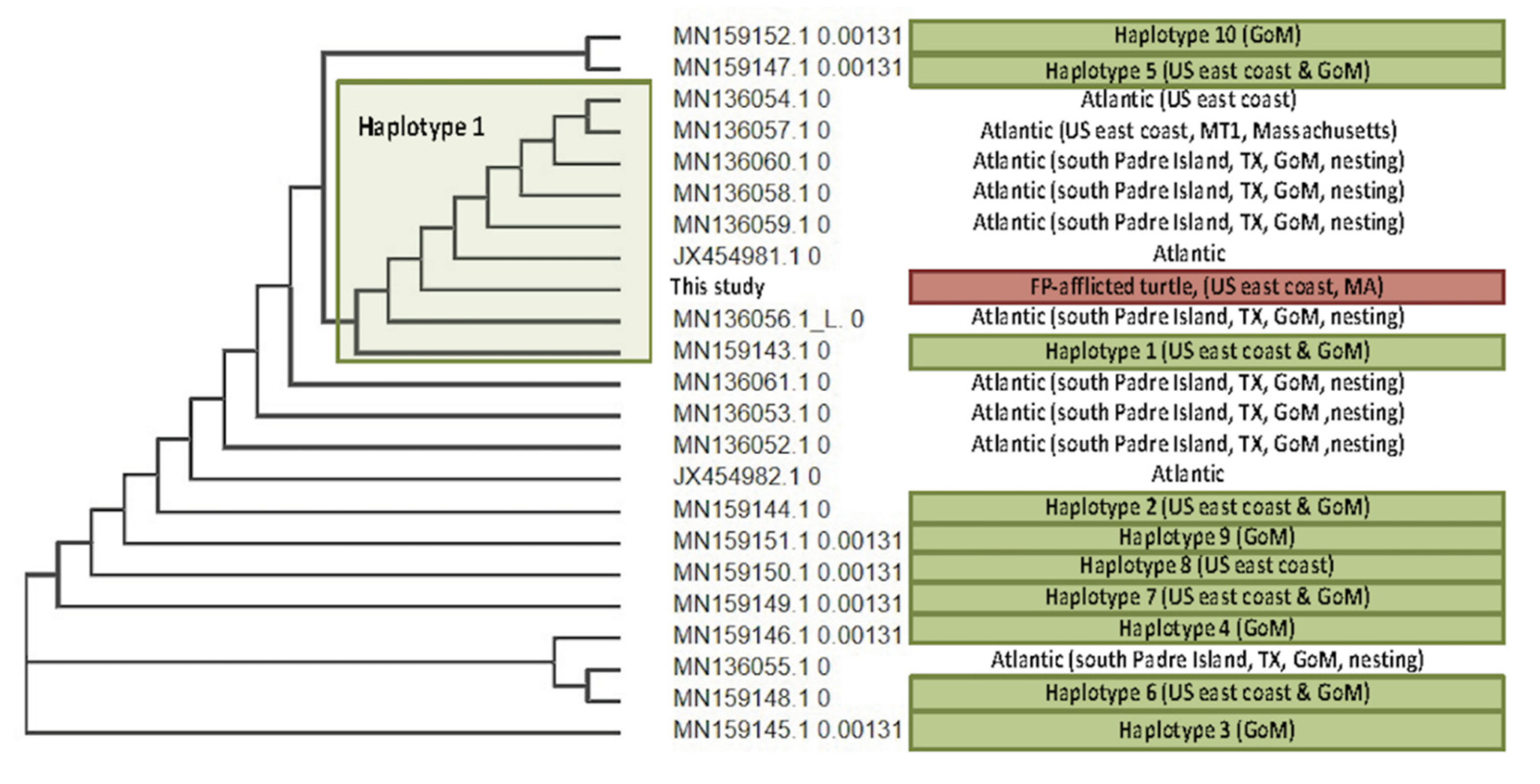
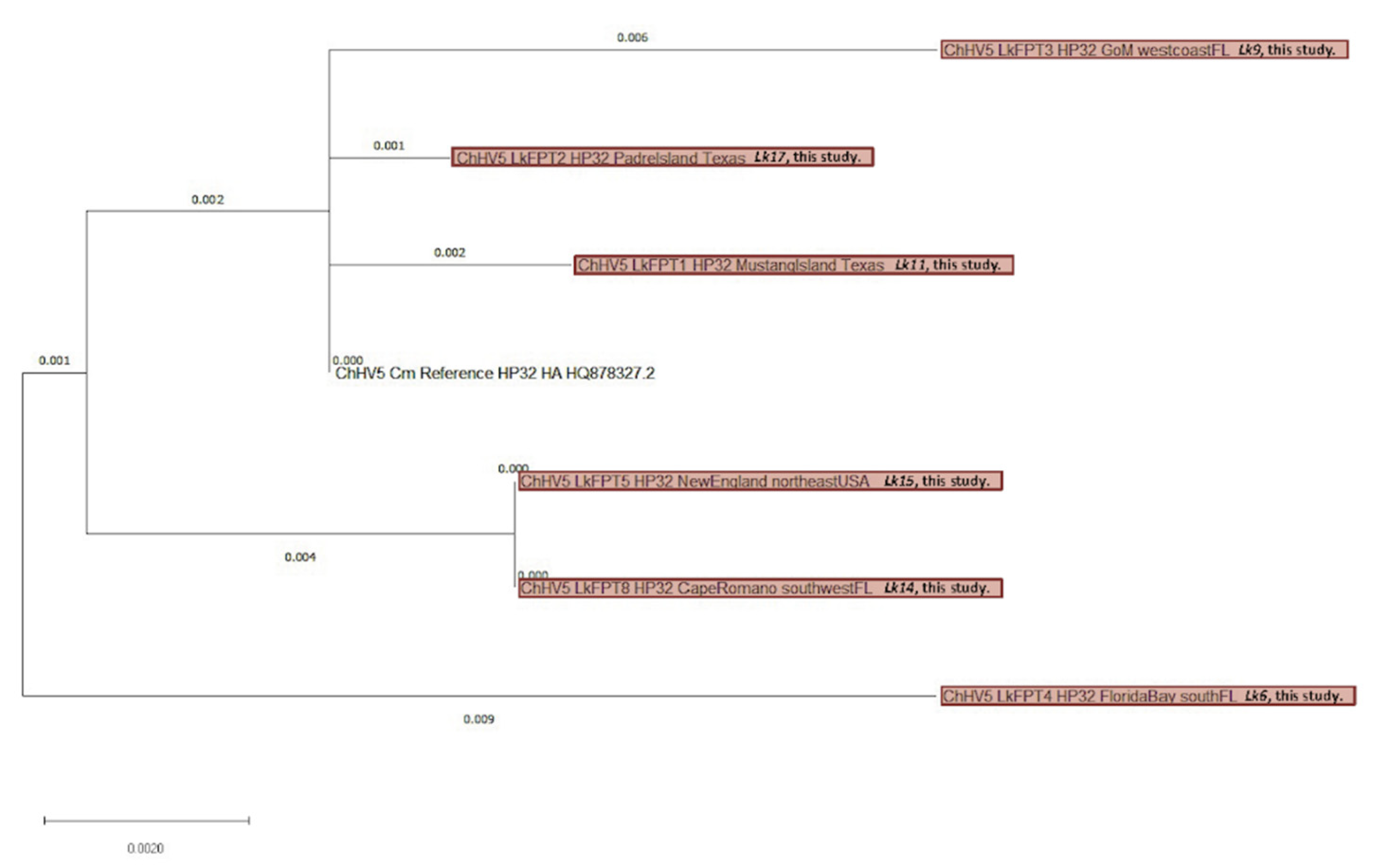
3.4. Viral Phylogenetics
4. Discussion
4.1. Epidemiology of FP in Kemp’s Ridley Turtles
4.2. Molecular Diagnostics for ChHV5
4.3. ChHV5 Phylogenetics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Marine Fisheries Service (NMFS); U.S. Fish and Wildlife Service (USFWS). Kemp’s Ridley Sea Turtle (Lepidochelys kempii) 5-Year Review: Summary and Evaluation; National Marine Fisheries Service, Office of Protected Resources: Silver Spring, MD, USA; U.S. Fish and Wildlife Service, Southwest Region: Albuquerque, NM, USA, 2015; 63p.
- National Marine Fisheries Service (NMFS); U.S. Fish and Wildlife Service (USFWS); Secretaria de Medio Ambiente y Recursos Naturales (SEMARNAT). Bi-National Recovery Plan for the Kemp’s Ridley Sea Turtle (Lepidochelys kempii), 2nd ed.; National Marine Fisheries Service: Silver Spring, MD, USA, 2011; 156p.
- United States Endangered Species Act (ESA), 35 FR 18319; Sirofchuck, C., Ed.; Federal Register, U.S. Government Publishing Office: Washington, DC, USA, 1970; Title 50 CFR, Part 17, Appendix A. Available online: https://www.govinfo.gov/content/pkg/CFR-2020-title50-vol2/html/CFR-2020-title50-vol2.htm (accessed on 12 June 2021).
- Wibbels, T.; Bevan, E. Lepidochelys kempii (errata version published in 2019). IUCN Red List Threat. Species 2019, e.T11533A155057916. [Google Scholar] [CrossRef]
- Seney, E.E.; Landry, A.M., Jr. Movements of Kemp’s ridley sea turtles nesting on the upper Texas coast: Implications for management. Endanger. Species Res. 2008, 4, 73–84. [Google Scholar] [CrossRef]
- Shaver, D.J. Analysis of the Kemp’s ridley imprinting and headstart project at Padre Island National Seashore, Texas, 1978–1988, with subsequent nesting and stranding records on the Texas coast. Chelonian Conserv. Biol. 2005, 4, 846–859. [Google Scholar]
- Bevan, E.; Wibbels, T.; Najera, B.M.Z.; Sarti, L.; Martinez, F.I.; Cuevas, J.M.; Gallaway, B.J.; Pena, L.J.; Burchfield, P.M. Estimating the historic size and current status of the Kemp’s ridley sea turtle (Lepidochelys kempii) population. Ecosphere 2016, 7, e01244. [Google Scholar] [CrossRef]
- Caillouet, C.W., Jr.; Putman, N.F.; Shaver, D.J.; Valverde, R.A.; Seney, E.E.; Lohmann, K.J.; Mansfield, K.L.; Gallaway, B.J.; Flanagan, J.P.; Godfrey, M.H. A call for evaluation of the contribution made by rescue, resuscitation, rehabilitation, and release translocations to Kemp’s ridley sea turtle (Lepidochelys kempii) population recovery. Herpetol. Conserv. Biol. 2016, 11, 486–496. [Google Scholar]
- Hunt, K.E.; Innis, C.; Merigo, C.; Burgess, E.A.; Norton, T.; Davis, D.; Kennedy, A.E.; Buck, C.L. Ameliorating transport-related stress in endangered Kemp’s ridley sea turtles (Lepidochelys kempii) with a recovery period in saltwater pools. Conserv. Physiol. 2019, 7, coy065. [Google Scholar] [CrossRef]
- Innis, C.I.; Finn, S.; Kennedy, A.; Burgess, E.; Norton, T.; Manire, C.A.; Harms, C.A. summary of sea turtles released from rescue and rehabilitation programs in the United States, with observations on re-encounters. Chel. Conserv. Biol. 2019, 18, 3–9. [Google Scholar] [CrossRef]
- Herbst, L.H. Fibropapillomatosis of marine turtles. Ann. Rev. Fish Dis. 1994, 4, 389–425. [Google Scholar] [CrossRef]
- Lackovich, J.K.; Brown, D.R.; Homer, B.L.; Garber, R.L.; Mader, D.R.; Moretti, R.H.; Patterrson, A.D.; Herbst, L.H.; Oros, J.; Jacobson, E.R.; et al. Association of herpesvirus with fibropapillomatosis of the green turtle Chelonia mydas and the loggerhead turtle Caretta caretta in Florida. Dis. Aquat. Org. 1999, 37, 89–97. [Google Scholar] [CrossRef]
- Herbst, L.H.; Klein, P.A. Experimental transmission of green turtle fibropapillomatosis using cell-free tumor extracts. Dis. Aquat. Org. 1995, 22, 1–12. [Google Scholar] [CrossRef]
- Farrell, J.A.; Yetsko, K.; Whitmore, L.; Whilde, L.; Whilde, J.; Eastman, C.B.; Ramia, D.R.; Thomas, R.; Linser, P.; Creer, S.; et al. Environmental DNA monitoring of oncogenic viral shedding and genomic profiling of sea turtle fibropapillomatosis reveals unusual viral dynamics. Commun. Biol. 2021, 4, 565. [Google Scholar] [CrossRef]
- Yetsko, K.; Farrell, J.; Stammnitz, M.R.; Whitmore, L.; Whilde, J.; Eastman, C.B.; Ramia, D.R.; Thomas, R.; Krstic, A.; Linser, P.; et al. Mutational, transcriptional and viral shedding dynamics of the marine turtle fibropapillomatosis tumor epizootic. bioRxiv 2020. [Google Scholar] [CrossRef]
- Foley, A.M.; Schroeder, B.A.; Redlow, A.E.; Fick-Child, K.J.; Teas, W.G. Fibropapillomatosis in stranded green turtles (Chelonia mydas) from the eastern United States (1980–1998): Trends and associations with environmental factors. J. Wildl. Dis. 2005, 41, 29–41. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Chabot, R.; Stacy, N.I.; Schenk, A.; Valverde, R.A.; Stewart, S.; Coppenrath, C.; Manire, C.A.; Herbst, L.H.; Gregory, C.R.; et al. Comprehensive health assessment of adult female green turtles (Chelonia mydas) nesting in southeastern Florida. Endanger. Species Res. 2020, 42, 21–35. [Google Scholar] [CrossRef]
- Shaver, D.J.; Walker, J.S.; Backof, T.F. Fibropapillomatosis prevalence and distribution in green turtles Chelonia mydas in Texas (USA). Dis. Aquat. Org. 2019, 135, 175–182. [Google Scholar] [CrossRef]
- Tristan, T.; Shaver, D.J.; Kimbro, J.; deMaar, T.; Metz, T.; George, J.; Amos, A. Identification of fibropapillomatosis in green sea turtles (Chelonia mydas) on the Texas coast. J. Herpetol. Med. Surg. 2010, 20, 109–112. [Google Scholar] [CrossRef]
- Barragan, A.R.; Sarti, M.L. A possible case of fibropapilloma in Kemp’s ridley turtle (Lepidochelys kempii). Mar. Turtle Newsl. 1994, 67, 27. [Google Scholar]
- Aguirre, A.A.; Spraker, T.R.; Chaves, A.; Toit, L.; Eure, W.; Balazs, G.H. Pathology of fibropapillomatosis in olive ridley turtles Lepidochely olivacea nesting in Costa Rica. J. Aquat. Anim. Health 1999, 11, 283–289. [Google Scholar] [CrossRef]
- D′Amato, A.F.; Moraes-Neto, M. First documentation of fibropapillomas verified by histopathology in Eretmochelys imbricata. Mar. Turtle Newsl. 2000, 89, 12–13. [Google Scholar]
- Diaz-Delgado, J.; Gomes-Borges, J.C.; Silveira, A.M.; Einhardt-Vergara, J.; Groch, K.R.; Cirqueira, C.S.; Sansone, M.; Gattamorta, M.A.; Matushima, E.R.; Catao-Dias, J.L. Primary multicentric pulmonary low-grade fibromyxoid sarcoma and chelonid alphaherpesvirus 5 detection in a leatherback sea turtle (Dermochelys coriacea). Comp. Pathol. 2019, 168, 1–7. [Google Scholar] [CrossRef]
- Ene, A.; Su, M.; Lemaire, S.; Rose, C.; Schaff, S.; Moretti, R.; Lenz, J.; Herbst, L.H. Distribution of chelonid fibropapilloma-associated herpesvirus variants in Florida: Molecular genetic evidence for infection of turtles following recruitment to neritic developmental habitats. J. Wildl. Dis. 2005, 41, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A review of fibropapillomatosis in green turtles (Chelonia mydas). Vet. J. 2016, 212, 48–57. [Google Scholar] [CrossRef]
- Limpus, C.J.; Couper, P.J.; Couper, K.L.D. Crab Island revisited: Reassessment of the world’s largest flatback turtle rookery after twelve years. Mem. Qld. Mus. 1993, 33, 227–289. [Google Scholar]
- Page-Karjian, A.; Norton, T.M.; Harms, C.A.; Mader, D.; Herbst, L.H.; Stedman, N.L.; Gottdenker, N.L. Case descriptions of fibropapillomatosis in rehabilitating loggerhead sea turtles Caretta caretta in the southeastern USA. Dis. Aquat. Org. 2015, 115, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Quackenbush, S.L.; Casey, R.N.; Murcek, R.J.; Paul, T.A.; Work, T.M.; Limpus, C.J.; Chaves, A.; duToit, L.; Aguirre, A.A.; Spraker, T.R.; et al. Quantitative analysis of herpesvirus sequences from normal tissue and fibropapillomas of marine turtles with real-time PCR. Virology 2001, 287, 105–111. [Google Scholar] [CrossRef][Green Version]
- Hargrove, S.; Work, T.; Brunson, S.; Foley, A.M.; Balazs, G.H. NOAA-TM-NMFS-PIFSC-54. In Proceedings of the 2015 Summit on Fibropapillomatosis: Global Status, Trends, and Population Impacts, Honolulu, HI, USA, 11–14 June 2015; U.S. Department of Commerce, National Oceanographic and Atmospheric Administration, National Marine Fisheries Service: Silver Spring, MD, USA, 2015; p. 87. [Google Scholar]
- Guillen, L.; Pena Villalobos, J. Papillomas in Kemp’s ridely turtles. NMFS-SEFSC-443, 237. In Proceedings of the 19th Annual Symposium on Sea Turtle Conservation & Biology, South Padre Island, TX, USA, 2–6 March 1999; Kalb, H., Wibbels, T., Eds.; U.S. Department of Commerce, National Oceanographic and Atmospheric Administration Technical Memorandum: Silver Spring, MD, USA, 2000. [Google Scholar]
- Florida Fish & Wildlife Conservation Commission (FWC). Fibropapillomatosis and Its Effect on Green Turtles. Available online: https://myfwc.com/research/wildlife/sea-turtles/threats/fibropapillomatosis/ (accessed on 15 March 2020).
- Page-Karjian, A.; Norton, T.M.; Krimer, P.; Groner, M.; Nelson, S.E.; Gottdenker, N.L. Factors affecting survivorship in rehabilitating green sea turtles (Chelonia mydas) with fibropapillomatosis. J. Zoo Wildl. Med. 2014, 45, 507–519. [Google Scholar] [CrossRef]
- Bjorndal, K.A.; Bolten, A.B.; Chaloupka, M.Y. Green turtle somatic growth model: Evidence for density dependence. Ecol. Appl. 2000, 10, 269–282. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Norton, T.M.; Ritchie, B.; Brown, C.; Mancia, C.; Jackwood, M.; Gottdenker, N.L. Quantifying chelonid herpesvirus 5 in symptomatic and asymptomatic rehabilitating green sea turtles. Endanger. Species Res. 2015, 28, 135–146. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.; et al. The Galaxy platform for accessible, reproducible, and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Morrison, C.L.; Iwanowicz, L.; Work, T.M.; Fahsbender, E.; Breitbart, M.; Adams, C.; Iwanowicz, D.; Sanders, L.; Ackermann, M.; Cornman, R.S. Genomic evolution, recombination, and inter-strain diversity of chelonid alphaherpesvirus 5 from Florida and Hawaii green sea turtles with fibropapillomatosis. PeerJ 2018, 6, e4386. [Google Scholar] [CrossRef] [PubMed]
- Avens, L.; Goshe, L.R.; Coggins, L.; Shaver, D.J.; Higgins, B.; Landry, A.M., Jr.; Bailey, R. Variability in age and size at maturation, reproductive longevity, and long-term growth dynamics for Kemp’s ridley sea turtles in the Gulf of Mexico. PLoS ONE 2017, 12, e0173999. [Google Scholar] [CrossRef]
- Craven, S.K.; Hodgson, J.Y.S.; Shaver, D.J.; Walker, J.S.; Villalba-Guerra, M.R.; Owens, D.W. Evaluation of gonadal tissue to validate size at reproductive maturity in Kemp’s ridley sea turtles found stranded in Texas, USA. Diversity 2019, 11, 76. [Google Scholar] [CrossRef]
- Work, T.M.; Balazs, G.H. Relating tumor score to hematology in green turtles with fibropapillomatosis in Hawaii. J. Wildl. Dis. 1999, 35, 804–807. [Google Scholar] [CrossRef]
- Innis, C.J.; Ravich, J.B.; Tlusty, M.F.; Hoge, M.S.; Wunn, D.S.; Boerner-Neville, L.B.; Merigo, C.; Weber, E.S., III. Hematologic and plasma biochemical findings in cold-stunned Kemp’s ridley turtles: 176 cases (2001–2005). J. Am. Vet. Med. Assoc. 2009, 235, 426–432. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Serrano, M.E.; Cartzendafner, J.; Morgan, A.; Ritchie, B.W.; Gregory, C.R.; Perrault, J.R.; Christiansen, E.F.; Harms, C.A. Molecular assessment of chelonid alphaherpesvirus 5 infection in tumor-free green (Chelonia mydas) and loggerhead (Caretta caretta) sea turtles in North Carolina, USA. Animals 2020, 10, 1964. [Google Scholar] [CrossRef]
- Frandsen, H.R.; Figueroa, D.F.; George, J.A. Mitochondrial genomes and genetic structure of the Kemp’s ridley sea turtle (Lepidochelys kempii). Ecol. Evol. 2020, 10, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Dutton, P.H.; Shaver, D.J.; Walker, J.S.; Rubio, C. Kemp’s ridley Lepidochelys kempii nesting abundance in Texas, USA: A novel approach using genetics to improve population census. Endang. Species Res. 2014, 23, 63–71. [Google Scholar] [CrossRef]
- Duchene, S.; Frey, A.; Alfaro-Nuñez, A.; Dutton, P.H.; Gilbert, M.T.P.; Morin, P.A. Marine turtle mitogenome phylogenetics and evolution. Mol. Phylogenet. Evol. 2012, 65, 241–250. [Google Scholar] [CrossRef]
- Ackermann, M.; Koriabine, M.; Hartmann-Fritsch, F.; de Jong, P.J.; Lewis, T.D.; Schetle, N.; Work, T.M.; Dagenais, J.; Balazs, G.H.; Leong, J.-A.C. The genome of chelonid herpesvirus 5 harbors atypical genes. PLoS ONE 2012, 7, e46623. [Google Scholar] [CrossRef]
- Herbst, L.H.; Ene, A.; Su, M.; Desalle, R.; Lenz, J. Tumor outbreaks in marine turtles are not due to recent herpesvirus mutations. Curr. Biol. 2004, 14, R697–R699. [Google Scholar] [CrossRef] [PubMed]
- Patrício, A.R.; Herbst, L.H.; Duarte, A.; Velez-Zuazo, X.; Loureiro, N.S.; Pereira, N.; Tavares, L.; Toranzos, G. Global phylogeography and evolution of the chelonid fibropapilloma-associated herpesvirus. J. Gen. Virol. 2012, 93, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Bean, S.B.; Logan, J.M. Stable isotope analysis of cold-stunned Kemp’s ridley (Leipdochelys kempii) sea turtles at the northern extent of their coastal range. Mar. Biol. 2019, 166, 64. [Google Scholar] [CrossRef]
- Schmid, J.R.; Witzell, W.N. Seasonal migrations of immature Kemp’s ridley turtles (Lepidochelys kempii Garman) along the west coast of Florida. Gulf Mex. Sci. 2006, 24, 28–40. [Google Scholar] [CrossRef]
- Shaver, D.J.; Tissot, P.E.; Streich, M.M.; Walker, J.S.; Rubio, C.; Amos, A.F.; Pasawicz, M.R. Hypothermic stunning of green sea turtles in a western Gulf of Mexico foraging habitat. PLoS ONE 2017, 12, e0173920. [Google Scholar] [CrossRef]
- Blackburn, N.B.; Leandro, A.C.; Nahvi, N.; Devlin, M.A.; Leandro, M.; Escobedo, I.M.; Peralta, J.M.; George, J.; Stacy, B.A.; deMaar, T.W.; et al. Transcriptomic profiling of fibropapillomatosis in green sea turtles (Chelonia mydas) from south Texas. Front. Immunol. 2021, 12, 630988. [Google Scholar] [CrossRef] [PubMed]
- Gredzens, C.; Shaver, D.J. Satellite tracking can inform population-level dispersal to foraging grounds of post-nesting Kemp’s ridley sea turtles. Front. Mar. Sci. 2020, 7, 559. [Google Scholar] [CrossRef]
- Herbst, L.H.; Jacobson, E.R.; Klein, P.A.; Balazs, G.H.; Moretti, R.; Brown, T.; Sundberg, J.P. Comparative pathology and pathogenesis of spontaneous and experimentally induced fibropapillomas of green turtles (Chelonia mydas). Vet. Pathol. 1999, 36, 551–554. [Google Scholar] [CrossRef]
- Work, T.M.; Balazs, G.H.; Wolcott, M.; Morris, R. Bacteraemia in free-ranging Hawaiian green turtles Chelonia mydas with fibropapillomatosis. Dis. Aquat. Org. 2003, 53, 41–46. [Google Scholar] [CrossRef]
- Hirama, S.; Ehrhart, L.M. Description, prevalence and severity of green turtle fibropapillomatosis in three developmental habitats on the east coast of Florida. Fla. Sci. 2007, 70, 435–448. [Google Scholar]
- Hirama, S.; Ehrhart, L.M.; Rea, L.D.; Kiltie, R.A. Relating fibropapilloma tumor severity to blood parameters in green turtles Chelonia mydas. Dis. Aquat. Org. 2014, 111, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gorham, J.C.; Bresette, M.J.; Guertin, J.R.; Shamblin, B.M.; Nairn, C.J. Green turtles (Chelonia mydas) in an urban estuary system: Lake Worth Lagoon, Florida. Fla. Sci. 2016, 79, 14–27. [Google Scholar]
- Perrault, J.R.; Stacy, N.I.; Lehner, A.F.; Mott, C.R.; Hirsch, S.; Gorham, J.C.; Buchweitz, J.P.; Bresette, M.J.; Walsh, C.J. Potential effects of brevetoxins and toxic elements on various health variables in Kemp’s ridley (Lepidochelys kempii) and green (Chelonia mydas) sea turtles after a red tide bloom event. Sci. Total Environ. 2017, 605, 967–979. [Google Scholar] [CrossRef]
- Chabot, R.M.; Welsh, R.C.; Mott, C.R.; Guertin, J.R.; Shamblin, B.M.; Witherington, B.E. A sea turtle population assessment for Florida’s Big Bend, northeastern Gulf of Mexico. Gulf Caribb. Res. 2021, 32, 19–33. [Google Scholar] [CrossRef]
- Ritchie, B. Virology. In Reptile Medicine & Surgery, 2nd ed.; Mader, D.R., Ed.; Saunders Elsevier: St. Louis, MO, USA, 2006; pp. 391–417. [Google Scholar]
- Renaud, M.L.; Williams, J.A. Kemp’s ridley sea turtle movements and migrations. Chelonian Conserv. Biol. 2005, 4, 808–816. [Google Scholar]
- Shaver, D.J.; Hart, K.M.; Fujisaki, I.; Rubio, C.; Sartain, A.R. Movement mysteries unveiled: Spatial ecology of juvenile green sea turtles. In Reptiles in Research: Investigations of Ecology, Physiology, and Behavior from Desert to Sea; Lutterschmidt, W., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 463–484. [Google Scholar]
- Shaver, D.J.; Rubio, C.; Walker, J.S.; George, J.; Amos, A.F.; Reich, K.; Jones, C.; Shearer, T. Kemp’s ridley sea turtle (Lepidochelys kempii) nesting on the Texas coast: Geographic, temporal, and demographic trends through 2014. Gulf Mex. Sci. 2016, 33, 158–178. [Google Scholar] [CrossRef]
- Howell, L.N.; Reich, K.J.; Shaver, D.J.; Landry, A.M., Jr.; Gorga, C.C. Ontogenetic shifts in diet and habitat of juvenile green sea turtles in the northwestern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2016, 559, 217–229. [Google Scholar] [CrossRef]
- Jones, K.; Burgess, G.; Budd, A.M.; Huerlimann, R.; Mashkour, N.; Ariel, E. Molecular evidence for horizontal transmission of chelonid alphaherpesvirus 5 at green turtle (Chelonia mydas) foraging grounds in Queensland, Australia. PLoS ONE 2020, 15, e0227268. [Google Scholar] [CrossRef] [PubMed]




| Turtle ID | Year | Age Class | Sex | How Encountered | Location | FP Tumor Score | # Tumors | Tumor Location(s) |
|---|---|---|---|---|---|---|---|---|
| Lk1 | 2006 | Juvenile | Undetermined | Stranded, rehabilitation | Florida | 3 | 8 | Neck, LF and LH flippers, carapace, plastron, tail |
| Lk2 | 2006 | Juvenile | Undetermined | Stranded, rehabilitation | Florida | 1 | 1 | LF flipper |
| Lk3 | 2011 | Juvenile | Undetermined | Stranded, rehabilitation | Georgia | 1 | 8 | L/R inguinal regions, tail base |
| Lk4 | 2011 | Juvenile | Undetermined | Incidental capture, rehabilitation | Georgia | 1 | 2 | LF and RF flippers |
| Lk5 | 2012 | Adult | Female | Nesting, rehabilitation | Texas | 2 | 1 | R inguinal region |
| Lk6a,c | 2014 | Adult | Female | Stranded dead | Florida | 3 | 9 | Neck, carapace, R inguinal region, tail, skeletal muscle, L/R lungs |
| Lk7 | 2014 | Juvenile | Undetermined | Stranded, rehabilitation | North Carolina | 1 | 1 | Tail |
| Lk8 | 2014 | Juvenile | Undetermined | Incidental capture, rehabilitation | North Carolina | 1 | 1 | LF flipper |
| Lk9a,c | 2015 | Juvenile | Female | Stranded dead | Florida | 2 | 8 | Neck, LF and RF flippers, carapace |
| Lk10 | 2015 | Adult | Female | Stranded, rehabilitation | Florida | 3 | 11 | R eye, neck, LF and RF flippers, R inguinal region, L/R lungs |
| Lk11a,c | 2017 | Juvenile | Undetermined | Stranded dead | Texas | 1 | 1 | L inguinal region |
| Lk12 | 2017 | Adult | Female | Stranded dead | Texas | 2 | 10 | Neck, LF and RF flipper bases |
| Lk13a | 2019 | Adult | Female | Incidental capture, rehabilitation | Texas | 1 | 2 | Neck, RF flipper base |
| Lk14a,c | 2019 | Adult | Female | Stranded dead | Florida | 1 | 2 | Neck |
| Lk15a,c | 2019 | Juvenile | Undetermined | Stranded, rehabilitation | Massachusetts | 2 | 7 | Neck, RF flipper, RF and LH flipper bases, R inguinal region |
| Lk16a,c | 2019 | Juvenile | Female | Stranded dead | Florida | 1 | 2 | Neck, LF flipper |
| Lk17a,b,c | 2019 | Adult | Female | Nesting | Texas | 1 | 1 | LF flipper * |
| Lk18a | 2020 | Adult | Female | Nesting | Texas | 1 | 2 | Neck |
| Lk19a,b | 2020 | Adult | Female | Nesting | Texas | 1 | 1 | Neck |
| Lk20a,b | 2020 | Adult | Female | Nesting | Texas | 1 | 2 | L eye, neck |
| Lk21a | 2020 | Adult | Female | Stranded, rehabilitation | North Carolina | 1 | 2 | RF flipper base |
| Lk22a | 2020 | Adult | Female | Stranded dead | Florida | 3 | 4 | L shoulder, L/R lungs |
| Sample Type | N | Positive for ChHV5 DNA N (Prevalence) | Mean (± SD) ChHV5 DNA Copy Number (Range) |
|---|---|---|---|
| Whole blood | 51 | 4 (7.8%) | 649 ± 516 (210–2393) |
| FP tumors | 12 | 11 (91.7%) | 6,809,552 ± 5,446,113 (50–17,261,001) |
| Sample | Location | Viral Enrichment (VE) | Total Reads | % ChHV5 Alignment | Total ChHV5 Aligning Reads | ChHV5 Reads per 10 Million Total Reads | ChHV5 Genome Coverage (x) | Number of SNPs | Sample Acquisition |
|---|---|---|---|---|---|---|---|---|---|
| Lk11 FP Tumor 1 | Mustang Island, TX | VE | 34,533,962 | 14.61 | 4,222,944 | 1,461,284 | 9580.69 | 106 | Necropsy |
| Lk17 FP Tumor 2 | N. Padre Island, TX | VE | 14,641,416 | 2.20 | 301,155 | 219,649 | 683.24 | 88 | Nesting |
| Lk9 FP Tumor 3 | Marco Island, SW FL | VE | 58,980,497 | 13.10 | 6,527,585 | 1,309,565 | 14,809.28 | 438 | Necropsy |
| Lk6 FP Tumor 4 | Florida Bay, SW FL | VE | 36,964,405 | 23.20 | 7,180,057 | 2,320,447 | 16,289.56 | 341 | Necropsy |
| Lk15 FP Tumor 5 | Brewster, MA | VE | 111,730,494 | 0.01 | 9062 | 811 | 20.56 | 2223 | Surgery |
| Lk16-1 FP Tumor 6 | Sanibel, SW FL | Non-VE | 129,584,639 | <0.01 | 140 | 11 | 0.31 | N/A | Necropsy |
| Lk16-2 FP Tumor 7 | Sanibel, SW FL | Non-VE | 197,167,284 | <0.01 | 30 | 2 | 0 | N/A | Necropsy |
| Lk14 FP Tumor 8 | Cape Romano, SW FL | Non-VE | 150,443,070 | 0.01 | 14,929 | 992 | 33.87 | 2284 | Necropsy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page-Karjian, A.; Whitmore, L.; Stacy, B.A.; Perrault, J.R.; Farrell, J.A.; Shaver, D.J.; Walker, J.S.; Frandsen, H.R.; Rantonen, E.; Harms, C.A.; et al. Fibropapillomatosis and Chelonid Alphaherpesvirus 5 Infection in Kemp’s Ridley Sea Turtles (Lepidochelys kempii). Animals 2021, 11, 3076. https://doi.org/10.3390/ani11113076
Page-Karjian A, Whitmore L, Stacy BA, Perrault JR, Farrell JA, Shaver DJ, Walker JS, Frandsen HR, Rantonen E, Harms CA, et al. Fibropapillomatosis and Chelonid Alphaherpesvirus 5 Infection in Kemp’s Ridley Sea Turtles (Lepidochelys kempii). Animals. 2021; 11(11):3076. https://doi.org/10.3390/ani11113076
Chicago/Turabian StylePage-Karjian, Annie, Liam Whitmore, Brian A. Stacy, Justin R. Perrault, Jessica A. Farrell, Donna J. Shaver, J. Shelby Walker, Hilary R. Frandsen, Elina Rantonen, Craig A. Harms, and et al. 2021. "Fibropapillomatosis and Chelonid Alphaherpesvirus 5 Infection in Kemp’s Ridley Sea Turtles (Lepidochelys kempii)" Animals 11, no. 11: 3076. https://doi.org/10.3390/ani11113076
APA StylePage-Karjian, A., Whitmore, L., Stacy, B. A., Perrault, J. R., Farrell, J. A., Shaver, D. J., Walker, J. S., Frandsen, H. R., Rantonen, E., Harms, C. A., Norton, T. M., Innis, C., Yetsko, K., & Duffy, D. J. (2021). Fibropapillomatosis and Chelonid Alphaherpesvirus 5 Infection in Kemp’s Ridley Sea Turtles (Lepidochelys kempii). Animals, 11(11), 3076. https://doi.org/10.3390/ani11113076








