Salinity as a Key Factor on the Benthic Fauna Diversity in the Coastal Lakes
Abstract
:Simple Summary
Abstract
1. Introduction
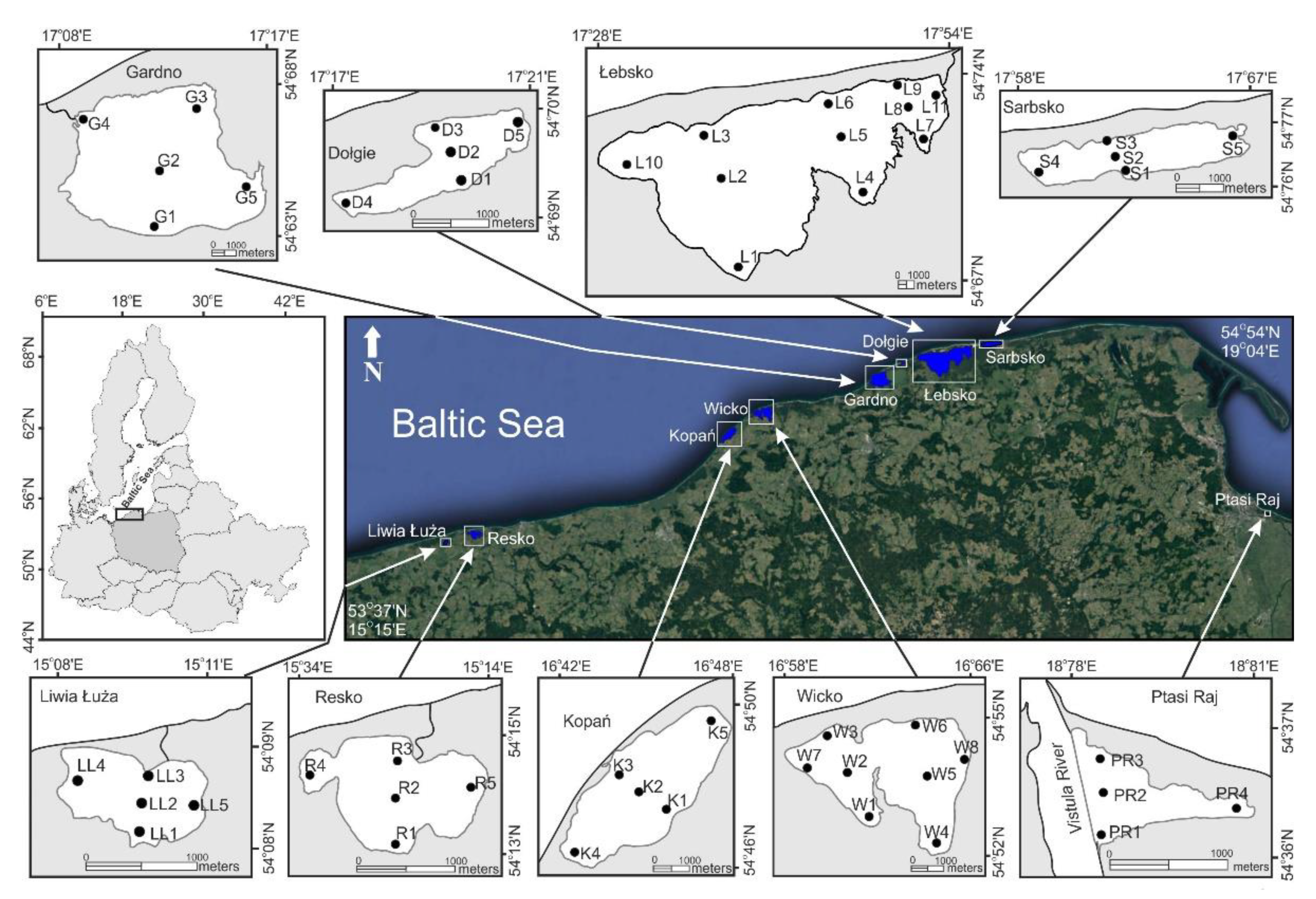
2. Materials and Methods
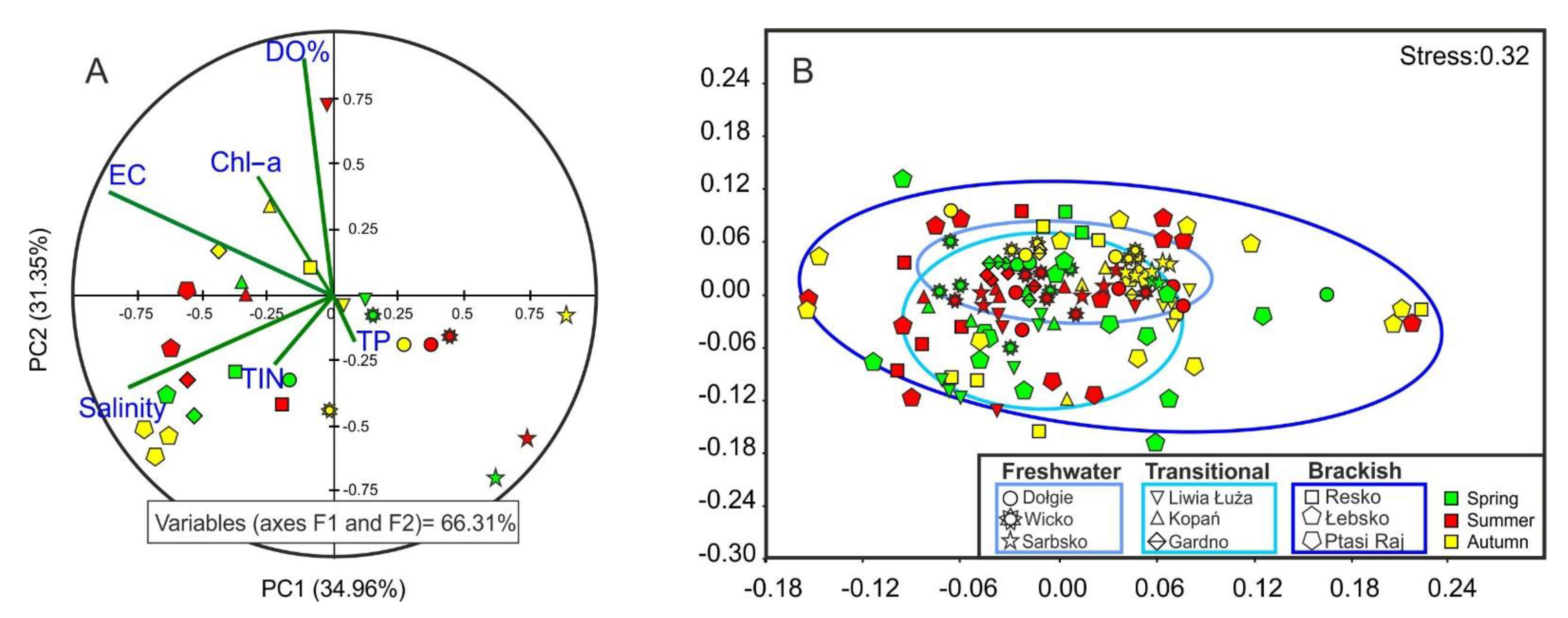
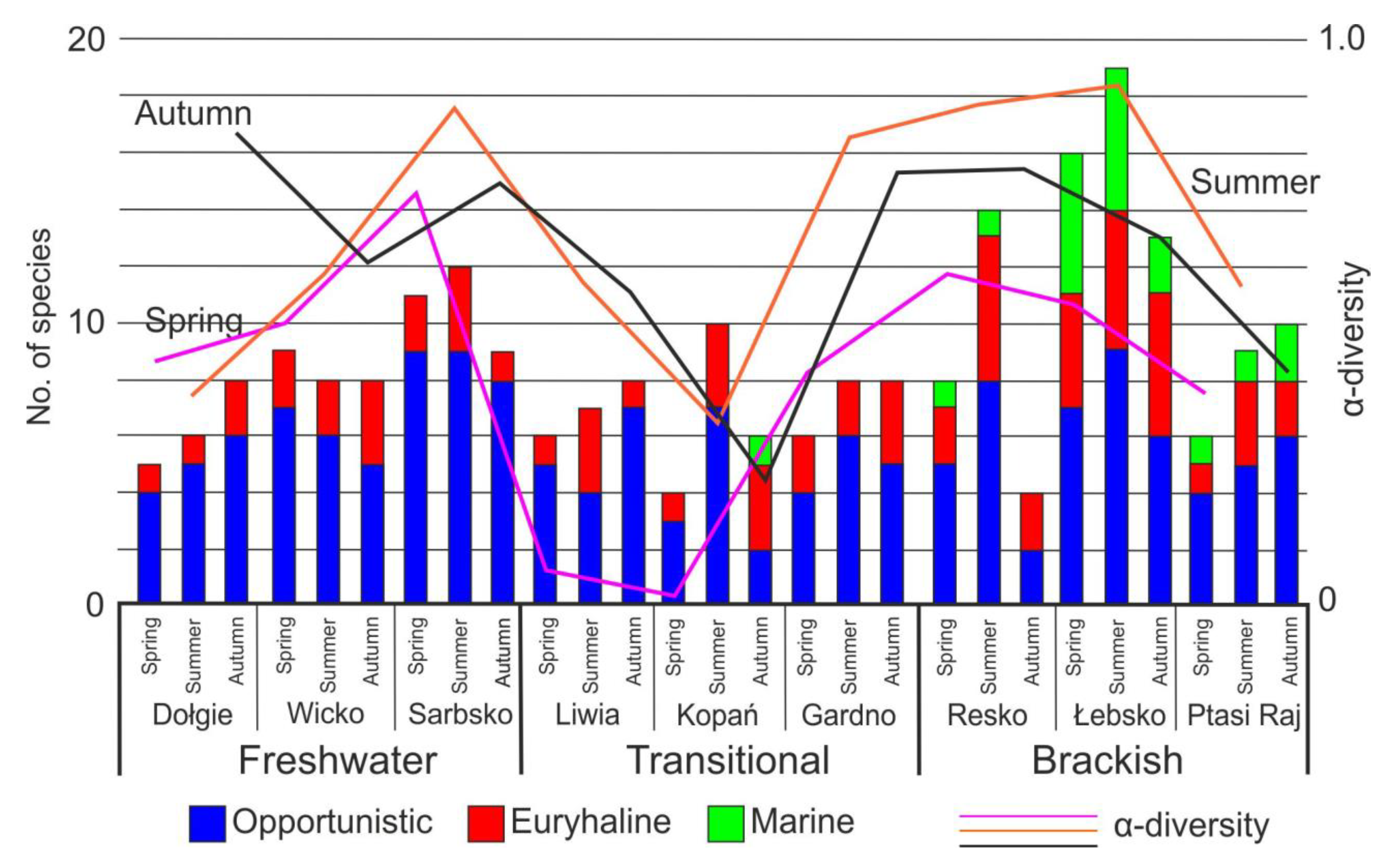
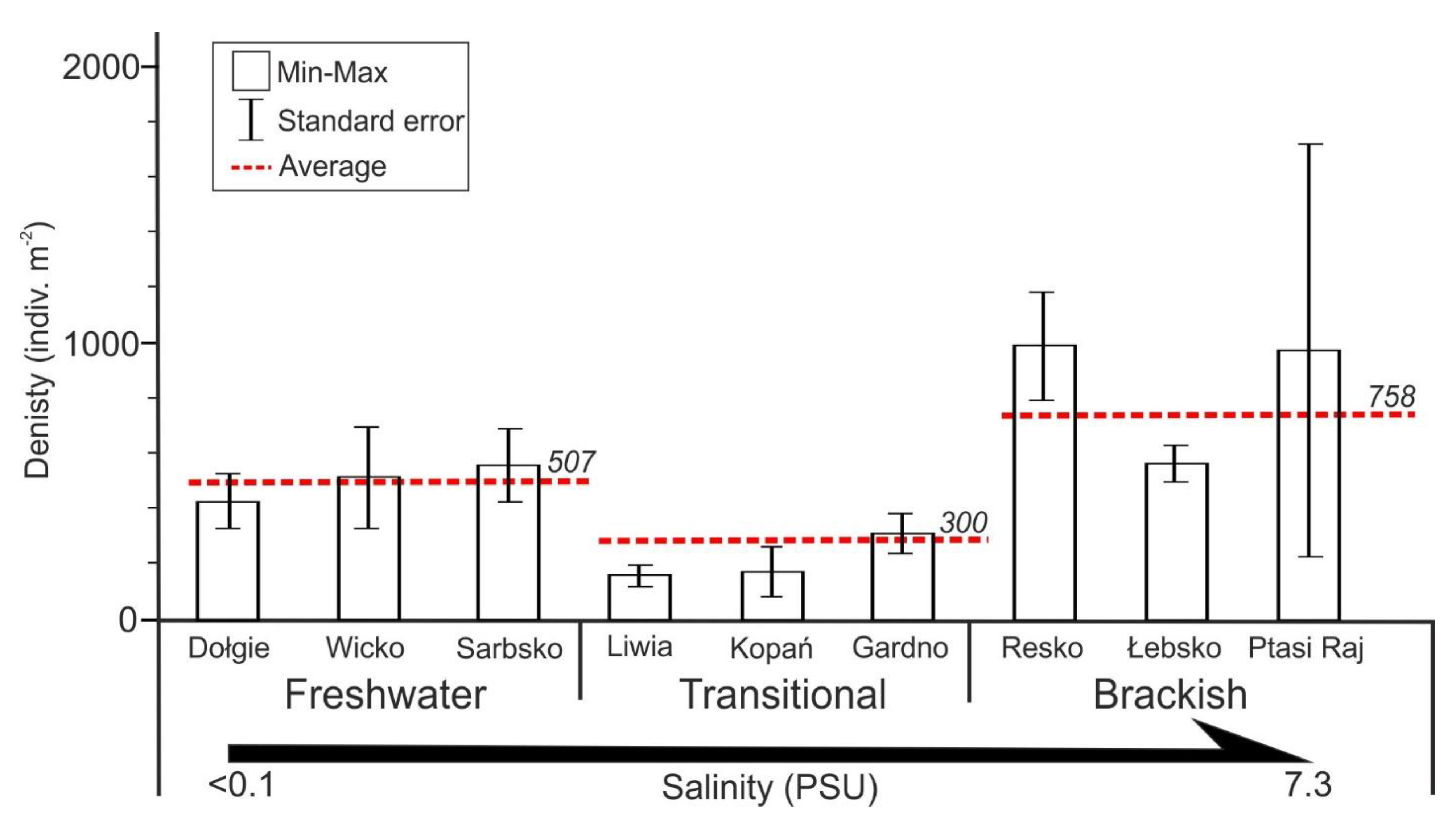
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tagliapietra, D.; Sigovini, M.; Volpi Ghirardini, A. A review of terms and definitions to categorise estuaries, lagoons and associated environments. Mar. Freshw. Res. 2009, 60, 497–509. [Google Scholar] [CrossRef]
- Netto, S.A.; Fonseca, G. Regime shifts in coastal lagoons: Evidence from free-living marine nematodes. PLoS ONE 2017, 12, e0172366. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.; Dalrymple, R.; Zaitlin, B.A. Classification of clastic coastal depositional environments. Sediment. Geol. 1992, 80, 139–150. [Google Scholar] [CrossRef]
- Dalrymple, R.W.; Zaitlin, B.A.; Boyd, R. Estuarine facies models: Conceptual basis and stratigraphic implications. J. Sediment. Res. 1992, 62, 1130–1146. [Google Scholar] [CrossRef]
- Heap, A.; Bryce, S.; Ryan, D.; Radke, L.; Smith, C.; Smith, R.; Harris, P.; Heggie, D. Australian Estuaries and Coastal Waterways: A Geoscience Perspective for Improved and Integrated Resource Management; AGSO Record 2001/07; Australian Geological Survey Organisation: Canberra, Australia, 2001. [Google Scholar]
- Shadrin, N.V. The alternative saline lake ecosystem states and adaptive environmental management. J. Oceanol. Limnol. 2018, 36, 2010–2017. [Google Scholar] [CrossRef]
- Obolewski, K.; Glińska-Lewczuk, K. Connectivity and complexity of coastal lakes as determinants for their restoration—A case study of the southern Baltic Sea. Ecol. Eng. 2020, 155, 105948. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.R. Catastrophic regime shifts in ecosystems: Linking theory to observation. Trends Ecol. Evol. 2003, 18, 648–656. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Attrill, M.J. A testable linear model for diversity trends in estuaries. J. Anim. Ecol. 2002, 71, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Schletterer, M.; Humpesch, U.H. Zoobenthos diversity in the Volga delta—A transect from Astrakhan to the Caspian Sea. Lauterbornia 2015, 79, 61–74. [Google Scholar]
- Catalina Stoica, C.; Stefania Gheorghe, S.; Petre, J.; Lucaciu, I.; Nita-Lazar, M. Tools for assessing Danube delta systems with macro invertebrates. Environ. Eng. Manag. J. 2014, 13, 2243–2252. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Evol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Holling, C.S. Understanding the complexity of economic, ecological, and social systems. Ecosystems 2001, 4, 390–405. [Google Scholar] [CrossRef]
- Astel, A.M.; Bigus, K.; Obolewski, K.; Glińska-Lewczuk, K. Spatiotemporal assessment of water chemistry in intermittently open/closed coastal lakes of Southern Baltic. Estuar. Coast. Shelf Sci. 2016, 182, 47–59. [Google Scholar] [CrossRef]
- Obolewski, K.; Glińska-Lewczuk, K.; Szymańska, M.; Mrozińska, N.; Bąkowska, M.; Astel, A.; Lew, S.; Paturej, E. Patterns of salinity regime in coastal lakes based on structure of benthic invertebrates. PLoS ONE 2018, 13, e0207825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruk, M.; Paturej, E.; Obolewski, K. Zooplankton predator–prey network relationships indicates the saline gradient of coastal lakes. Machine learning and meta-network approach. Ecol. Indic. 2021, 125, 107550. [Google Scholar] [CrossRef]
- Bazaïri, H.; Bayed, A.; Glémarec, M.; Hily, C. Spatial organisation of macrozoobenthic communities in response to environmental factors in a coastal lagoon of the NW African coast (Merja Zerga, Morocco). Oceanol. Acta 2003, 26, 457–471. [Google Scholar] [CrossRef] [Green Version]
- Reizopoulou, S.; Nicolaidou, A. Benthic diversity of coastal brackish-water lagoons in western Greece. Aquat. Conserv. 2004, 14, 93–102. [Google Scholar] [CrossRef]
- Mrozińska, N.; Bąkowska, M. Effects of heavy metals in lake water and sediments on bottom invertebrates inhabiting the brackish coastal Lake Łebsko on the Southern Baltic. Int. J. Environ. Res. Public Health 2020, 17, 6848. [Google Scholar] [CrossRef]
- Guélorget, O.; Perthuisot, J.P. Paralic ecosystems. Biological organization and functioning. Vie Milieu 1992, 42, 215–251. [Google Scholar]
- Reizopoulou, S.; Simboura, N.; Barbone, E.; Aleffi, F.; Basset, A.; Nicolaidou, A. Biodiversity in transitional waters: Steeper ecotone, lower diversity. Mar. Ecol. 2013, 35, 78–84. [Google Scholar] [CrossRef]
- Dobrowolski, Z. Occurrence of macrobentos in different littoral habitats of the polymictic Łebsko lake. Ekol. Pol. 1994, 42, 19–40. [Google Scholar]
- Bamber, R.N.; Batten, S.D.; Bridgwater, N.D. The brackish ponds at Killingholme, Humberside, UK. Aquat. Conser. 1991, 1, 173–181. [Google Scholar] [CrossRef]
- Cognetti, G. Colonization of stressed coastal environment. Mar. Pollut. Bull. 1992, 24, 12–14. [Google Scholar] [CrossRef]
- Andersen, J.H.; Al-Hamdani, Z.; Harvey, T.E.; Kallenbach, E.; Murraya, C.; Stock, A. Relative impacts of multiple human stressors in estuaries and coastal waters in the North Sea–Baltic Sea transition zone. Sci. Total Environ. 2020, 704, 135316. [Google Scholar] [CrossRef] [PubMed]
- Trojanowski, J.; Trojanowska, C.; Korzeniewski, K. Trophic state of coastal lakes. Pol. Arch. Hydrobiol. 1991, 38, 23–34. [Google Scholar]
- HELCOM, Baltic Marine Environment Protection Commission 2017–2018. Available online: http://stateofthebalticsea.helcom.fi (accessed on 15 May 2021).
- MarBEF. 2004. Available online: http://www.marbef.org/data/erms.php (accessed on 1 June 2021).
- Wetzel, R.G. Limnology—Lake and River Ecosystems; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- APHA. Standard methods for the examination of water and waste water, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Wagner, R.J.; Boulger, R.W.; Oblinger, C.J.; Smith, B.A. Guidelines and Standard Procedures for Continuous Water-Quality Monitors: Station Operation, Record Computation, and Data Reporting; Techniques and Methods Report 1-D3; U.S. Geological Survey Public, Warehouse: Reston, VA, USA, 2006. [Google Scholar] [CrossRef]
- McLusky, D.S.; Elliott, M. The Estuarine Ecosystem: Ecology, Threats and Management; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- McLusky, D.S. Marine and estuarine gradients. An overview. Neth. J. Aquat. Ecol. 1993, 27, 489–493. [Google Scholar] [CrossRef]
- Obolewski, K.; Glińska-Lewczuk, K.; Sidoruk, M.; Szymańska, M.M. Response of benthic fauna to habitat heterogeneity in a shallow temperate lake. Animals 2021, 11, 2488. [Google Scholar] [CrossRef]
- Netto, A.S.; Domingos, A.M.; Kurtz, M. Effects of artificial breaching of a temporarily open/closed estuary on benthic macroinvertebrates (Camacho Lagoon, Southern Brazil). Estuar. Coast. Shelf Sci. 2012, 35, 1069–1081. [Google Scholar] [CrossRef]
- Kornijów, R. Ecosystem of the Polish part of the Vistula Lagoon from the perspective of alternative stable states concept, with implications for management issues. Oceanologia 2018, 60, 390–404. [Google Scholar] [CrossRef]
- Holland, A.F.; Shaughnessy, A.; Heigel, M.H. Long-term variation in mesohaline Chesapeake Bay benthos: Spatial and temporal patterns. Estuaries 1987, 10, 227–245. [Google Scholar] [CrossRef]
- Attrill, M.J.; Rundle, S.D. Ecotone or ecocline: Ecological boundaries in estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 929–936. [Google Scholar] [CrossRef]
- Ysebaert, T.; Herman, P.M.J.; Meire, P.; Craeymeersch, J.; Verbeek, H.; Heip, C.H.R. Large-scale spatial patterns in estuaries: Estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuar. Coast. Shelf Sci. 2003, 57, 335–355. [Google Scholar] [CrossRef]
- Savoca, S.; Grifó, G.; Panarello, G.; Albano, M.; Giacobbe, S.; Capillo, G.; Spanó, N.; Consolo, G. Modelling prey-predator interactions in Messina beachrock pools. Ecol. Model. 2020, 434, 109206. [Google Scholar] [CrossRef]
- Wijnhoven, S.; Sistermans, W.; Hummel, H. Historic developments in macrozoobenthos of the Rhine–Meuse estuary: From a tidal inlet to a freshwater lake. Estuar. Coast. Shelf Sci. 2008, 76, 95–110. [Google Scholar] [CrossRef]
- Millet, B.; Guelorget, O. Spatial and seasonal variability in the relationships between benthic communities and physical environment in a lagoon ecosystem. Mar. Ecol. Prog. Ser. 1994, 108, 161–174. [Google Scholar] [CrossRef]
- Sigala, K.; Reizopoulou, S.; Basset, A.; Nicolaidou, A. Functional diversity in three Mediterranean transitional water ecosystems. Estuar. Coast. Shelf Sci. 2012, 110, 202–209. [Google Scholar] [CrossRef]
- Nicolaidou, A. Lack of temporal variability in the benthos of a coastal brackish water lagoon in Greece. Mediterr. Mar. Sci. 2007, 8, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Cieśliński, R. Geographical Determinants of Hydrochemical Variability of the Lakes of the Southern Baltic Coast; Uniwersytet Gdański Press: Gdańsk, Poland, 2011. (In Polish) [Google Scholar]
- Obolewski, K.; Glińska-Lewczuk, K.; Astel, A. Lost connectivity between a coastal lagoon and the sea e implications of floodgate closure for benthic macroinvertebrates. Estuar. Coast. Shelf Sci. 2018, 211, 77–89. [Google Scholar] [CrossRef]
- Zettler, M.L.; Schiedek, D.; Bobertz, B. Benthic biodiversity indices versus salinity gradient in the southern Baltic Sea. Mar. Pollut. Bull. 2007, 55, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Dauvin, J.C. Paradox of estuarine quality: Benthic indicators and indices, consensus or debate for the future. Mar. Pollut. Bull. 2007, 55, 271–281. [Google Scholar] [CrossRef]
- Elliott, M.; Quintino, V. Estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar. Pollut. Bull. 2007, 54, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Ceia, R.F.; Patrícioa, J.; Francoa, J.; Pintoa, R.; Fernández-Boob, S.; Losic, V.; Marquesa, C.J.; Neto, M.J. Assessment of estuarine macrobenthic assemblages and ecological quality status at a dredging site in a southern Europe estuary. Ocean Coast. Manag. 2013, 72, 80–92. [Google Scholar] [CrossRef]
- Ojaveer, H.; Andres Jaanus, A.; MacKenzie, B.R.; Martin, G.; Olenin, S.; Radziejewska, T.; Telesh, I.; Zettler, M.L.; Zaiko, A. Status of biodiversity in the Baltic Sea. PLoS ONE 2010, 5, e12467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olenin, S.; Leppäkoski, E. Non-native animals in the Baltic Sea: Alteration of benthic habitats in coastal inlets and lagoons. Hydrobiologia 1999, 393, 233–243. [Google Scholar] [CrossRef]
- Perus, J.; Bonsdorff, E.; Bäck, S.; Lax, H.-G.; Villnäs, A.; Westberg, V. Zoobenthos as indicators of ecological status in coastal brackish waters: A comparative study from the Baltic Sea. Ambio 2007, 3, 250–256. [Google Scholar] [CrossRef]

| Freshwater | Transitional | Brackish | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dołgie | Wicko | Sarbsko | Liwia | Kopań | Gardno | Resko | Łebsko | Ptasi Raj | |||
| Spring | Salinity (PSU) | 0.02 ± 0.00 | 0.07 ± 0.01 | 0.23 ± 0.04 | 0.47 ± 0.07 | 0.77 ± 0.08 | 0.81 ± 0.36 | 1.48 ± 0.43 | 2.20 ± 0.72 | 6.31 ± 0.54 | <0.0001 |
| EC (µS cm−1) | 47 ± 2 | 151 ± 17 | 483 ± 86 | 988 ± 144 | 1576 ± 154 | 1649 ± 735 | 2873 ± 819 | 4129 ± 1285 | 11088 ± 847 | <0.0001 | |
| O2 (%) | 96.3 ± 9.0 | 111.2 ± 14.8 | 80.5 ± 14.5 | 113.3 ± 10.3 | 112.4 ± 12.7 | 87.4 ± 9.9 | 96.1 ± 31.4 | 90.1 ± 19.2 | 109.3 ± 37.7 | <0.0001 | |
| Chl-a (mg L−1) | 21.1 ± 22.4 | 23.9 ± 24.5 | 25.9 ± 27.0 | 17.1 ± 19.1 | 25.7 ± 21.3 | 18.4 ± 18.5 | 14.0 ± 15.8 | 13.3 ± 12.2 | 14.7 ± 9.2 | 0.81 | |
| TP (mg L−1) | 0.32 ± 0.06 | 0.57 ± 0.23 | 0.32 ± 0.28 | 0.71 ± 0.37 | 0.43 ± 0.13 | 0.19 ± 0.05 | 0.36 ± 0.15 | 0.30 ± 0.12 | 0.50 ± 0.38 | <0.0001 | |
| NO3− (mg L−1) | 0.56 ± 0.30 | 0.86 ± 0.46 | 0.98 ± 1.04 | 1.35 ± 1.77 | 0.90 ± 0.44 | 0.58 ± 0.24 | 0.98 ± 0.61 | 0.97 ± 0.49 | 1.91 ± 1.82 | 0.03 | |
| NO2− (mg L−1) | 0.21 ± 0.17 | 0.52 ± 0.27 | 0.64 ± 0.33 | 0.72 ± 0.31 | 0.61 ± 0.26 | 0.51 ± 0.24 | 0.57 ± 0.22 | 0.74 ± 0.85 | 0.77 ± 0.40 | 0.005 | |
| NH4+ (mg L−1) | 0.36 ± 0.28 | 0.25 ± 0.16 | 0.24 ± 0.07 | 0.18 ± 0.12 | 0.18 ± 0.11 | 0.27 ± 0.19 | 0.06 ± 0.15 | 0.36 ± 0.25 | 0.68 ± 0.42 | <0.0001 | |
| TIN (mg L−1) | 1.14 ± 0.64 | 1.55 ± 0.83 | 1.86 ± 1.28 | 2.25 ± 1.96 | 1.68 ± 0.64 | 1.36 ± 0.41 | 1.60 ± 0.9 | 2.08 ± 0.89 | 3.36 ± 2.56 | 0.02 | |
| Summer | Salinity (PSU) | 0.02 ± 0.00 | 0.13 ± 0.07 | 0.31 ± 0.24 | 0.54 ± 0.12 | 1.26 ± 0.23 | 1.29 ± 1.04 | 2.76 ± 1.14 | 2.80 ± 0.64 | 7.12 ± 0.45 | <0.0001 |
| EC (µS cm−1) | 50 ± 3 | 282 ± 139 | 630 ± 468 | 1107 ± 237 | 2560 ± 451 | 2535 ± 1938 | 5113 ± 1997 | 5204 ± 1109 | 12415 ± 758 | <0.0001 | |
| O2 (%) | 107.5 ± 30.2 | 109.7 ± 21.3 | 95.8 ± 33.0 | 148.1 ± 30.1 | 111.0 ± 24.8 | 93.2 ± 20.1 | 90.3 ± 19.4 | 82.0 ± 18.8 | 83.9 ± 35.8 | <0.0001 | |
| Chl-a (mg L−1) | 12.3 ± 7.3 | 18.6 ± 16.8 | 28.3 ± 25.5 | 16.8 ± 18.7 | 26.5 ± 24.1 | 17.3 ± 14.9 | 22.5 ± 2262 | 14.8 ± 16.3 | 12.0 ± 9.2 | 0.78 | |
| TP (mg L−1) | 0.33 ± 0.13 | 0.37 ± 0.28 | 0.35 ± 0.37 | 0.38 ± 0.22 | 0.32 ± 0.19 | 0.16 ± 0.12 | 0.42 ± 0.27 | 0.25 ± 0.12 | 0.62 ± 0.23 | <0.002 | |
| NO3− (mg L−1) | 0.56 ± 0.24 | 0.81 ± 0.48 | 0.79 ± 0.73 | 0.42 ± 0.20 | 0.87 ± 0.53 | 0.94 ± 0.44 | 0.99 ± 0.75 | 1.02 ± 0.61 | 1.40 ± 0.72 | 0.03 | |
| NO2− (mg L−1) | 0.33 ± 0.19 | 0.53 ± 0.36 | 0.43 ± 0.32 | 0.34 ± 0.13 | 0.53 ± 0.26 | 0.56 ± 0.21 | 0.56 ± 0.24 | 0.83 ± 1.42 | 0.73 ± 0.38 | 0.19 | |
| NH4+ (mg L−1) | 0.31 ± 0.25 | 0.61 ± 1.18 | 0.27 ± 0.19 | 0.16 ± 0.20 | 0.21 ± 0.08 | 0.55 ± 0.46 | 0.01 ± 0.01 | 0.36 ± 0.20 | 0.57 ± 0.41 | <0.0001 | |
| TIN (mg L−1) | 1.20 ± 0.55 | 2.07 ± 1.30 | 1.48 ± 0.91 | 0.93 ± 0.22 | 1.61 ± 0.73 | 2.05 ± 0.95 | 1.56 ± 0.79 | 2.20 ± 1.62 | 2.71 ± 1.46 | 0.005 | |
| Autumn | Salinity (PSU) | 0.02 ± 0.00 | 0.15 ± 0.09 | 0.46 ± 0.20 | 0.57 ± 0.23 | 1.27 ± 0.29 | 1.33 ± 0.57 | 3.17 ± 1.43 | 2.95 ± 0.74 | 7.28 ± 0.16 | <0.0001 |
| EC (µS cm−1) | 50 ± 2 | 325 ± 182 | 921 ± 395 | 1156 ± 443 | 2522 ± 511 | 2642 ± 1111 | 5797 ± 2502 | 5455 ± 1284 | 12681 ± 280 | <0.0001 | |
| O2 (%) | 106.6 ± 13.1 | 91.8 ± 8.9 | 117.6 ± 16.5 | 112.0 ± 16.7 | 127.5 ± 16.6 | 118.2 ± 9.1 | 117.9 ± 16.7 | 73.1 ± 34.8 | 75.1 ± 17.8 | <0.0001 | |
| Chl-a (mg L−1) | 20.3 ± 17.8 | 20.4 ± 19.4 | 32.8 ± 34.6 | 16.6 ± 21.3 | 28.9 ± 25.1 | 20.1 ± 20.3 | 11.1 ± 9.0 | 12.7 ± 12.2 | 17.4 ± 11.7 | 0.60 | |
| TP (mg L−1) | 0.27 ± 0.12 | 0.46 ± 0.18 | 0.31 ± 0.28 | 0.40 ± 0.46 | 0.30 ± 0.20 | 0.14 ± 0.06 | 0.42 ± 0.22 | 0.30 ± 0.16 | 0.58 ± 0.27 | 0.0003 | |
| NO3− (mg L−1) | 0.64 ± 0.39 | 0.82 ± 0.39 | 0.68 ± 0.80 | 0.86 ± 0.28 | 1.05 ± 0.61 | 1.61 ± 1.39 | 1.62 ± 0.99 | 0.74 ± 0.39 | 1.21 ± 0.60 | <0.05 | |
| NO2− (mg L−1) | 0.40 ± 0.22 | 0.49 ± 0.23 | 0.48 ± 0.55 | 0.58 ± 0.12 | 0.66 ± 0.23 | 0.60 ± 0.20 | 0.56 ± 0.21 | 0.58 ± 0.21 | 0.80 ± 0.50 | 0.30 | |
| NH4+ (mg L−1) | 0.34 ± 0.27 | 0.33 ± 0.27 | 0.24 ± 0.46 | 0.25 ± 0.35 | 0.14 ± 0.09 | 0.48 ± 0.48 | 0.05 ± 0.08 | 0.29 ± 0.15 | 0.53 ± 0.29 | <0.0001 | |
| TIN (mg L−1) | 1.37 ± 0.57 | 1.60 ± 0.74 | 1.12 ± 1.20 | 1.70 ± 0.37 | 1.85 ± 0.75 | 2.70 ± 1.32 | 2.23 ± 1.21 | 1.61 ± 0.53 | 2.54 ± 1.35 | <0.04 | |
| GS | Freshwater | Transitional | Brackish | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dołgie | Wicko | Sarbsko | Liwia | Kopań | Gardno | Resko | Łebsko | Ptasi Raj | ||
| Oligochaeta | + | + | + | + | + | + | + | + | + | |
| Polychaeta | ||||||||||
| Hediste diversicolor | M | + | + | |||||||
| Pygospio elegans | M | + | ||||||||
| Crustacea | ||||||||||
| Asellus aquaticus | O | + | + | + | ||||||
| Gammarus duebeni | E | + | + | + | + | + | + | |||
| Gammarus oceanicus | M | + | + | + | + | |||||
| Corophium volutator | E | + | ||||||||
| Praunus flexuosus | E | + | ||||||||
| Idotea balthica | M | + | ||||||||
| Neomysis integer | M | + | ||||||||
| Palaemon elegans | E | + | ||||||||
| Hirudinea | ||||||||||
| Glossiphonia complanata | O | + | + | |||||||
| Erpobdella octoculata | O | + | + | |||||||
| Piscicola geometra | E | + | ||||||||
| Diptera larvae | ||||||||||
| Chironomus f.l. plumosus | O | + | + | + | + | + | + | + | + | + |
| Chironomus f.l. thummi | E | + | ||||||||
| Glyptotendipes e.g., gripekoveni | O | + | + | + | ||||||
| Procladius spp. | O | + | + | + | + | + | + | + | + | + |
| Polypedilum nubeculosum | O | + | + | + | + | + | + | + | + | + |
| Polypedilum e.g., scalaeum | O | + | + | + | + | + | ||||
| Psectrocladius barbimanus | E | + | + | + | + | + | + | + | + | + |
| Bezzia nobilis | E | + | + | + | + | + | + | + | ||
| Microtendipes e.g., chloris | O | + | + | |||||||
| Sergentia coracina | O | + | + | + | + | + | + | + | + | + |
| Einfeldia e.g., carbonaria | O | + | + | + | ||||||
| Clunio sp. | M | + | ||||||||
| Chaoborus sp. | O | + | ||||||||
| Diamesa campestris | E | + | + | + | ||||||
| Tanatyrus e.g., mancus | O | + | ||||||||
| Chironomidae n.det. | + | + | + | + | + | + | + | |||
| Hemiptera | ||||||||||
| Sigara sp. | O | + | ||||||||
| Corixa sp. | O | + | ||||||||
| Trichoptera larvae | ||||||||||
| Triaenodes bicolor | E | + | ||||||||
| Limnephilus sp. | O | + | ||||||||
| Limnephilus bipunctatus | O | + | + | |||||||
| Ecnomus tenellus | O | + | + | |||||||
| Ephemeroptera larvae | ||||||||||
| Caenis macrura | O | + | ||||||||
| Gastropoda | ||||||||||
| Bithynia tentaculata | O | + | + | + | + | + | + | |||
| Planorbis planorbis | O | + | ||||||||
| Valvata piscinalis | O | + | + | + | ||||||
| Theodoxus fluviatilis | E | + | + | + | + | |||||
| Potamopyrgus jenkinsi | E | + | + | |||||||
| Hydrobia ulvae | M | + | + | |||||||
| Bivalvia | ||||||||||
| Dreissena polymorpha | E | + | + | + | + | + | + | |||
| Pisidium sp. | O | + | + | |||||||
| Unio tumidus | O | + | + | |||||||
| Unio pictorum | O | + | + | |||||||
| Anodonta anatina | O | + | + | + | ||||||
| Number of taxa | 12 | 17 | 18 | 16 | 15 | 12 | 21 | 27 | 18 | |
| Number of taxa in type of lakes | 24 | 25 | 36 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrozińska, N.; Glińska-Lewczuk, K.; Obolewski, K. Salinity as a Key Factor on the Benthic Fauna Diversity in the Coastal Lakes. Animals 2021, 11, 3039. https://doi.org/10.3390/ani11113039
Mrozińska N, Glińska-Lewczuk K, Obolewski K. Salinity as a Key Factor on the Benthic Fauna Diversity in the Coastal Lakes. Animals. 2021; 11(11):3039. https://doi.org/10.3390/ani11113039
Chicago/Turabian StyleMrozińska, Natalia, Katarzyna Glińska-Lewczuk, and Krystian Obolewski. 2021. "Salinity as a Key Factor on the Benthic Fauna Diversity in the Coastal Lakes" Animals 11, no. 11: 3039. https://doi.org/10.3390/ani11113039
APA StyleMrozińska, N., Glińska-Lewczuk, K., & Obolewski, K. (2021). Salinity as a Key Factor on the Benthic Fauna Diversity in the Coastal Lakes. Animals, 11(11), 3039. https://doi.org/10.3390/ani11113039








