Immune-Related Gene Expression Profiling of Broiler Chickens Fed Diets Supplemented with Vinification Byproducts: A Valorization Approach II
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Broilers’ Trial
2.2. Vinification Byproducts Processing and Diets Formulation
2.3. Determination of Total Antioxidant Capacity of Vinification Byproducts
2.4. Determination of Total b-1,3-1,6-Glucans of Wine Lees
2.5. Determination of Performance Parameters
2.6. Sample Collection
2.7. Molecular Analysis
2.7.1. RNA Isolation and cDNA Synthesis
2.7.2. Primers’ Design
2.7.3. Real-Time Quantitative PCR
2.8. Statistics
3. Results
3.1. Grape Byproducts Total Antioxidant Capacity and Wine Lee b-Glucan Content
3.2. Broilers’ Performance
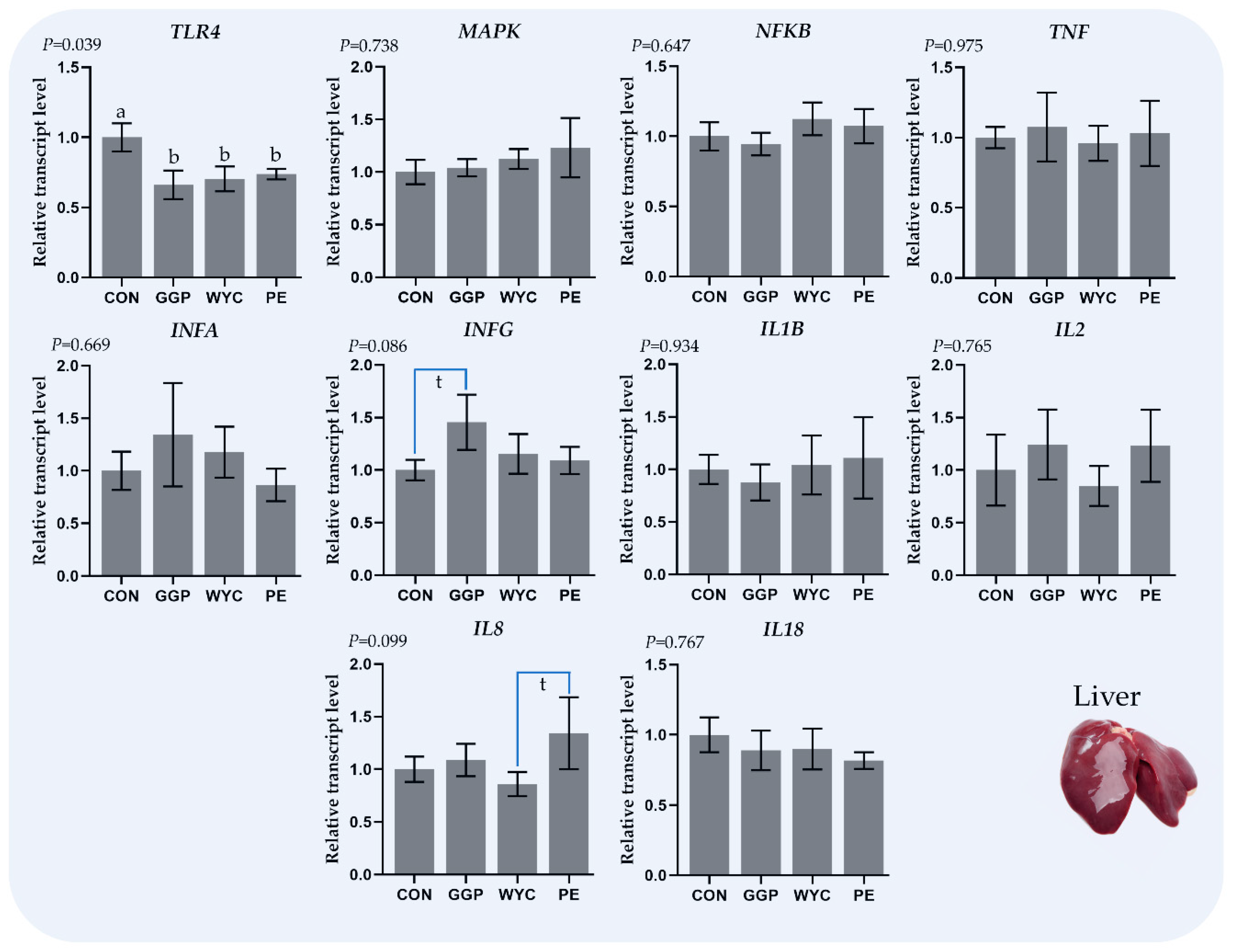
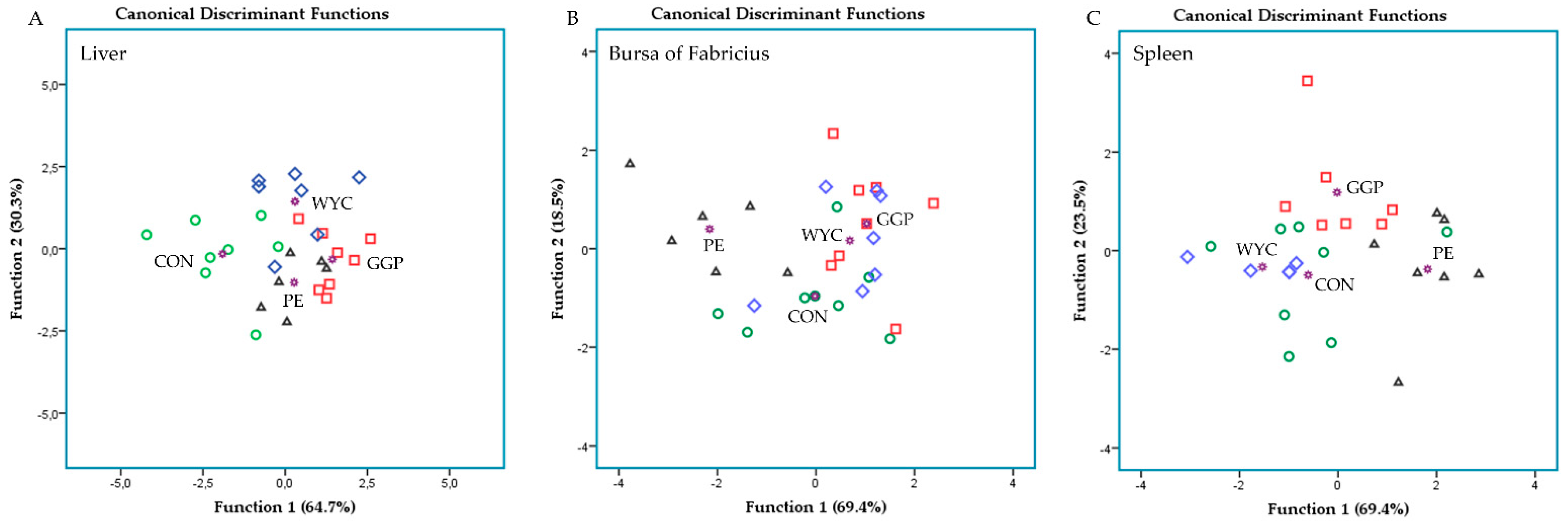
3.3. Relative Transcript Levels of Genes Regulating the Immune System in Liver
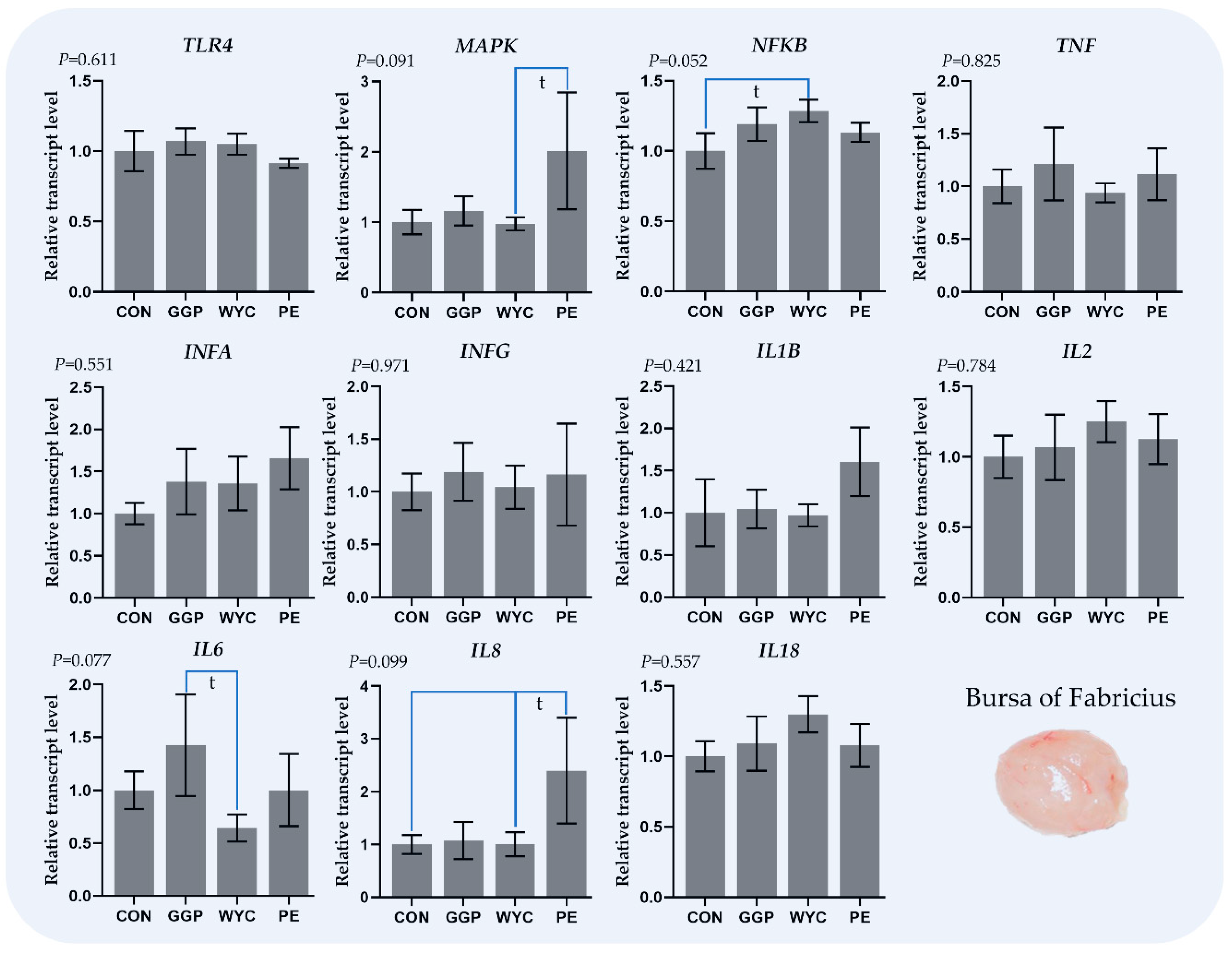
3.4. Relative Transcript Levels of Genes Regulating the Immune System in the Bursa of Fabricius
3.5. Relative Transcript Levels of Genes Regulating the Immune System in the Spleen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. STAT-FAO Statistical Database. 2010. Available online: http://faostat3.fao.org (accessed on 25 August 2021).
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of grape stalks and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind. Crop. Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goñi, I.; Centeno, C.; Sáyago-Ayerdy, S.G.; Arija, I.; Saura-Calixto, F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult. Sci. 2008, 87, 307–316. [Google Scholar] [CrossRef]
- Righi, F.; Pitino, R.; Manuelian, C.L.; Simoni, M.; Quarantelli, A.; De Marchi, M.; Tsiplakou, E. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins on Poultry Performances, Health, and Oxidative Status: A Review of the Literature in the Last 20 Years. Antioxidants 2021, 10, 659. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Giamouri, E.; Myrtsi, E.D.; Evergetis, E.; Filippi, K.; Papapostolou, H.; Koulocheri, S.D.; Zoidis, E.; Pappas, A.C.; Koutinas, A.; et al. Antioxidant Status of Broiler Chickens Fed Diets Supplemented with Vinification Byproducts: A Valorization Approach. Antioxidants 2021, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T. Natural compounds as modulators of NADPH oxidases. Oxidative Med. Cell. Longev. 2013, 2013, 271602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.S.; Jung, H.Y.; Park, E.Y.; Kim, J.; Lee, W.J.; Bae, Y.S. Cutting edge: Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 2004, 173, 3589–3593. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.C.; Zhu, Y.R.; Zhao, Z.H.; Jiang, P.; Yin, F.Q. Effects of Dietary Supplementation of Algae-Derived Polysaccharides on Morphology, Tight Junctions, Antioxidant Capacity and Immune Response of Duodenum in Broilers under Heat Stress. Animals 2021, 11, 2279. [Google Scholar] [CrossRef]
- Liu, W.C.; Ou, B.H.; Liang, Z.L.; Zhang, R.; Zhao, Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021, 100, 101139. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Kidd, M.T. Redox Homeostasis in Poultry: Regulatory Roles of NF-κB. Antioxidants 2021, 10, 186. [Google Scholar] [CrossRef]
- Burdick Sanchez, N.C.; Broadway, P.R.; Carroll, J.A. Influence of Yeast Products on Modulating Metabo-lism and Immunity in Cattle and Swine. Animals 2021, 11, 371. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Cuevas, A.; Saavedra, N.; Salazar, L.A.; Abdalla, D.S.P. Modulation of Immune Function by Polyphenols: Possible Contribution of Epigenetic Factors. Nutrients 2013, 5, 2314–2332. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [Green Version]
- Myrtsi, E.D.; Koulocheri, S.D.; Iliopoulos, V.; Haroutounian, S.A. High-Throughput Quantifica-tion of 32 Bioactive Antioxidant Phenolic Compounds in Grapes, Wines and Vinification Byproducts by LC-MS/MS. Antioxidants 2021, 10, 1174. [Google Scholar] [CrossRef]
- Nitschke, J.; Modick, H.; Busch, E.; von Rekowski, R.W.; Altenbach, H.J.; Mölleken, H. A new colorimetric method to quantify β-1,3-1,6-glucans in comparison with total β-1,3-glucans in edible mushrooms. Food Chem. 2011, 127, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Tsiplakou, E.; Mavrommatis, A.; Skliros, D.; Sotirakoglou, K.; Flemetakis, E.; Zervas, G. The effects of dietary supplementation with rumen-protected amino acids on the expression of several genes involved in the immune system of dairy sheep. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1437–1449. [Google Scholar] [CrossRef]
- Qin, N.; Shan, X.; Sun, X.; Liswaniso, S.; Chimbaka, I.M.; Xu, R. Evaluation and Validation of the Six Housekeeping Genes for Normalizing Mrna Expression in the Ovarian Follicles and Several Tissues in Chicken. Braz. J. Poult. Sci. 2020, 22, 3. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Mitsiopoulou, C.; Christodoulou, C.; Karabinas, D.; Nenov, V.; Zervas, G.; Tsiplakou, E. Dietary Supplementation of a Live Yeast Product on Dairy Sheep Milk Performance, Oxidative and Immune Status in Peripartum Period. J. Fungi 2020, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Csernus, B.; Biró, S.; Babinszky, L.; Komlósi, I.; Jávor, A.; Stündl, L.; Remenyik, J.; Bai, P.; Oláh, J.; Pesti-Asbóth, G.; et al. Effect of Carotenoids, Oligosaccharides and Antho-cyanins on Growth Performance, Immunological Parameters and Intestinal Morphology in Broiler Chickens Challenged with Escherichia coli Lipopolysaccharide. Animals 2020, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Ahmadipour, B.; Hassanpour, H.; Khajali, F. Evaluation of hepatic lipogenesis and antioxidant status of broiler chickens fed mountain celery. BMC Vet. Res. 2018, 14, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Liu, F. Polyphenol extracts from Punica granatum and Terminalia chebula are an-ti-inflammatory and increase the survival rate of chickens challenged with Escherichia coli. Biol. Pharm. Bull. 2014, 37, 1575–1582. [Google Scholar]
- Han, H.; Zhang, J.; Chen, Y.; Shen, M.; Yan, E.; Wei, C.; Yu, C.; Zhang, L.; Wang, T. Dietary taurine supplementation attenuates lipopolysaccharide-induced inflammatory responses and oxidative stress of broiler chickens at an early age. J. Anim. Sci. 2020, 98, skaa311. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Regassa, A.; Kim, J.H.; Kim, W.K. The effect of dietary fructooligosaccharide supple-mentation on growth performance, intestinal morphology, and immune responses in broiler chickens challenged with Salmonella Enteritidis lipopolysaccharides. Poult. Sci. 2015, 94, 2887–2897. [Google Scholar] [CrossRef]
- Deng, J.; Guo, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Wang, X.; Zhao, L. Oxidative stress and inflammatory responses involved in dietary nickel chloride (NiCl2)-induced pulmonary toxicity in broiler chickens. Toxicol. Res. 2016, 5, 1421–1433. [Google Scholar] [CrossRef] [Green Version]
- Paraskeuas, V.V.; Mountzouris, K.C. Modulation of broiler gut microbiota and gene expression of Toll-like receptors and tight junction proteins by diet type and inclusion of phytogenics. Poult. Sci. 2019, 98, 2220–2230. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G. Effect of Raw and Fermented Grape Pomace on the Growth Performance, Antioxidant Status, Intestinal Morphology, and Selected Bacterial Species in Broiler Chicks. Animals 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, S.K.; Navidshad, B.; Farhoomand, P.; Aghjehgheshlagh, F.M. Effects of grape pomace and vitamin E on performance, antioxidant status, immune response, gut morphology and histopathological responses in broiler chickens. S. Afr. J. Anim. Sci. 2018, 48, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Hasted, T.L.; Sharif, S.; Boerlin, P.; Diarra, M.S. Immunostimulatory Potential of Fruits and Their Extracts in Poultry. Front. Immunol. 2021, 12, 641696. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Yi, R.; Chen, X.; Li, W.; Mu, J.; Tan, F.; Zhao, X. Preventive effect of insect tea primary leaf (Malus sieboldii (Regal) Rehd.) extract on D-galactose-induced oxidative damage in mice. Food Sci. Nutr. 2020, 8, 5160–5171. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Palade, L.M.; Pelmus, R.S.; Dragomir, C.; Taranu, I. Red Grape Pomace Rich in Polyphenols Diet Increases the Antioxidant Status in Key Organs—Kidneys, Liver, and Spleen of Piglets. Animals 2019, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, N.; Nasti, T.H.; Meleth, S.; Elmets, C.A. Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol. Carcinog. 2009, 48, 713–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 278. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Biswas, D.; Lingbeck, J.; Koo, O.K.; Ricke, S.C. Enhancement of chicken macrophage cytokine response to Salmonella Typhimurium when combined with bacteriophage P22. FEMS Microbiol. Lett. 2013, 339, 137–144. [Google Scholar] [CrossRef]
- Wang, Y.; Zeigler, M.M.; Lam, G.K. The role of the NADPH oxidase complex, p38 MAPK, and Akt in regulating human monocyte/macrophage survival. Am. J. Respir. Cell Mol. Biol. 2007, 36, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Quilen, C.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Pinent, M.; Ardévol, A.; Blay, M.T.; Terra, X. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Qin, D.; Wang, X.; Feng, Y.; Yang, X.; Yao, J. Effect of immune stress on growth performance and energy metabolism in broiler chickens. Food Agric. Immunol. 2015, 26, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003, 4, 117. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.W.; Tan, X.; Sun, J.Y.; Gu, C.M.; Liu, C.; Guo, X. Catechin attenuates TNF-α induced inflammatory response via AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS ONE 2019, 14, e0217090. [Google Scholar] [CrossRef] [Green Version]
- Afolabi, O.K.; Aderibigbe, F.A.; Folarin, D.T.; Arinola, A.; Wusu, A.D. Oxidative stress and inflammation following sub-lethal oral exposure of cypermethrin in rats: Mitigating potential of epicatechin. Heliyon 2019, 5, e02274. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, B.; Vetvicka, V. Review: β-glucans as Effective Antibiotic Alternatives in Poultry. Molecules 2021, 26, 3560. [Google Scholar] [CrossRef]

| Gene | Sequence | Amplicon bp | Accession No.* | References |
|---|---|---|---|---|
| GAPDH | F: 5′- GCTGGCATTGCACTGAATGAC -3′ | 113 | NM_204305.1 | [23] |
| R: 5′- CACTCCTTGGATGCCATGT -3′ | ||||
| ACTB | F: 5′- AGCGAACGCCCCCAAAGTTCT -3′ | 139 | NM_205518.1 | [24] |
| F: 5′- AGCTGGGCTGTTGCCTTCACA -3′ | ||||
| TLR4 | R: 5′- ACCCGAACTGCAGTTTCTGGAT -3′ | 120 | NM_001030693.1 | [23] |
| R: 5′- AGGTGCTGGAGTGAATTGGC -3′ | ||||
| MAPK | F: 5′- GAACGTGCGCTTCATCTACG -3′ | 137 | XM_040649449.1 | |
| R: 5′- CCACGGGCTTAAACGCTTTC -3′ | ||||
| NFKB | F: 5′- GAAGGAATCGTACCGGGAACA -3′ | 131 | NM_205134 131 | [25] |
| R: 5′- CTCAGAGGGCCTTGTGACAGTAA -3′ | ||||
| TNF | F: 5′- CCCCTACCCTGTCCCACAA -3′ | 67 | NM204267 | [26] |
| R: 5′- TGAGTACTGCGGAGGGTTCAT -3′ | ||||
| INFA | F: 5′- ACTTCAGCTGCCTCCACACCTT -3′ | 92 | AM049251.1 | [23] |
| R: 5′- CAGGAACCAGGCACGAGCTT -3′ | ||||
| INFG | F: 5′- AACAACCTTCCTGATGGCGTGA -3′ | 89 | NM_205149.1 | [23] |
| R: 5′- GCTTTGCGCTGGATTCTCAAGT -3′ | ||||
| IL1B | F: 5′- TGCTTCGTGCTGGAGTCACCC -3′ | 98 | XM_015297469.1 | [23] |
| R: 5′- GGCCGGTACAGCGCAATGTT -3′ | ||||
| IL2 | F: 5′- CGTAAGTGGATGGTTTTCCTCT -3′ | 161 | NM204153 | [27] |
| R: 5′- GGCTAAAGCTCACCTGGGTC -3′ | ||||
| IL6 | F: 5′- AGCGAAAAGCAGAACGTCGAGTC -3′ | 107 | XM_015281283.2 | [23] |
| R: 5′- GCCGAGTCTGGGATGACCACTTC -3′ | ||||
| IL8 | F: 5′- CTGGCCCTCCTCCTGGTT-3′ | 105 | HM179639 | [28] |
| R: 5′- GCAGCTCATTCCCCATCTTTAC -3′ | ||||
| IL18 | F: 5′- GTTGTTCGATTTAGGGAAGGAG -3′ | 146 | NM204608.1 | [29] |
| R: 5′- TCAAAGGCCAAGAACATTCC -3′ |
| Dietary Treatment | |||
|---|---|---|---|
| GGP | WYC | PE | |
| FRAP | 0.95 ± 0.07 | 2.6 ± 0.1 | 2.1 ± 0.2 |
| DPPH | 1.56 ± 0.01 | 1.846 ± 0.001 | 1.860 ± 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrommatis, A.; Simitzis, P.E.; Kyriakaki, P.; Giamouri, E.; Myrtsi, E.D.; Evergetis, E.; Filippi, K.; Papapostolou, H.; Koulocheri, S.D.; Pappas, A.C.; et al. Immune-Related Gene Expression Profiling of Broiler Chickens Fed Diets Supplemented with Vinification Byproducts: A Valorization Approach II. Animals 2021, 11, 3038. https://doi.org/10.3390/ani11113038
Mavrommatis A, Simitzis PE, Kyriakaki P, Giamouri E, Myrtsi ED, Evergetis E, Filippi K, Papapostolou H, Koulocheri SD, Pappas AC, et al. Immune-Related Gene Expression Profiling of Broiler Chickens Fed Diets Supplemented with Vinification Byproducts: A Valorization Approach II. Animals. 2021; 11(11):3038. https://doi.org/10.3390/ani11113038
Chicago/Turabian StyleMavrommatis, Alexandros, Panagiotis E. Simitzis, Panagiota Kyriakaki, Elisavet Giamouri, Eleni D. Myrtsi, Epameinondas Evergetis, Katiana Filippi, Harris Papapostolou, Sofia D. Koulocheri, Athanasios C. Pappas, and et al. 2021. "Immune-Related Gene Expression Profiling of Broiler Chickens Fed Diets Supplemented with Vinification Byproducts: A Valorization Approach II" Animals 11, no. 11: 3038. https://doi.org/10.3390/ani11113038
APA StyleMavrommatis, A., Simitzis, P. E., Kyriakaki, P., Giamouri, E., Myrtsi, E. D., Evergetis, E., Filippi, K., Papapostolou, H., Koulocheri, S. D., Pappas, A. C., Koutinas, A., Haroutounian, S. A., & Tsiplakou, E. (2021). Immune-Related Gene Expression Profiling of Broiler Chickens Fed Diets Supplemented with Vinification Byproducts: A Valorization Approach II. Animals, 11(11), 3038. https://doi.org/10.3390/ani11113038












