Long-Tailed Pygmy Rice Rats Modify Their Behavioural Response and Faecal Corticosterone Metabolites in Response to Culpeo Fox but Not to Lesser Grison
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Simulation of Predation Risk by Faecal Odour
2.4. Data Collection
2.5. Faeces Collection and Quantification of Faecal Corticosterone Metabolites
2.6. Statistical Analysis
3. Results
3.1. Behavioural Response: Capturability
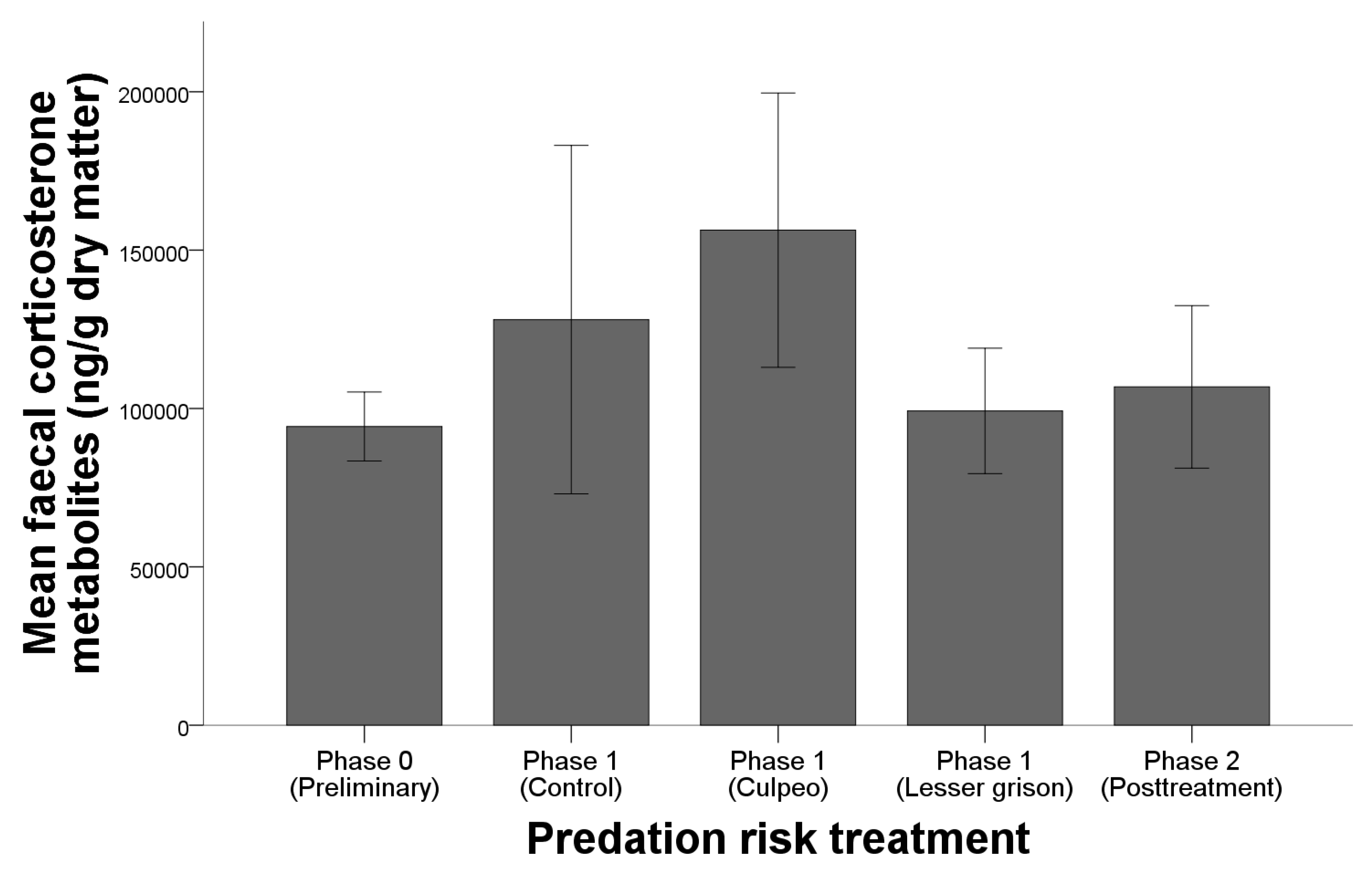
3.2. Physiological Stress Response
4. Discussion
4.1. Behavioural Response
4.2. Physiological Stress Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, P.A. The Evolution of Predator-Prey Interactions: Theory and Evidence. Annu. Rev. Ecol. Syst. 2000, 31, 79–105. [Google Scholar] [CrossRef]
- Johnson, J.B.; Belk, M.C. Predators as Agents of Selection and Diversification. Diversity 2020, 12, 415. [Google Scholar] [CrossRef]
- Barbosa, P.; Castellanos, I. Ecology of Predator-Prey Interactions; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Davies, N.B.; Krebs, J.R.; West, S.A. An Introduction to Behavioural Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Scherer, A.E.; Smee, D.L. A review of predator diet effects on prey defensive responses. Chemoecology 2016, 26, 83–100. [Google Scholar] [CrossRef]
- Lima, S.L. Nonlethal effects in the ecology of predator-prey interactions. Bioscience 1998, 48, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-González, B.; Barja, I.; Navarro-Castilla, Á. Wood mice modify food intake under different degrees of predation risk: Influence of acquired experience and degradation of predator’s faecal volatile compounds. Chemoecology 2017, 27, 115–122. [Google Scholar] [CrossRef]
- Navarro-Castilla, A.; Barja, I.; Diaz, M. Foraging, feeding, and physiological stress responses of wild wood mice to increased illumination and common genet cues. Curr. Zool. 2018, 64, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Hayes, R.A.; Nahrung, H.F.; Wilson, J.C. The response of native Australian rodents to predator odours varies seasonally: A by-product of life history variation? Anim. Behav. 2006, 71, 1307–1314. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, B.; Planillo, A.; Navarro-Castilla, A.; Barja, I. The concentration of fear: Mice’s behavioural and physiological stress responses to different degrees of predation risk. Naturwissenschaften 2018, 105, 16. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.C.; Navarro-Castilla, Á.; Planillo, A.; Sánchez-González, B.; Barja, I. The landscape of fear: Why some free-ranging rodents choose repeated live-trapping over predation risk and how it is associated with the physiological stress response. Behav. Process. 2018, 157, 125–132. [Google Scholar] [CrossRef]
- Romero, L.M. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef]
- Casas, F.; Benítez-López, A.; Tarjuelo, R.; Barja, I.; Viñuela, J.; García, J.T.; Morales, M.B.; Mougeot, F. Changes in behaviour and faecal glucocorticoid levels in response to increased human activities during weekends in the pin-tailed sandgrouse. Sci. Nat. 2016, 103, 91. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Bonier, F.; Martin, P.R.; Moore, I.T.; Wingfield, J.C. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 2009, 24, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, J.C.; Romero, L.M. Adrenocortical responses to stress and their modulation in free-living vertebrates. Compr. Physiol. 2010, 23, 211–234. [Google Scholar]
- Stewart, P.M.; Krone, N.P. The adrenal cortex. In Williams Textbook of Endocrinology; Larsen, P.R., Kronenberg, H., Melmed, S., Polonsky, K.S., Eds.; Saunders: Philadelphia, PA, USA, 2003. [Google Scholar]
- Gálvez, N.; Meniconi, P.; Infante, J.; Bonacic, C. Response of mesocarnivores to anthropogenic landscape intensification: Activity patterns and guild temporal interactions. J. Mammal. 2021, 102, 1149–1164. [Google Scholar] [CrossRef]
- Lantschner, M.V.; Rusch, V.; Hayes, J.P. Habitat use by carnivores at different spatial scales in a plantation forest landscape in Patagonia, Argentina. Ecol. Manag. 2012, 269, 271–278. [Google Scholar] [CrossRef]
- Guntiñas, M.; Lozano, J.; Cisneros, R.; Malo, A.F. Ecology of the culpeo (Lycalopex culpaeus): A synthesis of existing knowledge. Hystrix 2021, 32. [Google Scholar] [CrossRef]
- Zúñiga, A.; Muñoz-Pedreros, A.; Fierro, A. Dieta de Lycalopex griseus (Gray, 1837)(Mammalia: Canidae) en la depresión intermedia del sur de Chile. Gayana 2008, 72, 113–116. (In Spanish) [Google Scholar] [CrossRef] [Green Version]
- Zúñiga, A.H.; Fuenzalida, V. Dieta del zorro culpeo (Lycalopex culpaeus Molina 1782) en un área protegida del sur de Chile. Mastozoología Neotrop. 2016, 23, 201–205. (In Spanish) [Google Scholar]
- Moreira-Arce, D.; Vergara, P.M.; Boutin, S.; Simonetti, J.A.; Briceño, C.; Acosta-Jamett, G. Native forest replacement by exotic plantations triggers changes in prey selection of mesocarnivores. Biol. Conserv. 2015, 192, 258–267. [Google Scholar] [CrossRef]
- Rubio, A.V.; Fredes, F.; Simonetti, J.A. Exotic Pinus radiata plantations do not increase Andes Hantavirus prevalence in rodents. EcoHealth 2019, 16, 659–670. [Google Scholar] [CrossRef]
- Ebensperger, L.A.; Mella, J.E.; Simonetti, J.A. Trophic-niche relationships among Galictis cuja, Dusicyon culpaeus, and Tyto alba in central Chile. J. Mammal. 1991, 72, 820–823. [Google Scholar] [CrossRef]
- Yensen, E.; Tarifa, T. Galictis cuja. Mamm. Species 2003, 2003, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Delibes, M.; Travaini, A.; Zapata, S.C.; Palomares, F. Alien mammals and the trophic position of the lesser grison (Galictis cuja) in Argentinean Patagonia. Can. J. Zool. 2003, 81, 157–162. [Google Scholar] [CrossRef]
- Sade, S.; Rau, J.R.; Orellana, J.I. Dieta del quique (Galictis cuja Molina 1782) en un remanente de bosque valdiviano fragmentado del sur de Chile. Gayana 2012, 76, 112–116. (In Spanish) [Google Scholar] [CrossRef] [Green Version]
- Kasper, C.B.; Peters, F.B.; Christoff, A.U.; de Freitas, T.R.O. Trophic relationships of sympatric small carnivores in fragmented landscapes of southern Brazil: Niche overlap and potential for competition. Mammalia 2016, 80, 143–152. [Google Scholar] [CrossRef]
- Luengos Vidal, E.M.; Castillo, D.F.; Caruso, N.C.; Casanave, E.B.; Lucherini, M. Field capture, chemical immobilization, and morphometrics of a little-studied South American carnivore, the lesser grison. Wild. Soc. Bull. 2016, 40, 400–405. [Google Scholar] [CrossRef]
- Cantoni, G.; Padula, P.; Calderón, G.; Mills, J.; Herrero, E.; Sandoval, P.; Martinez, V.; Pini, N.; Larrieu, E. Seasonal variation in prevalence of antibody to hantaviruses in rodents from southern Argentina. Trop. Med. Int. Health 2001, 6, 811–816. [Google Scholar] [CrossRef]
- Saavedra, B.; Simonetti, J.A. Small mammals of Maulino forest remnants, a vanishing ecosystem of south-central Chile. Mammalia 2005, 69, 337–348. [Google Scholar] [CrossRef]
- González-Ittig, R.E.; Salazar-Bravo, J.; Barquez, R.M.; Gardenal, C.N. Phylogenetic relationships among species of the genus Oligoryzomys (Rodentia, Cricetidae) from Central and South America. Zool. Scr. 2010, 39, 511–526. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Temporal Variation in Danger Drives Antipredator Behavior: The Predation Risk Allocation Hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Coldwell, V. An Analysis of Methodologies Used to Study Medium and Large Mammals in the Valdivian Temperate Rainforests of Central-Southern Chile; Imperial College: London, UK, 2008. [Google Scholar]
- Galvez, N. Priority Habitats for Carnivores Surrounding Protected Areas in Andean Temperate Forests of Chile; University of Kent: Canterbury, UK, 2009. [Google Scholar]
- Murúa, R.; González, L.; Meserve, P. Population ecology of Oryzomys longicaudatus philippii (Rodentia: Cricetidae) in southern Chile. J. Anim. Ecol. 1986, 281–293. [Google Scholar] [CrossRef]
- Polop, F.; Levis, S.; Pini, N.; Enría, D.; Polop, J.; Provensal, M.C. Factors associated with hantavirus infection in a wild host rodent from Cholila, Chubut Province, Argentina. Mammal. Biol. 2018, 88, 107–113. [Google Scholar] [CrossRef]
- Polop, F.J.; Provensal, M.C.; Pini, N.; Levis, S.C.; Priotto, J.W.; Enría, D.; Calderón, G.E.; Costa, F.; Polop, J.J. Temporal and spatial host abundance and prevalence of Andes hantavirus in southern Argentina. EcoHealth 2010, 7, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Quintana, V.; Yáñez, J.; Valdebenito, M.; Iriarte, A. Orden carnívora. In Mamíferos de Chile; Pedreros, A.M., Yañez, J., Eds.; Ediciones CEA: Valdivia, Chile, 2000. (In Spanish) [Google Scholar]
- Apfelbach, R.; Blanchard, C.D.; Blanchard, R.J.; Hayes, R.A.; McGregor, I.S. The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neurosci. Biobehav. Rev. 2005, 29, 1123–1144. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Castilla, Á.; Barja, I. Does predation risk, through moon phase and predator cues, modulate food intake, antipredatory and physiological responses in wood mice (Apodemus sylvaticus)? Behav. Ecol. Sociobiol. 2014, 68, 1505–1512. [Google Scholar] [CrossRef]
- Barja, I.; Silvan, G.; Illera, J.C. Relationships between sex and stress hormone levels in feces and marking behavior in a wild population of Iberian wolves (Canis lupus signatus). J. Chem. Ecol. 2008, 34, 697–701. [Google Scholar] [CrossRef]
- Barja, I.; Silvan, G.; Martinez-Fernandez, L.; Illera, J.C. Physiological stress responses, fecal marking behavior, and reproduction in wild European pine martens (Martes martes). J. Chem. Ecol. 2011, 37, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.A.; Morelli, T.L.; Wright, P.C. Volatile components of lemur scent secretions vary throughout the year. Am. J. Primatol. 2006, 68, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Scordato, E.S.; Dubay, G.; Drea, C.M. Chemical composition of scent marks in the ringtailed lemur (Lemur catta): Glandular differences, seasonal variation, and individual signatures. Chem. Senses 2007, 32, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.; Barja, I.; López, P. Chemical scent constituents in feces of wild Iberian wolves (Canis lupus signatus). Biochem. Syst. Ecol. 2010, 38, 1096–1102. [Google Scholar] [CrossRef]
- Navarro-Castilla, Á.; Barja, I. Stressful living in lower-quality habitats? Body mass, feeding behavior and physiological stress levels in wild wood mouse populations. Integr. Zool. 2019, 14, 114–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barja, I.; Escribano-Ávila, G.; Lara-Romero, C.; Virgós, E.; Benito, J.; Rafart, E. Non-invasive monitoring of adrenocortical activity in European badgers (Meles meles) and effects of sample collection and storage on faecal cortisol metabolite concentrations. Anim. Biol. 2012, 62, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, J.A.; Lacey, E.A.; Bentley, G.E.; Kriegsfeld, L.J. Effects of social environment on baseline glucocorticoid levels in a communally breeding rodent, the colonial tuco-tuco (Ctenomys sociabilis). Horm. Behav. 2013, 64, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, B.; Barja, I.; Pineiro, A.; Hernandez-Gonzalez, M.C.; Silvan, G.; Illera, J.C.; Latorre, R. Support vector machines for explaining physiological stress response in Wood mice (Apodemus sylvaticus). Sci. Rep. 2018, 8, 2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003, 130, 267–278. [Google Scholar] [CrossRef]
- Navarro-Castilla, Á.; Barja, I. Antipredatory response and food intake in wood mice (Apodemus sylvaticus) under simulated predation risk by resident and novel carnivorous predators. Ethology 2014, 120, 90–98. [Google Scholar] [CrossRef]
- Brzeziński, M.; Pyrlik, J.; Churski, M.; Komar, E.; Zalewski, A. The influence of American mink odour on the spatial distribution and behaviour of water voles. Ethology 2019, 125, 791–801. [Google Scholar] [CrossRef]
- Navarro-Castilla, Á.; Sánchez-González, B.; Barja, I. Latrine behaviour and faecal corticosterone metabolites as indicators of habitat-related responses of wild rabbits to predation risk. Ecol. Indic. 2019, 97, 175–182. [Google Scholar] [CrossRef]
- Fishman, M.A. Predator inspection: Closer approach as a way to improve assessment of potential threats. J. Theor. Biol. 1999, 196, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Fincel, M.; Chipps, S.; Voldseth, R. Chemically-mediated predator inspection behavior by fathead minnow (Pimephales promelas). J. Freshwr. Ecol. 2010, 25, 279–283. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.C.; Bshary, R.; Cardoso, S.C.; Côté, I.M.; Oliveira, R.F. Face your fears: Cleaning gobies inspect predators despite being stressed by them. PLoS ONE 2012, 7, e39781. [Google Scholar] [CrossRef] [Green Version]
- Nolte, D.L.; Mason, J.R.; Epple, G.; Aronov, E.; Campbell, D.L. Why are predator urines aversive to prey? J. Chem. Ecol. 1994, 20, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Berton, F.; Vogel, E.; Belzung, C. Modulation of mice anxiety in response to cat odor as a consequence of predators diet. Physiol. Behav. 1998, 65, 247–254. [Google Scholar] [CrossRef]
- Kats, L.B.; Dill, L.M. The scent of death: Chemosensory assessment of predation risk by prey animals. Écoscience 1998, 5, 361–394. [Google Scholar] [CrossRef]
- Murray, D.L.; Jenkins, C.L. Perceived predation risk as a function of predator dietary cues in terrestrial salamanders. Anim. Behav. 1999, 57, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Dickman, C.R.; Doncaster, C.P. Responses of small mammals to Red fox (Vulpes vulpes) odour. J. Zool. 1984, 204, 521–531. [Google Scholar] [CrossRef]
- Dickman, C.R. Predation and Habitat Shift in the House Mouse Mus Domesticus. Ecology 1992, 73, 313–322. [Google Scholar] [CrossRef]
- Zapata, S.C.; Travaini, A.; Delibes, M.; Martínez-Peck, R. Annual food habits of the lesser grison (Galictis cuja) at the southern limit of its range. Mammalia 2005, 69, 85–88. [Google Scholar] [CrossRef]
- Cowan, P. Neophobia and neophilia: New-object and new-place reactions of three Rattus species. J. Comp. Physiol. Psychol. 1977, 91, 63–71. [Google Scholar] [CrossRef]
- Barnett, S.A. Exploring, sampling, neophobia, and feeding. In Rodent Pest Management; Prakash, I., Ed.; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Best, I.N.; Shaner, P.L.; Lo, H.Y.; Pei, K.J.; Kuo, C.C. Bigger doesn’t mean bolder: Behavioral variation of four wild rodent species to novelty and predation risk following a fast-slow continuum. Front. Zool. 2020, 17, 27. [Google Scholar] [CrossRef]
- Figueroa, R.; Corales, S.; Cerda, J.; Saldivia, H. Roedores, Rapaces y Carnívoros de Aysén; Servicio Agrícola y Ganadero, Gobierno Regional de Aysén: Coyhaique, Chile, 2001. (In Spanish) [Google Scholar]
- Hernández, M.C.; Jara-Stapfer, D.M.; Muñoz, A.; Bonacic, C.; Barja, I.; Rubio, A.V. Behavioral Responses of Wild Rodents to Owl Calls in an Austral Temperate Forest. Animals 2021, 11, 428. [Google Scholar] [CrossRef]
- Vasquez, R.A. Patch utilization by three species of Chilean rodents differing in body size and mode of locomotion. Ecology 1996, 77, 2343–2351. [Google Scholar] [CrossRef]
- Moore, Y.T. An Integrative Investigation of Convergent Bipedal Locomotion in Desert Rodents; Harvard University: Cambridge, MA, USA, 2016. [Google Scholar]
- Ylönen, H.; Eccard, J.A.; Jokinen, I.; Sundell, J. Is the antipredatory response in behaviour reflected in stress measured in faecal corticosteroids in a small rodent? Behav. Ecol. Sociobiol. 2006, 60, 350–358. [Google Scholar] [CrossRef]
- Boonstra, R.; Hik, D.; Singleton, G.R.; Tinnikov, A. The Impact of Predator-Induced Stress on the Snowshoe Hare Cycle. Ecol. Monog. 1998, 68, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Sheriff, M.J.; Thaler, J.S. Ecophysiological effects of predation risk; an integration across disciplines. Oecologia 2014, 176, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Hammerschlag, N.; Meÿer, M.; Seakamela, S.M.; Kirkman, S.; Fallows, C.; Creel, S. Physiological stress responses to natural variation in predation risk: Evidence from white sharks and seals. Ecology 2017, 98, 3199–3210. [Google Scholar] [CrossRef] [PubMed]
- Gyssels, F.G.M.; Stoks, R. Threat-Sensitive Responses to Predator Attacks in a Damselfly. Ethology 2005, 111, 411–423. [Google Scholar] [CrossRef]
- MacLean, S.A.; Bonter, D.N. The sound of danger: Threat sensitivity to predator vocalizations, alarm calls, and novelty in gulls. PLoS ONE 2013, 8, e82384. [Google Scholar] [CrossRef] [PubMed]
- Monclús, R.; Palomares, F.; Tablado, Z.; Martínez-Fontúrbel, A.; Palme, R. Testing the threat-sensitive predator avoidance hypothesis: Physiological responses and predator pressure in wild rabbits. Oecologia 2009, 158, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A.; Smith, J.M. Optimality theory in evolutionary biology. Nature 1990, 348, 27–33. [Google Scholar] [CrossRef]
- Orzack, S.H.; Sober, E. Optimality models and the test of adaptationism. Am. Nat. 1994, 143, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Rosen, R. Optimality Principles in Biology; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]

| Effect | F | df | p-Value |
|---|---|---|---|
| Intercept | 160.70 | 1 | <0.001 |
| Predation risk treatment | 18.94 | 4 | <0.001 |
| Plot | 3.75 | 2 | 1.153 |
| Effect | Estimate | Std. Error | df | t Value | p-Value |
|---|---|---|---|---|---|
| Intercept | 11.25 | 0.07 | 103.90 | 152.39 | <0.001 |
| Treatment—Control | 0.10 | 0.11 | 247.98 | 0.88 | 0.3817 |
| Treatment—Culpeo | 0.38 | 0.10 | 262.49 | 3.65 | <0.001 |
| Treatment—Lesser grison | 0.04 | 0.11 | 262.05 | 0.39 | 0.699 |
| Treatment—Posttreatment | 0.04 | 0.09 | 249.97 | 0.47 | 0.639 |
| Sex—Male | 0.11 | 0.07 | 57.24 | 1.53 | 0.132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, M.d.C.; Rubio, A.V.; Barja, I. Long-Tailed Pygmy Rice Rats Modify Their Behavioural Response and Faecal Corticosterone Metabolites in Response to Culpeo Fox but Not to Lesser Grison. Animals 2021, 11, 3036. https://doi.org/10.3390/ani11113036
Hernández MdC, Rubio AV, Barja I. Long-Tailed Pygmy Rice Rats Modify Their Behavioural Response and Faecal Corticosterone Metabolites in Response to Culpeo Fox but Not to Lesser Grison. Animals. 2021; 11(11):3036. https://doi.org/10.3390/ani11113036
Chicago/Turabian StyleHernández, María del Carmen, André V. Rubio, and Isabel Barja. 2021. "Long-Tailed Pygmy Rice Rats Modify Their Behavioural Response and Faecal Corticosterone Metabolites in Response to Culpeo Fox but Not to Lesser Grison" Animals 11, no. 11: 3036. https://doi.org/10.3390/ani11113036
APA StyleHernández, M. d. C., Rubio, A. V., & Barja, I. (2021). Long-Tailed Pygmy Rice Rats Modify Their Behavioural Response and Faecal Corticosterone Metabolites in Response to Culpeo Fox but Not to Lesser Grison. Animals, 11(11), 3036. https://doi.org/10.3390/ani11113036






