Classic and Non-Classic Effects of the Duration of Supplementation of 25-Hydroxicholecalciferol in Broiler Chicken Diets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, House, and Treatments
2.2. Growth Performance and Carcass Yield
2.3. Plasma Concentration of 25(OH)D3
2.4. Protein Deposition and Fat Content in the Breast Muscle
2.5. Tibia Breaking Strength Analysis
2.6. mRNA Expression of the mTOR Gene in the Breast Muscle
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Carcass and Cuts Yield
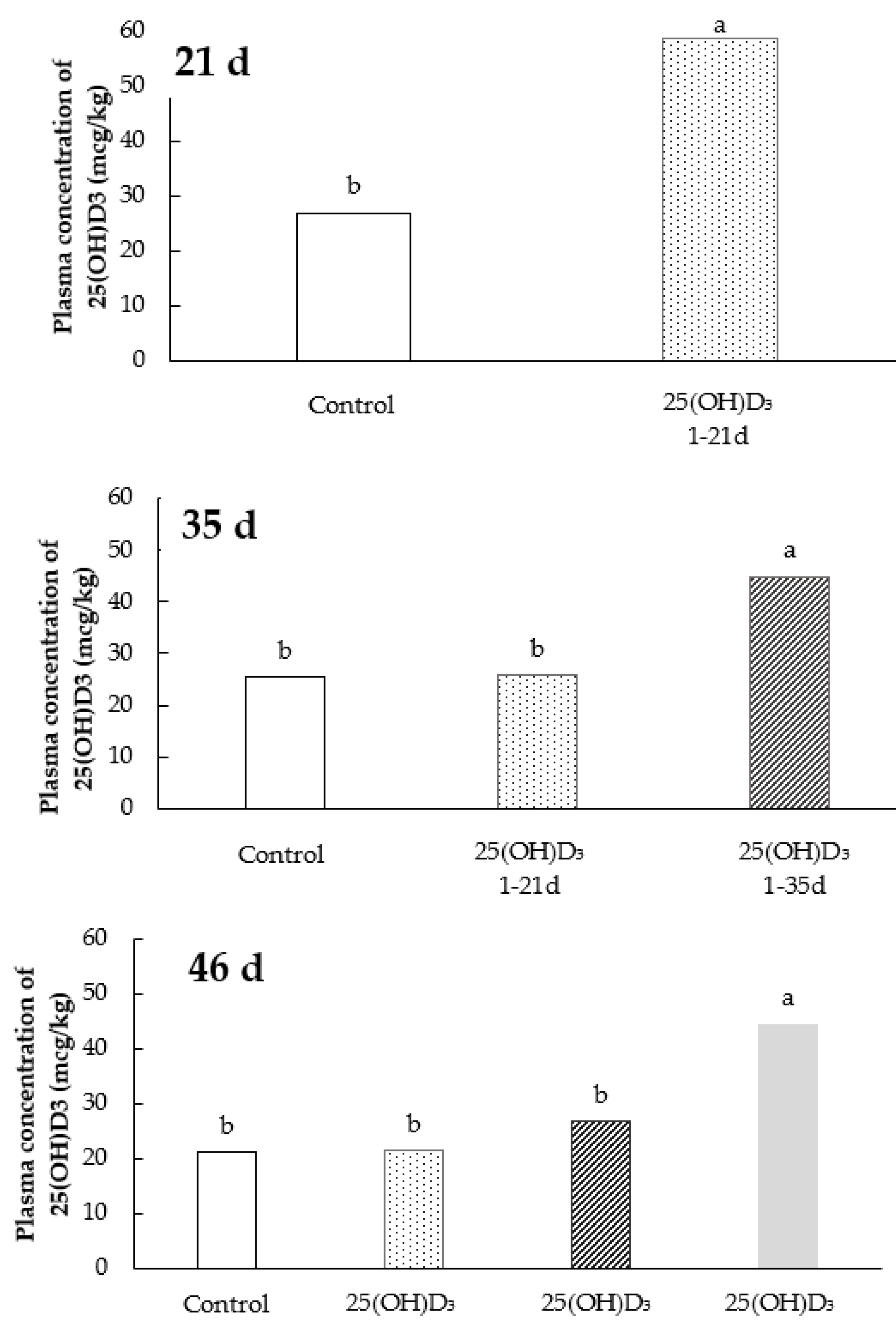
3.2. Plasma Concentration of 25(OH)D3
3.3. Protein Deposition and Fat Content in the Breast Muscle
3.4. Tibia Breaking Strength Analysis
3.5. mRNA Expression of the mTOR Gene in the Breast Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ABPA. Brazilian Association of Animal Protein. Annual Report. 2020. Available online: www.abpa.com.br (accessed on 2 June 2020).
- Edwards, H.M. Nutrition and Skeletal Problems in Poultry. Poult. Sci. 2000, 79, 1018–1023. [Google Scholar] [CrossRef]
- McDowell, L.R. (Ed.) Vitamin D. In Vitamin in Animal Nutrition; Academic Press: Gainesville, FL, USA, 1989; pp. 55–92. [Google Scholar]
- Garcia, A.F.Q.M.; Murakami, A.E.; do Duarte, C.R.A.; Rojas, I.C.O.; Picoli, K.P.; Puzotti, M.M. Use of Vitamin D3 and Its Metabolites in Broiler Chicken Feed on Performance, Bone Parameters and Meat Quality. Asian-Australas. J. Anim. Sci. 2013, 26, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Morris, A.; Shanmugasundaram, R.; McDonald, J.; Selvaraj, R.K. Effect of in vitro and in vivo 25-hydroxyvitamin D treatment on macrophages, T cells, and layer chickens during a coccidia challenge. J. Anim. Sci. 2016, 93, 2894–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.C.; Chen, G.H.; Wang, J.G.; Zhang, J.L.; Qu, H.X.; Zhang, C.M.; Yan, Y.F.; Cheng, Y.H. Evaluation of Relative Bioavailability of 25-Hydroxycholecalciferol to Cholecalciferol for Broiler Chickens. Asian-Australas. J. Anim. Sci. 2016, 29, 1145–1151. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, R.; White, D.; House, J.D.; Kim, W.K. Effects of additional dosage of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3 on calcium and phosphorus utilization, egg quality and bone mineralization in laying hens. Poult. Sci. 2020, 99, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Sharvit, M.; Noff, D.; Edelstein, S.; Hurwitz, S. Absorption and excretion of cholecalciferol and of 25-hydroxycholecalciferol and metabolites in birds. J. Nutr. 1980, 110, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, D.; Meredith, S.C.; Sitrin, M.D. Isolation and characterization of a 25-hydroxyvitamin D binding protein from rat enterocyte cytosol. J. Nutr. Biochem. 1997, 8, 195–200. [Google Scholar] [CrossRef]
- Bello, A.; Hester, P.Y.; Gerard, P.D.; Zhai, W.; Peebles, E.D. Effects of commercial in ovo injection of 25-hydroxycholecalciferol on bone development and mineralization in male and female broilers. Poult. Sci. 2014, 93, 2734–2739. [Google Scholar] [CrossRef] [PubMed]
- Levya-Jimenez, H.; Gardner, K.; AL-Jumaa, Y.; Padgett, J.C.; Bailey, C.A. Partial replacement of dietary cholecalciferol with 25-hydroxycholecalciferol on broiler chickens subjected to a coccidiosis vaccine challenge. J. Appl. Poul. Res. 2019, 28, 743–754. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, L.; Gong, H.; Celi, P.; Yan, L.; Ding, X.; Bai, S.; Zeng, Q.; Mao, X.; Xu, S.; et al. Effect of dietary 25-hydroxycholecalciferol supplementation and high stocking density on performance, egg quality, and tibia quality in laying hens. Poult. Sci. 2019, 99, 2608–2615. [Google Scholar] [CrossRef]
- Vignale, K.; Greene, E.S.; Caldas, J.V.; England, J.A.; Boonsinchai, N.; Sodsee, P.; Pollock, E.D.; Dridi, S.; Coon, C.N. 25-Hydroxycholecalciferol Enhances Male Broiler Breast Meat Yield through the mTOR Pathway. J. Nutr. 2015, 145, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Chou, S.H.; Chung, T.K.; Yu, B. Effects of supplemental 25-hydroxycholecalciferol on growth performance, small intestinal morphology, and immune response of broiler chickens. Poult. Sci. 2009, 88, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Oikeh, I.; Sakkas, P.; Blake, D.P.; Kyriazakis, I. Interactions between dietary calcium and phosphorus level, and vitamin D source on bone mineralization, performance, and intestinal morphology of coccidia-infected broilers. Poult. Sci. 2019, 98, 5679–5690. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Luo, M.; Pan, L.; Chen, Y.; Guo, S.; Luo, D.; Zhu, L.; Liu, Y.; Pan, L.; Xu, S.; et al. Vitamin D signaling maintains intestinal innate immunity and gut microbiota: Potential intervention for metabolic syndrome and NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Schadt, H.S.; Gossl, R.; Sibel, N.; Aebischer, C.P. Quantification of vitamin D3 in feed, food, and pharmaceuticals using high-performance liquid chromatography/tandem mass spectrometry. J. AOAC Int. 2012, 95, 1487–1494. [Google Scholar] [CrossRef]
- Sakomura, N.K.; Rostagno, H.S. Métodos de Pesquisa em Nutrição de Monogástricos, 2nd ed.; Funep: Jaboticabal, Brazil, 2016; p. 262. [Google Scholar]
- Weber, G.M.; Witschi, A.-K.M.; Wenk, C.; Martens, H. Triennial Growth Symposium—Effects of dietary 25-hydroxycholecalciferol and cholecalciferol on blood vitamin D and mineral status, bone turnover, milk composition, and reproductive performance of sows. J. Anim. Sci. 2014, 92, 899–909. [Google Scholar] [CrossRef]
- Seedor, J.G.; Quartuccio, H.A.; Thompson, D.D. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J. Bone Miner. Res. 1991, 6, 339–346. [Google Scholar] [CrossRef]
- Lee., J. Molecular Basis of Feed Efficiency in Meat-Type Chickens. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2012. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Salim, H.M.; Zaman, M.A.; Beg, M.A.H.; Khaleduzzaman, A.B.M. Effect of supplemental 25-hydroxycholecalciferol on live performance, bone development, and mineral utilization of broiler chickens fed low dietary Ca and P. EC Nutr. 2019, 14, 227–238. [Google Scholar]
- Hutton, K.C.; Vaughn, M.A.; Litta, G.; Turner, B.J.; Starkey, J.D. Effect of vitamin D status improvement with 25-hydroxycholecalciferol on skeletal muscle growth characteristics and satellite cell activity in broiler chickens. J. Anim. Sci. 2014, 92, 3291–3299. [Google Scholar] [CrossRef]
- Whitehead, C.C.; McCormack, H.A.; McTeir, L.; Fleming, R.H. High vitamin D3 requirements in broilers for bone quality and prevention of tibial dyschondroplasia and interactions with dietary calcium, available phosphorus, and vitamin A. Br. Poult. Sci. 2004, 45, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.R.; Raju, M.V.L.N.; Panda, A.K.; Sunder, G.S.; Sharma, R.P. Effect of High Concentrations of Cholecalciferol on Growth, Bone Mineralization, and Mineral Retention in Broiler Chicks Fed Suboptimal Concentrations of Calcium and Nonphytate Phosphorus. J. Appl. Poult. Res. 2006, 15, 493–501. [Google Scholar] [CrossRef]
- Angel, R.; Saylor, W.W.; Mitchell, A.D.; Powers, W.; Applegate, T.J. Effect of dietary phosphorus, phytase, and 25-hydroxycholecalciferol on broiler chicken bone mineralization, litter phosphorus, and processing yields. Poult. Sci. 2006, 85, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Colet, S.; Garcia, R.G.; Almeida Paz, I.C.L.; Caldara, F.R.; Borille, R.; Royer, A.F.B.; Nääs, I.A.; Sgavioli, S. Bone characteristics of broilers supplemented with vitamin D. Braz. J. Poult. Sci. 2015, 17, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, M.; Yalçin, S.; Koçer, B.; Tüzün, A.E.; Akşit, H.; Özkan, S.; Uygun, M.; Ege, G.; Güven, G.; Yildiz, O. Effects of enhancing vitamin D status by 25-hydroxycholecalciferol supplementation, alone or in combination with calcium and phosphorus, on sternum mineralization and breast meat quality in broilers. Br. Poult. Sci. 2017, 58, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, P.; Smith, S.; Hill, T.R.; Kyriazakis, I. A reassessment of the vitamin D requirements of modern broiler genotypes. Poult. Sci. 2019, 98, 330–340. [Google Scholar] [CrossRef]
- Fritts, C.A.; Waldroup, P.W. Effect of Source and Level of Vitamin D on Live Performance and Bone Development in Growing Broilers. J. Appl. Poult. Res. 2003, 12, 45–52. [Google Scholar] [CrossRef]
- Ovesen, L.; Brot, C.; Jakobsen, J. Food contents and biological activity of 25-hydroxyvitamin D: A vitamin D metabolite to be reckoned with? Ann. Nutr. Metab. 2003, 47, 107–113. [Google Scholar] [CrossRef]
- Moriuchi, S.; Deluca, H.F. Metabolism of vitamin D3 in the chick embryo. Arch. Biochem. Biophys. 1974, 164, 165–171. [Google Scholar] [CrossRef]
- Nechama, H.; Hoff, D.; Harrell, A.; Edelstein, S. The intestinal absorption of vitamin D and its metabolites. J. Mol. Med. 1977, 2, 416–422. [Google Scholar]
- Hassan-Smith, Z.K.; Jenkinson, C.; Smith, D.J.; Hernandez, I.; Morgan, S.A.; Crabtree, N.J.; Gittoes, N.J.; Keevil, B.G.; Stewart, P.M.; Hewison, M. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exert distinct effects on human skeletal muscle function and gene expression. PLoS ONE 2017, 12, e0170665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhang, Q.; Applegate, T.J. Impact of dietary branched chain amino acids concentration on broiler chicks during aflatoxicosis. Poult. Sci. 2016, 95, 1281–1289. [Google Scholar] [CrossRef] [PubMed]



| Treatments | Vitamin D | 25(OH)D3 1 | Total Vitamin D Activity | Period |
|---|---|---|---|---|
| (IU/kg) | (IU/kg) | (Days) | ||
| T1 | 3000 | - | 3000 | 0–46 |
| T2 | 3000 | 2760 | 5760 | 0–21 |
| 3000 | - | 3000 | 22–46 | |
| T3 | 3000 | 2760 | 5760 | 0–35 |
| 3000 | - | 3000 | 36–46 | |
| T4 | 3000 | 2760 | 5760 | 0–46 |
| Ingredients, % | Starter | Grower | Finisher |
|---|---|---|---|
| Corn 7.5% | 60.17 | 63.85 | 69.06 |
| Soybean meal 46.7% | 30.90 | 26.80 | 22.30 |
| Meat bone meal 48.5% | 4.500 | 3.600 | 2.900 |
| Soybean oil | 2.100 | 3.500 | 3.600 |
| Limestone | 0.580 | 0.560 | 0.560 |
| Salt | 0.200 | 0.190 | 0.200 |
| Sodium bicarbonate | 0.290 | 0.270 | 0.240 |
| Vitamin and mineral premix 1 | 0.300 | 0.300 | 0.300 |
| Choline 60% | 0.096 | 0.074 | 0.060 |
| DL-Methionine 98% | 0.369 | 0.352 | 0.320 |
| L-Lysine 50.7% | 0.292 | 0.320 | 0.322 |
| L-Threonine 98% | 0.099 | 0.100 | 0.082 |
| L-Valine 96.5% | 0.046 | 0.071 | 0.060 |
| Salinomycin 12% | - | 0.055 | - |
| Nicarbazin + Narasin 80/80 | 0.05 | - | - |
| Calculated nutritional composition | |||
| CP, % | 22.00 | 20.00 | 18.00 |
| ME, Kcal/kg | 3050 | 3180 | 3250 |
| Fat, % | 5.233 | 6.555 | 6.698 |
| CF, % | 2.359 | 2.210 | 2.061 |
| Calcium, % | 0.950 | 0.840 | 0.760 |
| AvP, % | 0.470 | 0.420 | 0.380 |
| Dig. Lys., % | 1.256 | 1.160 | 1.040 |
| Dig. AAS,% | 0.967 | 0.906 | 0.832 |
| Dig. Thr, % | 0.829 | 0.767 | 0.687 |
| Dig. Trp, % | 0.229 | 0.205 | 0.179 |
| Dig. Val, % | 0.954 | 0.894 | 0.801 |
| Na, % | 0.200 | 0.190 | 0.180 |
| Cl, % | 0.240 | 0.240 | 0.240 |
| Analyzed 25(OH)D3, mcg 2 | 67.9 | 63.4 | 63.6 |
| Treatment | BWG, g | FI, g | FCR |
|---|---|---|---|
| 0 to 7 d | |||
| Control 1 | 134.1 | 161.8 | 1.208 |
| 25(OH)D3 | 134.8 | 161.4 | 1.202 |
| CV, % | 4.51 | 3.55 | 6.28 |
| p value | 0.75 | 0.86 | 0.80 |
| 0 to 21 d | |||
| Control 1 | 975 | 1288 | 1.322 |
| 25(OH)D3 | 979 | 1284 | 1.312 |
| CV, % | 2.53 | 2.48 | 2.36 |
| p value | 0.63 | 0.80 | 0.47 |
| 0 to 35 d | |||
| Control 1 | 2395 | 3461 | 1.446 |
| 25(OH)D3 1–21 d 2 | 2384 | 3446 | 1.446 |
| 25(OH)D3 1–35 d 3 | 2390 | 3409 | 1.427 |
| CV, % | 3.65 | 3.28 | 1.62 |
| p value | 0.96 | 0.48 | 0.21 |
| 0 to 46 d | |||
| Control 1 | 3340 | 5365 | 1.606 |
| 25(OH)D3 1–21 d 2 | 3314 | 5300 | 1.602 |
| 25(OH)D3 1–35 d 3 | 3360 | 5283 | 1.574 |
| 25(OH)D3 1–46 d 4 | 3264 | 5186 | 1.590 |
| CV, % | 6.23 | 5.75 | 2.68 |
| p value | 0.79 | 0.66 | 0.45 |
| Regression | Ns | Ns | Ns |
| Characteristics | Control 1 | 25(OH)D3 ² | 25(OH)D3 ³ | 25(OH)D3 4 | CV, % | p Value | Regression |
|---|---|---|---|---|---|---|---|
| 1 to 21 Days | 1 to 35 Days | 1 to 46 Days | |||||
| Carcass, % | 80.6 a,b | 80.7 a,b | 80.2 b | 81.1 a | 2.8 | 0.04 | Ns |
| Breast (filet + sassami), % | 29.9 a,b | 29.7 b | 30.2 a,b | 30.5 a | 6.6 | 0.04 | Linear |
| Filet, % | 24.7 | 24.7 | 25.1 | 25.2 | 7.1 | 0.08 | Ns |
| Sassami, % | 5.2 | 5.1 | 5.1 | 5.2 | 10.2 | 0.07 | Ns |
| Legs, % | 31.6 | 31.7 | 31.1 | 31.3 | 6.2 | 0.09 | Ns |
| Wings, % | 9.9 | 9.7 | 9.6 | 9.6 | 10.3 | 0.17 | Ns |
| Back, % | 22.3 | 22.3 | 22.2 | 21.9 | 7.1 | 0.38 | Ns |
| Medallion, % | 1.9 | 1.9 | 1.9 | 1.9 | 14.7 | 0.54 | Ns |
| Skin, % | 2.3 | 2.3 | 2.3 | 2.3 | 16.8 | 0.76 | Ns |
| Breast flaps, % | 0.7 | 0.7 | 0.7 | 0.7 | 35.6 | 0.97 | Ns |
| Fat, % | 0.9 | 1.9 | 1.9 | 1.8 | 34.8 | 0.71 | Ns |
| Breast cartilage, % | 0.3 | 0.3 | 0.3 | 0.3 | 26.6 | 0.53 | Ns |
| Control 1 | 25(OH)D3 ² | 25(OH)D3 ³ | 25(OH)D3 4 | CV, % | p Value | Regression | |
|---|---|---|---|---|---|---|---|
| 1 to 21 d | 1 to 35 d | 1 to 46 d | |||||
| Resistance, kg | 43.8 | 48.3 | 47.6 | 47.0 | 19.0 | 0.09 | Ns |
| Weight, g | 33.0 | 33.4 | 32.7 | 32.5 | 12.4 | 0.74 | Ns |
| Diameter, mm | 15.8 | 16.0 | 15.7 | 16.0 | 9.8 | 0.85 | Ns |
| Length, mm | 121.7 | 119.7 | 122.3 | 120.8 | 4.7 | 0.09 | Ns |
| Seedor Index | 271.3 | 279.04 | 267.9 | 269.1 | 11.8 | 0.29 | Ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokoski, K.; Bittencourt, L.C.; Teixeira, L.V.; Bortoluzzi, C.; Vanroo, E.; Palma, S.; Fernandes, J.I.M. Classic and Non-Classic Effects of the Duration of Supplementation of 25-Hydroxicholecalciferol in Broiler Chicken Diets. Animals 2021, 11, 2971. https://doi.org/10.3390/ani11102971
Prokoski K, Bittencourt LC, Teixeira LV, Bortoluzzi C, Vanroo E, Palma S, Fernandes JIM. Classic and Non-Classic Effects of the Duration of Supplementation of 25-Hydroxicholecalciferol in Broiler Chicken Diets. Animals. 2021; 11(10):2971. https://doi.org/10.3390/ani11102971
Chicago/Turabian StyleProkoski, Karen, Leticia C. Bittencourt, Levy V. Teixeira, Cristiano Bortoluzzi, Elisangela Vanroo, Sabrina Palma, and Jovanir I. M. Fernandes. 2021. "Classic and Non-Classic Effects of the Duration of Supplementation of 25-Hydroxicholecalciferol in Broiler Chicken Diets" Animals 11, no. 10: 2971. https://doi.org/10.3390/ani11102971
APA StyleProkoski, K., Bittencourt, L. C., Teixeira, L. V., Bortoluzzi, C., Vanroo, E., Palma, S., & Fernandes, J. I. M. (2021). Classic and Non-Classic Effects of the Duration of Supplementation of 25-Hydroxicholecalciferol in Broiler Chicken Diets. Animals, 11(10), 2971. https://doi.org/10.3390/ani11102971





