Spatiotemporal Analysis of West Nile Virus Epidemic in South Banat District, Serbia, 2017–2019
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Sources of Information
2.2. Study Area
2.3. Mosquito Pooled Samples
2.4. Surveillance Targeting Dead Wild Birds
2.5. Blood Samples
2.6. WNV Genome Detection by Molecular Methods
2.7. Spatiotemporal Analysis
2.7.1. Kernel Density Estimation
2.7.2. Spatial Autocorrelation Analysis
2.7.3. Hot Spot Analysis
2.7.4. Space–Time Aggregation
3. Results
3.1. Epidemiological Characteristics of WNV Outbreaks in 2017, 2018, and 2019
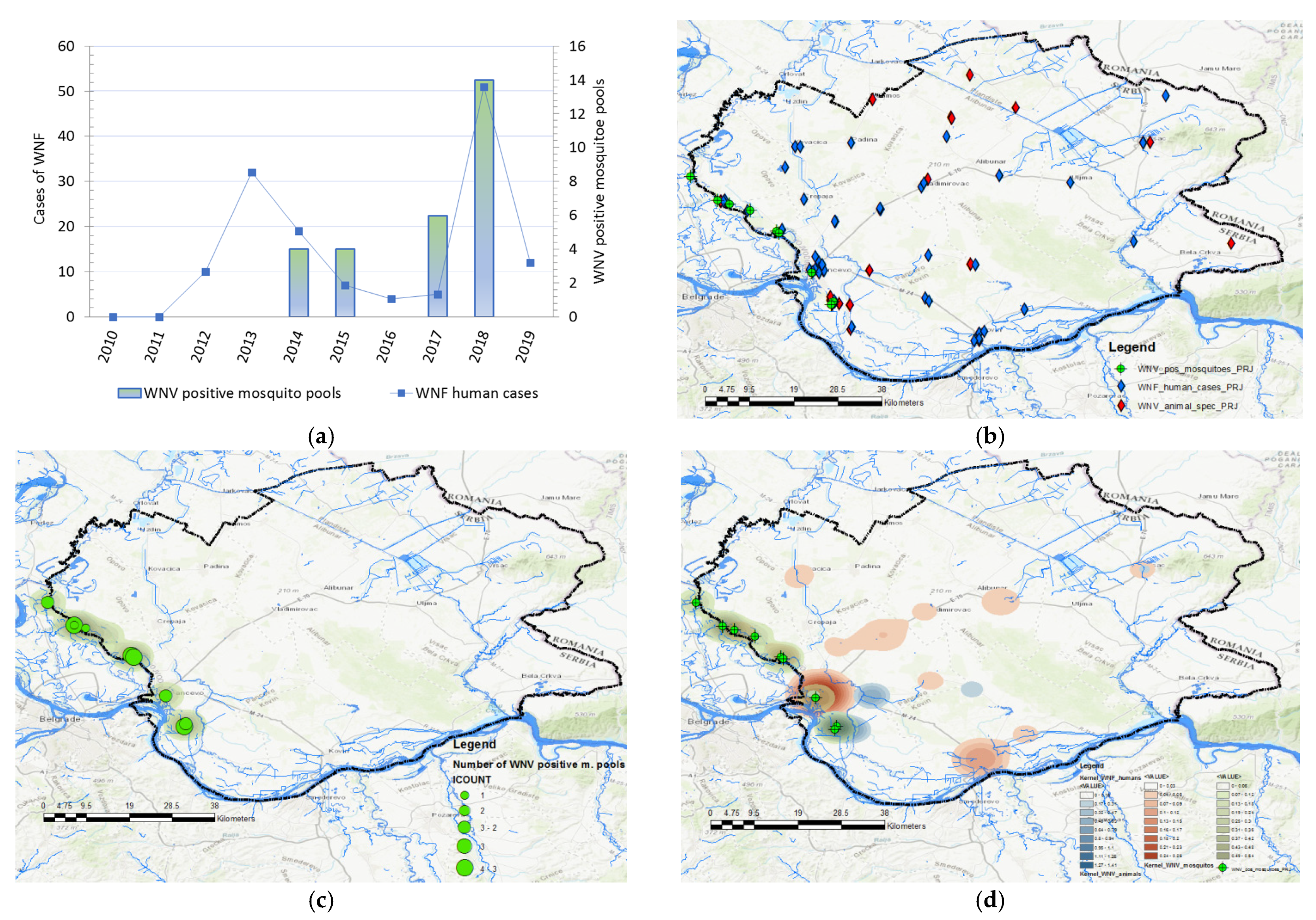
3.1.1. Descriptive Statistics
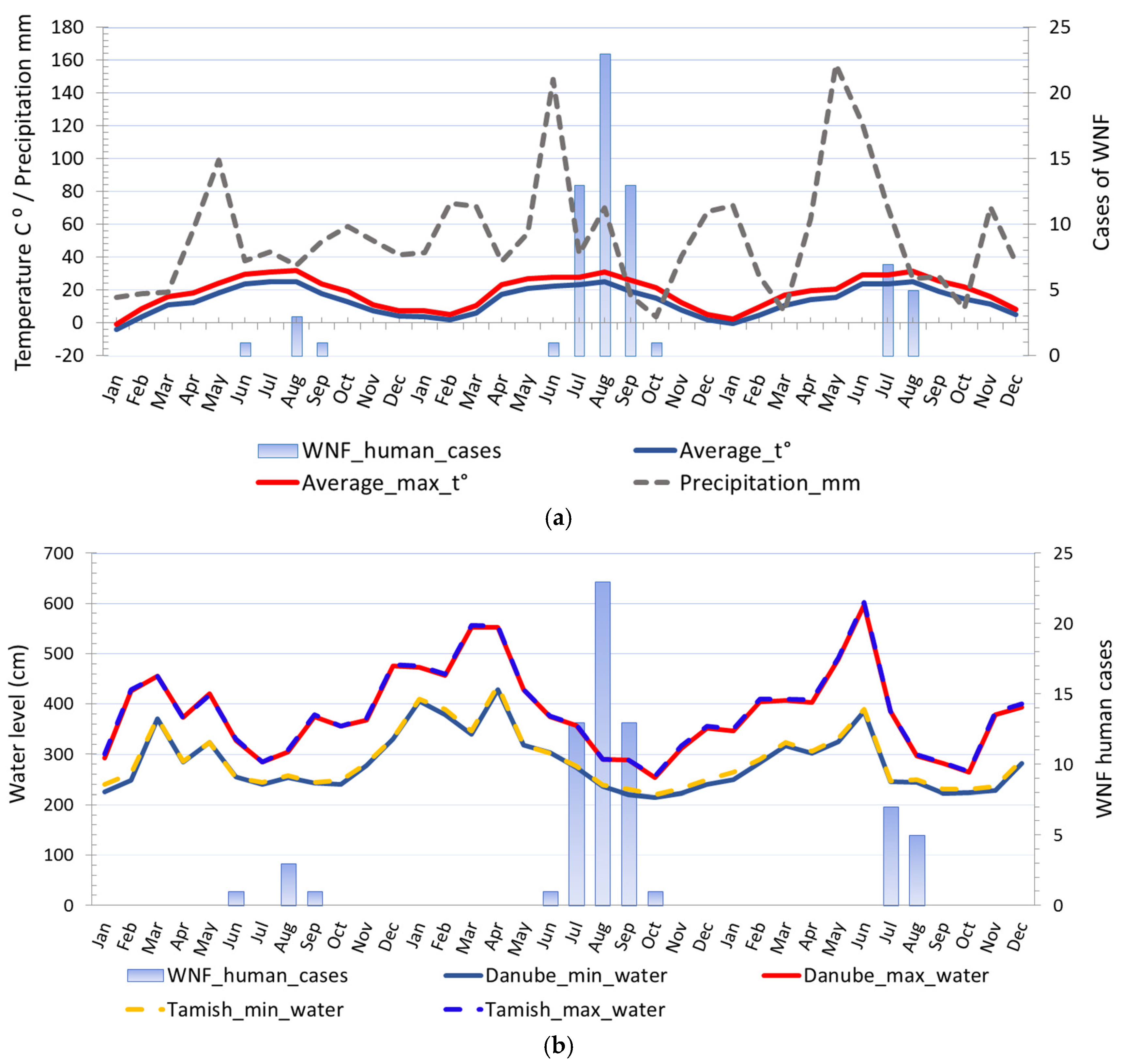
3.1.2. Seasonality
3.1.3. Geographical Distribution and Abundance of WNV-Positive Mosquitoes
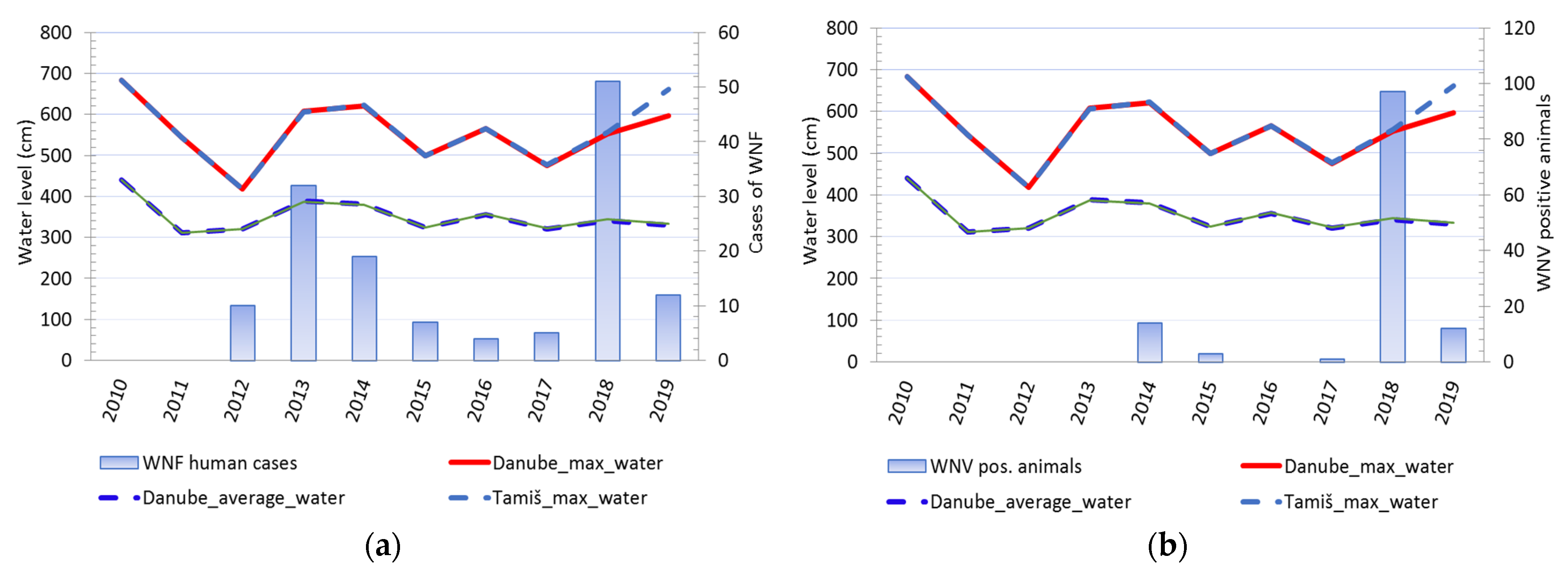
3.1.4. The Water Level of the Danube and Tamiš Rivers
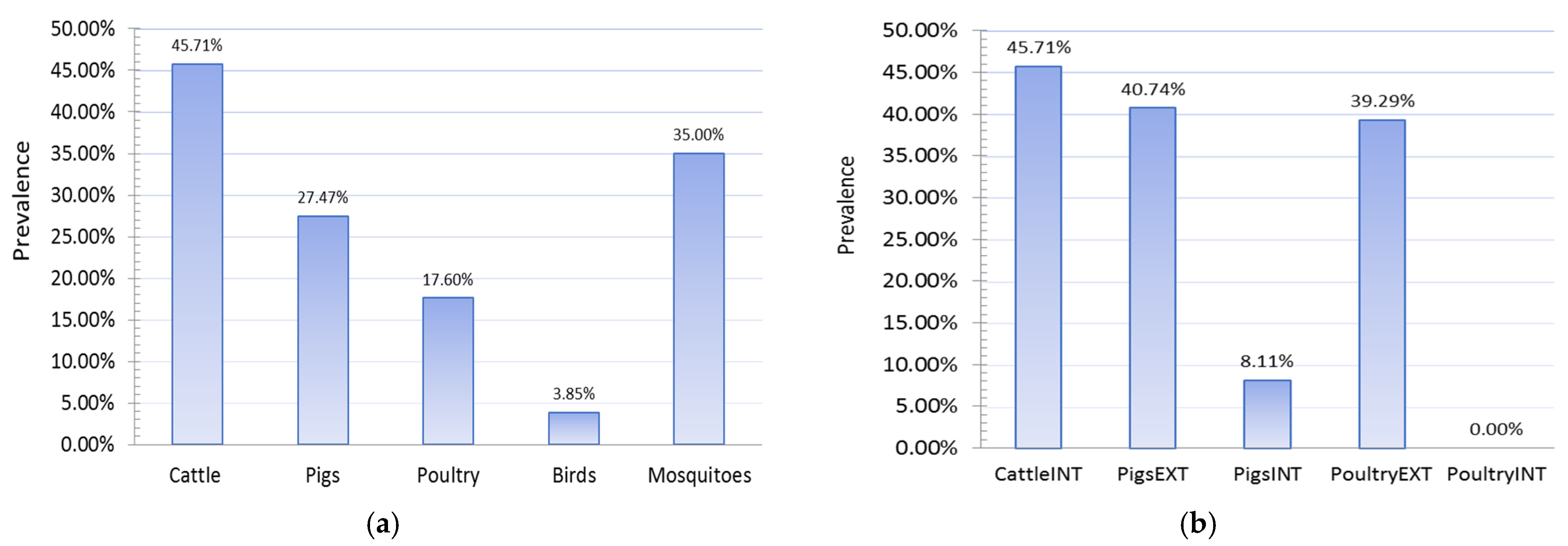
3.1.5. Results of Seroconversion Tests in Domestic and Wild Animals
3.2. Results of Spatiotemporal Analysis
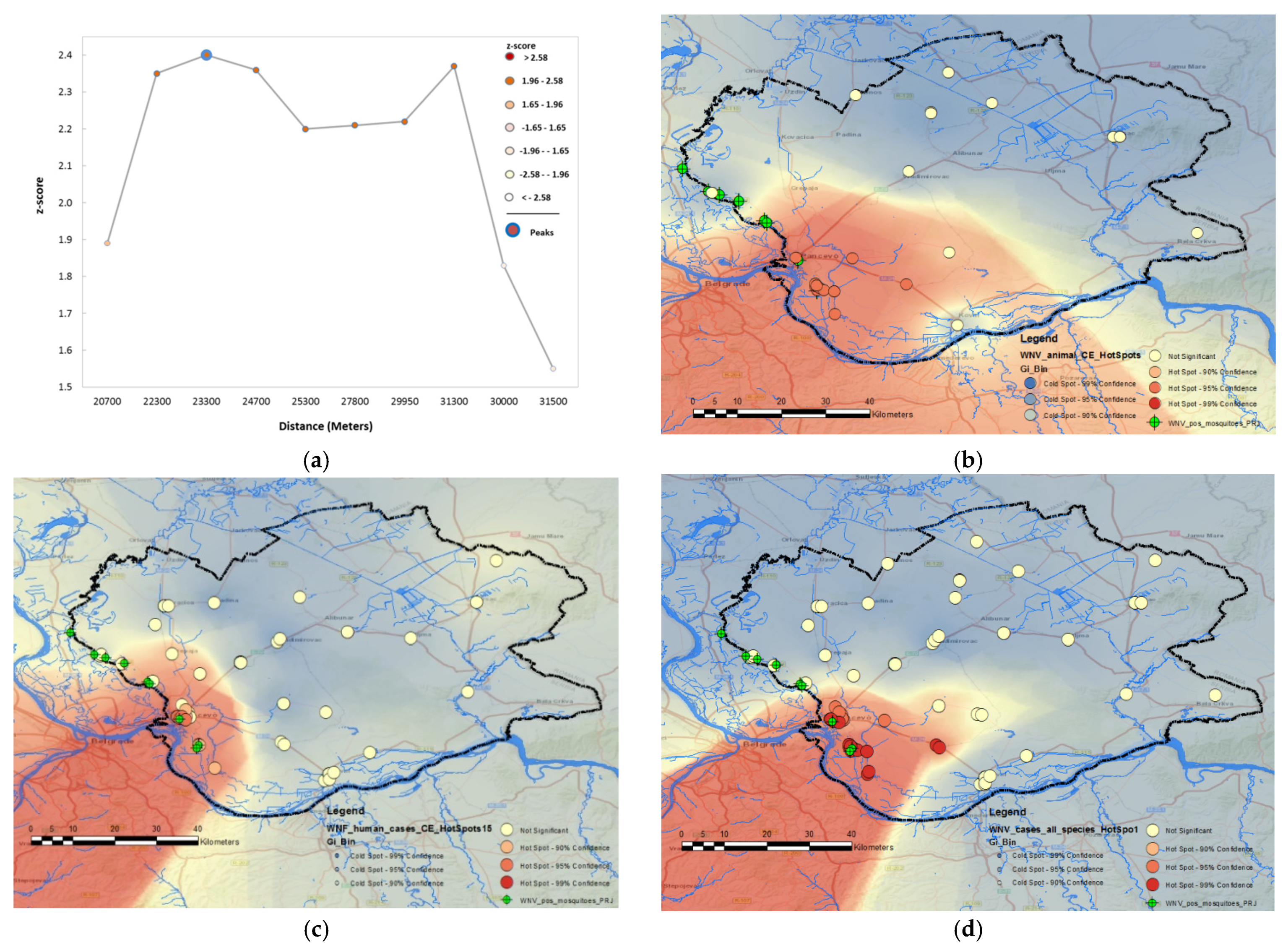
3.2.1. Cluster Analysis
3.2.2. Space–Time Aggregation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sfakianos, J.; Hecht, A. Deadly Diseases and Epidemics-West Nile, 2nd ed.; Chelsea House Publishers: New York, NY, USA, 2009; pp. 44–48. [Google Scholar]
- Troupin, A.; Colpitts, M.T. Overview of West Nile Virus Transmission and Epidemiology. In West Nile Virus Methods and Protocols; Colpitts, M.T., Ed.; Springer Science+Business Media: New York, NY, USA, 2016; pp. 15–18. [Google Scholar]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. Biomed Res. Int. 2015, 2015, 20. [Google Scholar] [CrossRef] [Green Version]
- Petersen, L.; Roehrig, J.; Sejvar, J. Global Epidemiology of West Nile Virus. In West Nile Encephalitis Virus Infection Viral Pathogenesis and the Host Immune Response; Diamond, S.M., Ed.; Springer Science+Business Media: New York, NY, USA, 2009; pp. 1–16. [Google Scholar]
- Medić, S.; Lazić, S.; Petrović, T.; Petrić, D.; Samojlović, M.; Lazić, G.; Lupulović, D. Evidence of the first clinical case of equine neuroinvasive West Nile disease in Serbia. Acta Vet.-Beogr. 2018, 69, 123–130. [Google Scholar] [CrossRef] [Green Version]
- OIE World Organisation for Animal Health. Available online: https://www.oie.int/en/animal-health-in-the-world/animal-diseases/West-Nile-fever/ (accessed on 21 February 2021).
- ECDC European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical (accessed on 18 May 2021).
- Petersen, L. West Nile Virus. In Emerging Infections 7; Scheld, W.M., Hooper, D.C., Hughes, J.M., Eds.; ASM Press American Society for Microbiology: Washington, DC, USA, 2007; pp. 99–119. [Google Scholar]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006, 4, e82. [Google Scholar] [CrossRef]
- Peterson, A.T.; Robbins, A.; Restifo, R.; Howell, J. Predictable ecology and geography of West Nile Virus transmission in the central United States. J. Vector. Ecol. 2008, 33, 342–352. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Hay, S.I.; Pigott, D.M.; Smith, D.L.; Wint, G.R.W.; Golding, N. Progress and Challenges in Infectious Disease Cartography. Trends Parasitol. 2016, 32, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Sudre, B.; Paz, S.; Rossi, M.; Desbrosse, A.; Chevalier, V.; Semenza, J.C. Environmental predictors of West Nile fever risk in Europe. Int. J. Health Geogr. 2014, 13, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, T.W.; Hockett, C.W.; Kightlinger, L.; Wimberly, M.C. Landscape-level spatial patterns of West Nile virus risk in the northern Great Plains. Am. J. Trop. Med. Hyg. 2012, 86, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, S.G.; Tullis, J.A.; Cothren, J. A remote sensing and GIS-assisted landscape epidemiology approach to West Nile virus. Appl. Geogr. 2013, 45, 241–249. [Google Scholar] [CrossRef]
- Rochlin, I.; Turbow, D.; Gomez, F.; Ninivaggi, D.V.; Campbell, S.R. Predictive Mapping of Human Risk for West Nile Virus (WNV) Based on Environmental and Socioeconomic Factors. PLoS ONE 2011, 6, e23280. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, C.; Ruiz, M.; McLafferty, S. Mosquito politics: Local vector control policies and the spread of West Nile Virus in the Chicago region. Health Place 2010, 16, 1188–1195. [Google Scholar] [CrossRef]
- Jones, R.C.; Weaver, K.N.; Smith, S.; Blanco, C.; Flores, C.; Gibbs, K.; Markowski, D.; Mutebi, J. Use of the vector index and geographic information system to prospectively inform West Nile virus interventions. J. Am. Mosq. Control Assoc. 2011, 27, 315–319. [Google Scholar] [CrossRef]
- De Groote, J.P.; Larson, S.R.; Zhang, Y.; Sugumaran, R. Application of geospatial technologies for understanding and predicting vector populations and vector-borne disease incidence. Geogr. Compass 2012, 6, 645–659. [Google Scholar] [CrossRef]
- Bousema, T.; Griffin, J.T.; Sauerwein, R.W.; Smith, D.L.; Churcher, T.S.; Takken, W.; Ghani, A.; Drakeley, C.; Gosling, R. Hitting Hotspots: Spatial Targeting of Malaria for Control and Elimination. PLoS Med. 2012, 9, e1001165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavod za Javno Zdravlje Pančevo. Available online: https://www.zjzpa.org.rs/nadzor-nad-groznicom-zapadnog-nila/ (accessed on 18 May 2021).
- Petrović, T.; Šekler, M.; Petrić, D.; Lazić, S.; Debeljak, Z.; Vidanović, D.; Ćupina, A.I.; Lazić, G.; Lupulović, D.; Kolarević, M.; et al. Methodology and results of integrated WNV surveillance programmes in Serbia. PLoS ONE 2018, 13, e0195439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GDI Gisdata, d.o.o. Available online: https://www.helloworld.rs/kompanija/GDI-Gisdata-d.o.o/242 (accessed on 15 May 2021).
- OpenStreetMap. Available online: https://www.openstreetmap.org (accessed on 15 May 2021).
- Copernicus Land Monitoring Service. Available online: https://land.copernicus.eu/pan-european/corine-land-cover (accessed on 15 May 2021).
- Republic Hydrometeorological Service of Serbia. Available online: http://www.hidmet.gov.rs/eng/hidrologija/index.php (accessed on 13 March 2021).
- South Banat District. Available online: https://juznobanatski.okrug.gov.rs/ (accessed on 11 November 2020).
- Statistical Office of the Republic of Serbia. Available online: https://data.stat.gov.rs/?caller=SDDB (accessed on 11 November 2020).
- Simić, D.; Puzović, S. Ptice Srbije i Područja od Međunarodnog Značaja; LOA—Liga za Ornitološku Akciju Srbije: Beograd, Srbija, 2008; pp. 38–39. [Google Scholar]
- Census of Agriculture 2012 Agriculture in the Republic of Serbia; Statistical Office of the Republic of Serbia: Belgrade, Serbia, 2012; ISBN 978-86-6161-078-3. Available online: www.popispoljoprivrede.stat.rs (accessed on 11 November 2020).
- Calzolari1, M.; Bonilauri, P.; Bellini, R.; Albieri, A.; Defilippo, F.; Maioli, G.; Galletti, G.; Gelati, A.; Barbieri, I.; Tamba, M.; et al. Evidence of Simultaneous Circulation of West Nile and Usutu Viruses in Mosquitoes Sampled in Emilia-Romagna Region (Italy) in 2009. PLoS ONE 2010, 5, e14324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKnight, K.P.; Messina, J.P.; Shortridge, A.M.; Burns, M.D.; Pigozzi, B.W. Using volunteered geographic information to assess the spatial distribution of West Nile Virus in Detroit, Michigan. IJAGR 2011, 2, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Shaw, I.G.R.; Robbins, P.F.; Jones, J.P. A bug’s life and the spatial ontologies of mosquito management. Ann. Assoc. Am. Geogr. 2010, 100, 373–392. [Google Scholar] [CrossRef]
- Prokopec, G.M.V.; Eng, J.L.V.; Kelly, R.; Mead, D.G.; Kolhe, P.; Howgate, J.; Kitron, U.; Burkot, T.R. The Risk of West Nile Virus Infection Is Associated with Combined Sewer Overflow Streams in Urban Atlanta, Georgia, USA. Environ. Health Persp. 2010, 118, 1382–1388. [Google Scholar] [CrossRef] [Green Version]
- ESRI. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/spatial-analyst/how-kernel-density-works.htm (accessed on 15 May 2021).
- Silverman, B.W. Monographs on Statistics and Applied Probability Density Estimation for Statistics and Data Analysis; Chapman and Hall: London, UK, 1986; pp. 1–22. [Google Scholar]
- Qin, J.; Yu, G.; Xia, T.; Li, Y.; Liang, X.; Wei, P.; Long, B.; Lei, M.; Wei, X.; Tang, X.; et al. Spatio-temporal variation of longevity clusters and the influence of social development level on lifespan in a Chinese Longevous area (1982–2010). Int. J. Environ. Res. Public Health 2017, 14, 812. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local Spatial Autocorrelation Statistics: Distributional Issues and an Application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Levine, N. CrimeStat III, Documentation, Part II: Spatial Description, Chapter 6—‘Hot Spot’ Analysis. Available online: http://www.icpsr.umich.edu/CrimeStat/download.html (accessed on 22 February 2021).
- ESRI. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/spatial-statistics/h-how-hot-spot-analysis-getis-ord-gi-spatial-stati.htm (accessed on 22 February 2021).
- Mitchell, A. The ESRI Guide to GIS Analysis: Spatial Measurements and Statistics; ESRI Press: New York, NY, USA, 2015; Volume 2, pp. 147–181. [Google Scholar]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Scott, L.; Warmerdam, N. Extend Crime Analysis with ArcGIS Spatial Statistics Tools. ArcUser 2015, 8, 1–4. Available online: https://www.esri.com/library/reprints/pdfs/arcuser_extend-crime-analysis.pdf (accessed on 15 May 2021).
- Mostashari, F.; Kulldorff, M.; Hartman, J.J.; Miller, J.R.; Kulasekera, V. Dead bird clusters as an early warning system for West Nile virus activity. Emerg. Infect. Dis. 2003, 9, 641–646. [Google Scholar] [CrossRef]
- Kulldorff, M.; Heffernan, R.; Hartman, J.; Assuncao, R.; Mostashari, F. A Space—Time Permutation Scan Statistic for Disease Outbreak Detection. PLoS Med. 2005, 2, e59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulldorff, M.; Huang, L.; Konty, K. A scan statistic for continuous data based on the normal probability model. Int. J. Health Geogr. 2009, 8, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulldorff, M. SaTScan User Guide for Version 9.0, Department of Ambulatory Care and Prevention; Harvard Medical School: Boston, MA, USA, July 2010; Available online: http://www.satscan.org/techdoc.html (accessed on 23 February 2021).
- Rochlin, I.; Ginsberg, H.S.; Campbell, S.R. Distribution and abundance of host-seeking Culex species at three proximate locations with different levels of West Nile virus activity. Am. J. Trop. Med. Hyg. 2009, 80, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M. A spatial scan statistic. Commun. Stat.-Theor. Methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Rozendaal, J.A. Relations between Anopheles darlingi breeding habitats, rainfall, river level and malaria transmission rates in the rain forest of Suriname. Med. Vet. Entomol. 1992, 6, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, E.; Gauthier-Clerc, M.; Bicout, D.; Sabatier, P. Bird Migration Routes and Risk for Pathogen Dispersion into Western Mediterranean Wetlands. Emerg. Infecti. Dis. 2007, 13, 365–372. [Google Scholar] [CrossRef]
- Davis, J.K.; Vincent, G.; Hildreth, M.B.; Kightlinger, L.; Carlson, C.; Wimberly, M.C. Integrating Environmental Monitoring and Mosquito Surveillance to Predict Vector-borne Disease: Prospective Forecasts of a West Nile Virus Outbreak. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Lockaby, G.; Noori, N.; Morse, W.; Zipperer, W.; Kalin, L.; Governo, R.; Sawant, R.; Ricker, M. Climatic, ecological, and socioeconomic factors associated with West Nile virus incidence in Atlanta, Georgia, U.S.A. J. Vector Ecol. 2016, 41, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Hartley, D.M.; Barker, C.M.; Le Menach, A.; Niu, T.; Gaff, H.D.; Reisen, W.K. Effects of Temperature on Emergence and Seasonality of West Nile Virus in California. Am. J. Trop. Med. Hyg. 2012, 86, 884–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, A.M.; Meola, M.A.; Moudy, R.M.; Kramer, L.D. Temperature, Viral Genetics, and the Transmission of West Nile Virus by Culex pipiens Mosquitoes. PLoS Pathog. 2008, 4, e1000092. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Gómez, A.; Amela, C.; Fernández-Carrión, E.; Martínez-Avilés, M.; Sánchez-Vizcaíno, J.M.; Sierra-Moros, M.J. Risk mapping of West Nile virus circulation in Spain. Acta Trop. 2015, 169, 163–169. [Google Scholar] [CrossRef]
- Kay, B.H.; Farrow, R.A. Mosquito (Diptera: Culicidae) dispersal: Implications for the epidemiology of Japanese and Murray Valley encephalitis viruses in Australia. J. Med. Entomol. 2000, 37, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Ji-Guang, M.; Hua, J.; Riley, J.R.; Reynolds, D.R.; Smith, A.D.; Ren-lai, W.; JI-YI, C.; Xia-Nian, C. Autumn southward “return” migration of the mosquito Culex tritaeniorhynchus in China. Med. Vet. Entomol. 1993, 7, 323–327. [Google Scholar] [CrossRef]
- Garrett-Jones, C. The possibility of active long-distance migrations by Anopheles pharoensis Theobald. Bull. World Health Organ. 1962, 27, 299–302. [Google Scholar]
- Asahina, S. Transoceanic flight of mosquitoes on the northwest Pacific. Jpn. J. Med. Sci. Biol. 1970, 23, 255–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horsfall, W.R. A migration of Aedes vexans Meigen. J. Econ. Entomol. 1954, 47, 544. [Google Scholar] [CrossRef]
- Chevillon, C.; Pasteur, N.; Marwuine, M.; Heyse, D.; Raymond, M. Population structure and dynamics of selected genes in the mosquito Culex pipiens. Evolution-US 1995, 49, 997–1007. [Google Scholar] [CrossRef]
- Verdonschot, P.F.; Besse-Lototskaya, A.A. Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica 2014, 45, 69–79. [Google Scholar] [CrossRef]

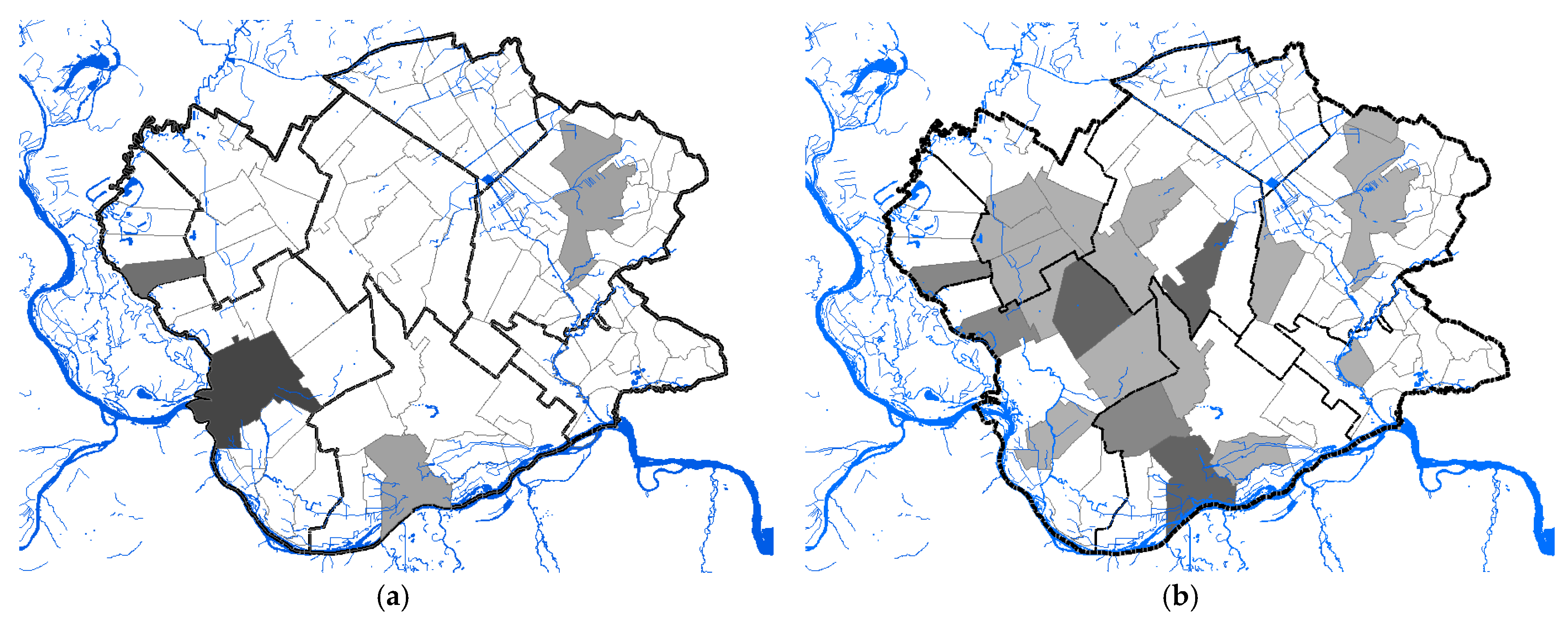
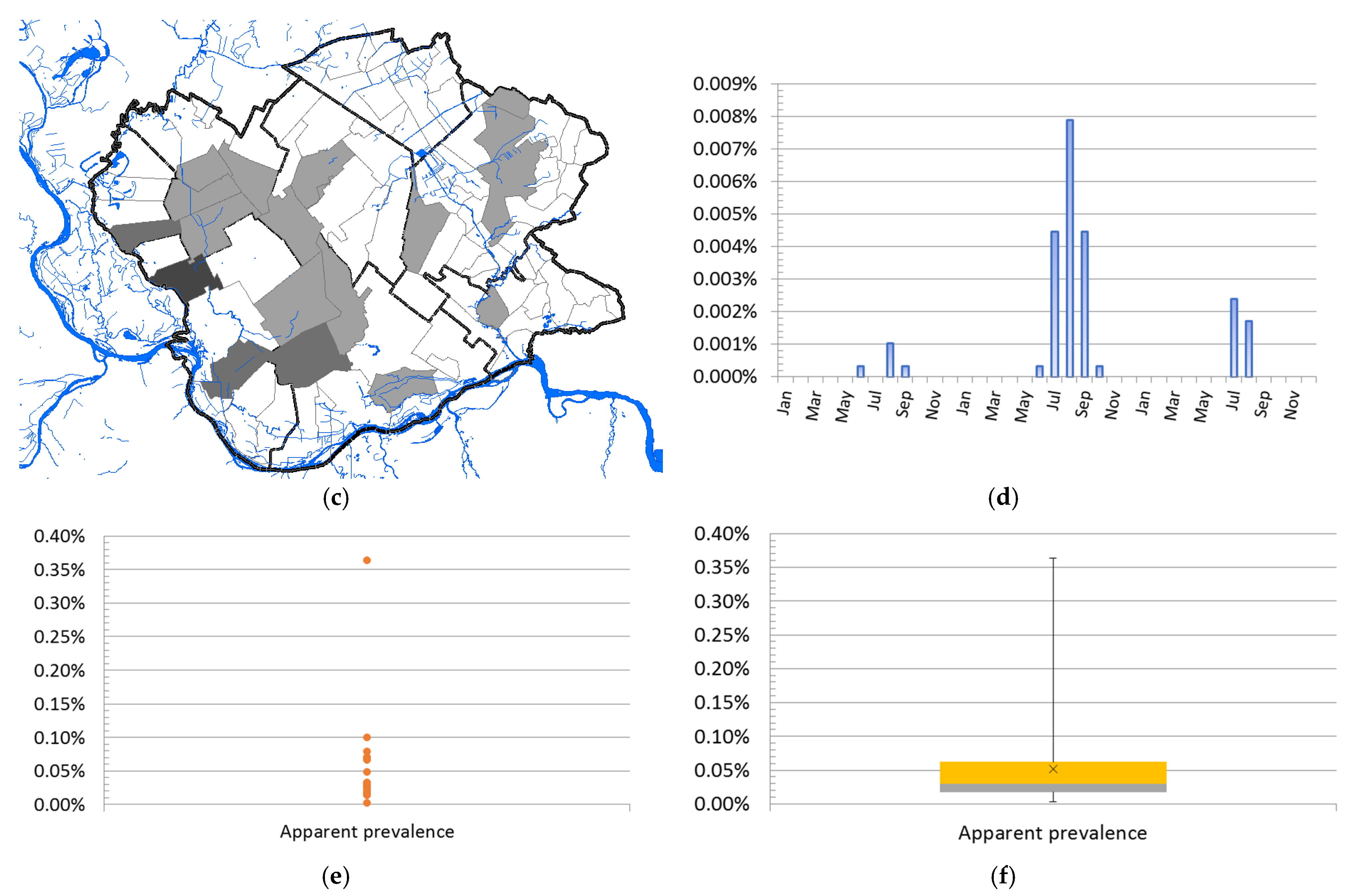
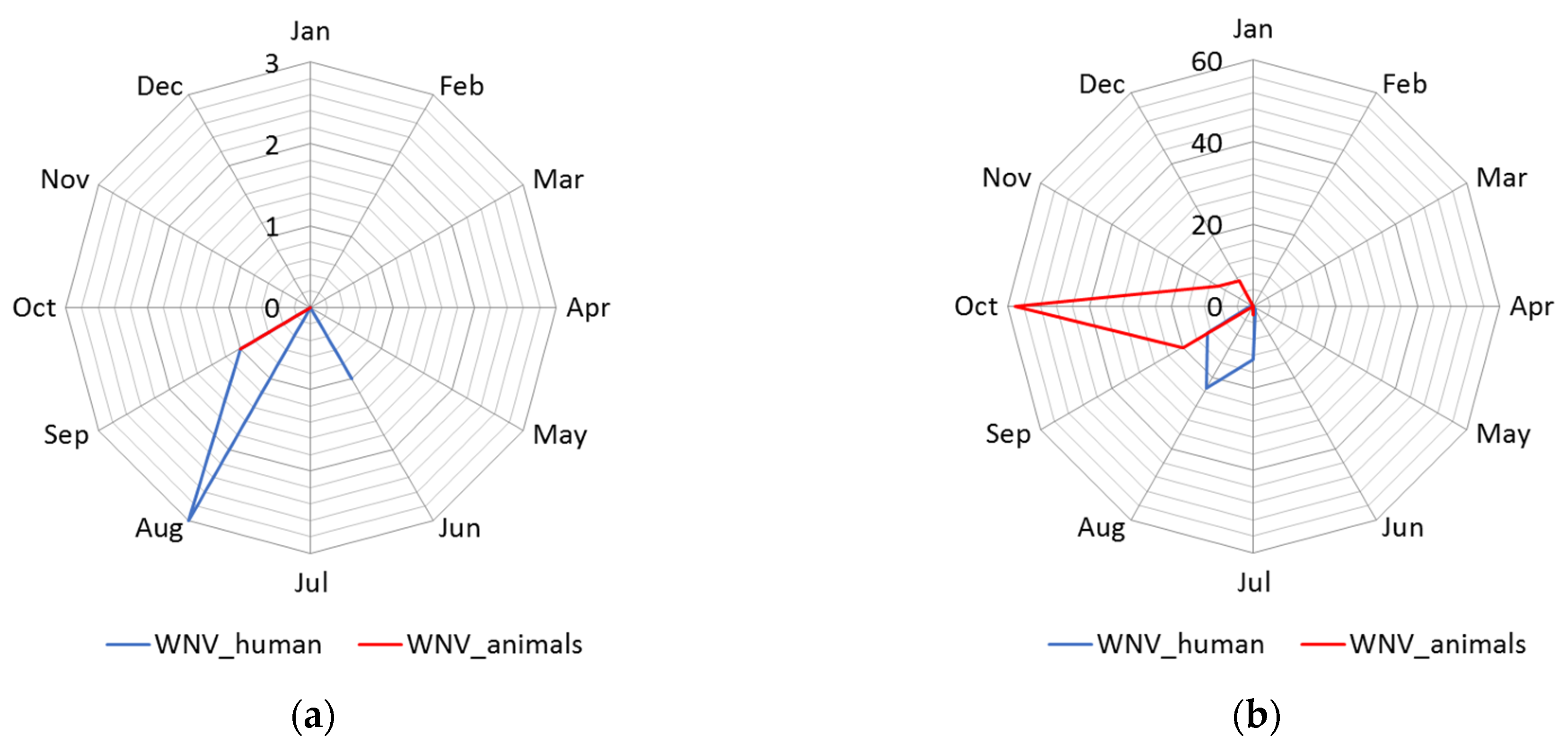
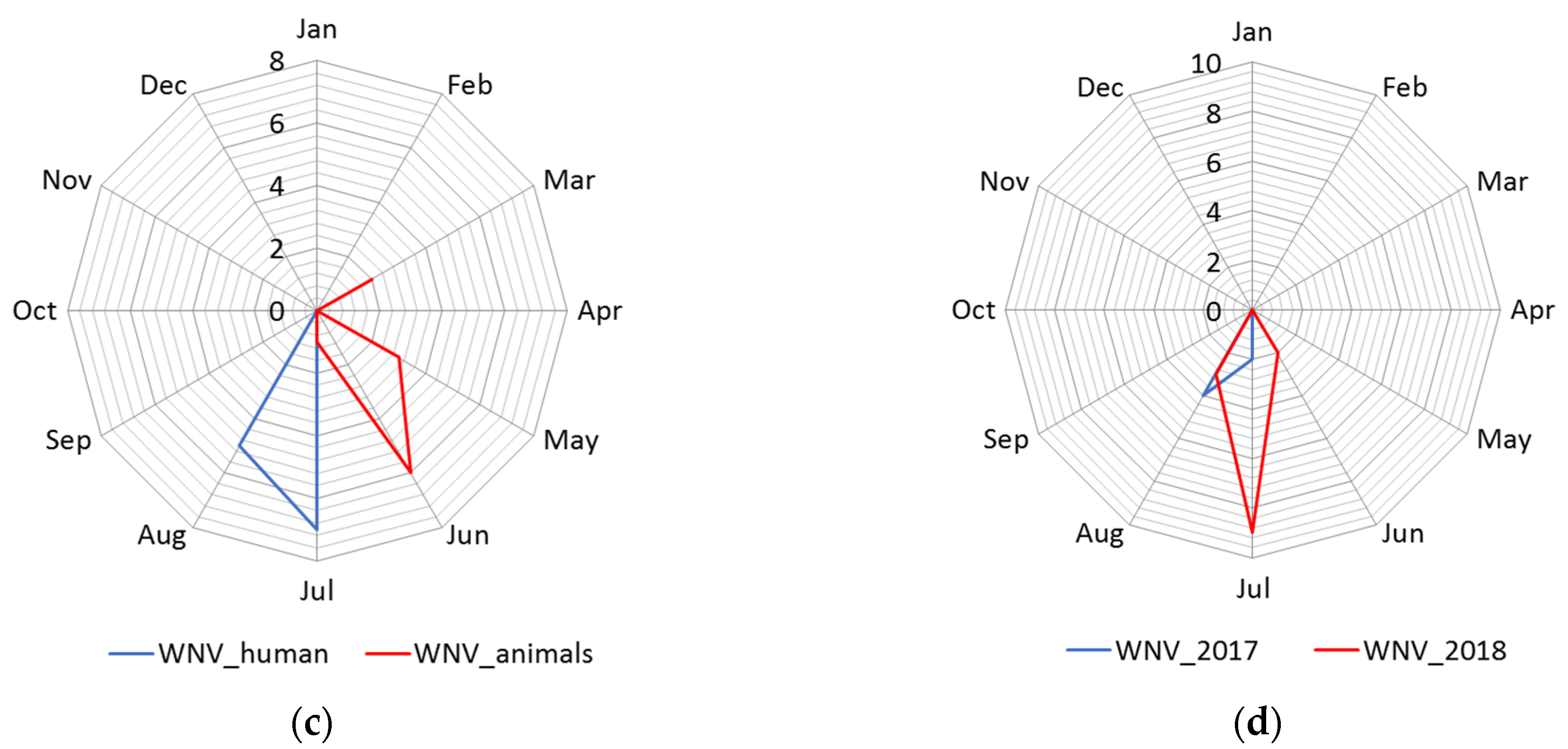
| Cluster | * Total Locations | Radius, km | Population | # WNV Cases | Expected Cases | O/E | RR | LLR | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 9.16 | 788 | 84 | 4.26 | 19.74 | 37.63 | 193.78 | <1 × 10−17 |
| 2 | 18 | 27.90 | 1907 | 39 | 10.30 | 3.78 | 4.60 | 25.92 | 2.1 × 10−10 |
| 3 | 5 | 12.67 | 29 | 7 | 0.16 | 44.28 | 46.12 | 19.83 | 5.7 × 10−8 |
| 4 | 4 | 12.82 | 777 | 17 | 4.20 | 4.05 | 4.38 | 11.47 | 0.00012 |
| 5 | 8 | 13.23 | 936 | 16 | 5.05 | 3.17 | 3.39 | 7.86 | 0.0035 |
| 6 | 3 | 15.93 | 38 | 3 | 0.20 | 14.73 | 14.97 | 5.30 | 0.037 |
| Cluster | * Total Locations | # WNV Cases | Expected Cases | O/E | RR | LLR | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | all | 76 | 2.78 | 27.35 | 48.21 | 197.04 | 0.001 |
| Cluster | * Total Locations | Radius, km | Start Date | End Date | Population | # WNV Cases | Expected Cases | O/E | RR | LLR | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 12.70 | 24 September 2018 | 15 October 2018 | 1023 | 48 | 0.07 | 677.35 | 939.16 | 272.35 | 1 × 10−17 |
| 2 | 3 | 5.11 | 5 September 2018 | 20 December 2018 | 680 | 39 | 2.85 | 13.70 | 17.42 | 70.09 | 1 × 10−17 |
| 3 | 6 | 15.59 | 17 August 2018 | 25 September 2018 | 1207 | 6 | 1.12 | 5.35 | 5.51 | 5.26 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radojicic, S.; Zivulj, A.; Petrovic, T.; Nisavic, J.; Milicevic, V.; Sipetic-Grujicic, S.; Misic, D.; Korzeniowska, M.; Stanojevic, S. Spatiotemporal Analysis of West Nile Virus Epidemic in South Banat District, Serbia, 2017–2019. Animals 2021, 11, 2951. https://doi.org/10.3390/ani11102951
Radojicic S, Zivulj A, Petrovic T, Nisavic J, Milicevic V, Sipetic-Grujicic S, Misic D, Korzeniowska M, Stanojevic S. Spatiotemporal Analysis of West Nile Virus Epidemic in South Banat District, Serbia, 2017–2019. Animals. 2021; 11(10):2951. https://doi.org/10.3390/ani11102951
Chicago/Turabian StyleRadojicic, Sonja, Aleksandar Zivulj, Tamas Petrovic, Jakov Nisavic, Vesna Milicevic, Sandra Sipetic-Grujicic, Dusan Misic, Malgorzata Korzeniowska, and Slavoljub Stanojevic. 2021. "Spatiotemporal Analysis of West Nile Virus Epidemic in South Banat District, Serbia, 2017–2019" Animals 11, no. 10: 2951. https://doi.org/10.3390/ani11102951
APA StyleRadojicic, S., Zivulj, A., Petrovic, T., Nisavic, J., Milicevic, V., Sipetic-Grujicic, S., Misic, D., Korzeniowska, M., & Stanojevic, S. (2021). Spatiotemporal Analysis of West Nile Virus Epidemic in South Banat District, Serbia, 2017–2019. Animals, 11(10), 2951. https://doi.org/10.3390/ani11102951







