Morphology of the Spleen in Oreochromis niloticus: Splenic Subregions and the Blood-Spleen Barrier
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Anatomy and Corrosion
2.3. Microscopic Observation
2.4. Ultrastructural Observation
2.5. Vital Staining
2.6. Statistical Analysis
3. Results
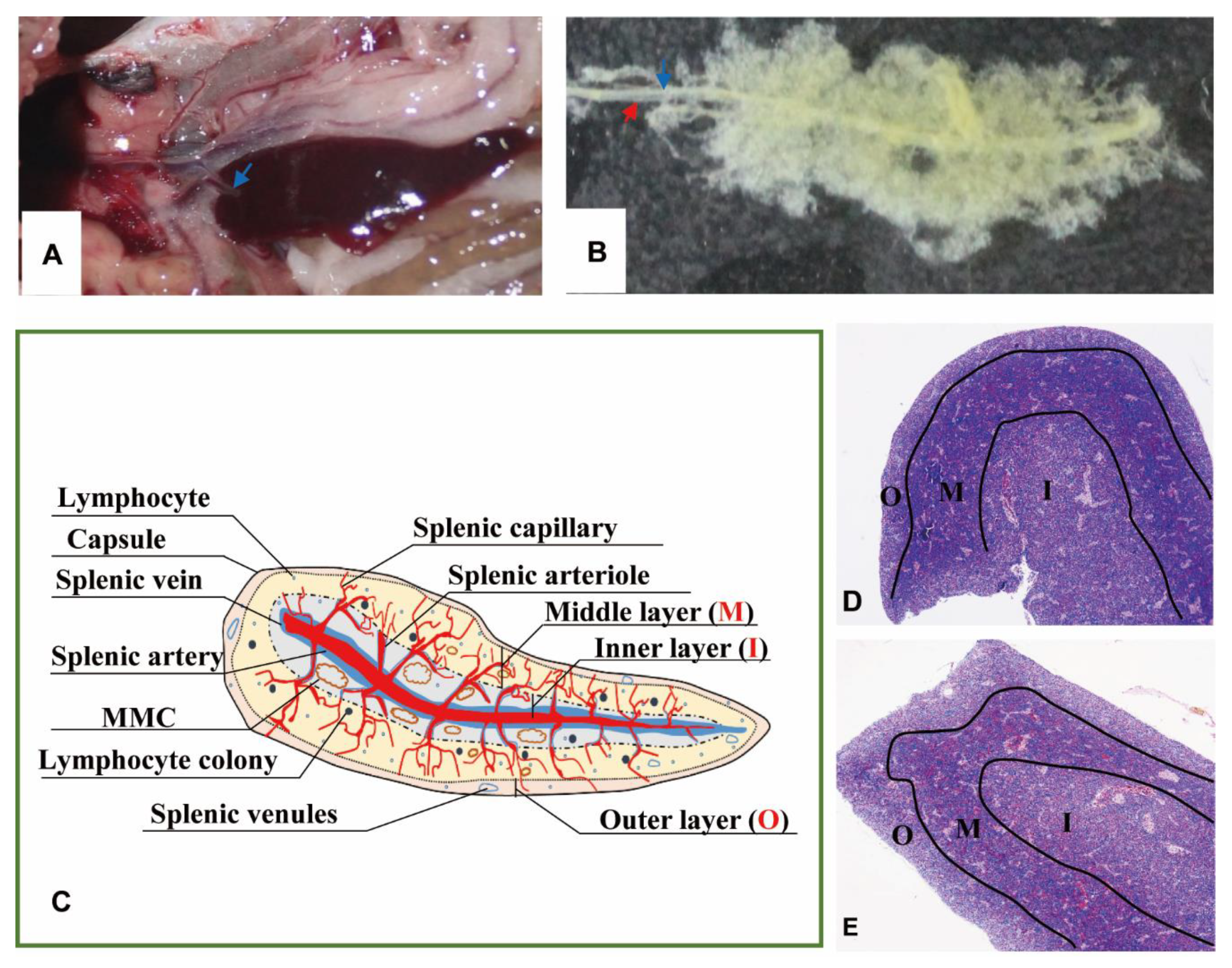
3.1. Macromorphology of Tilapia Spleen
3.2. Micromorphology of the Spleen
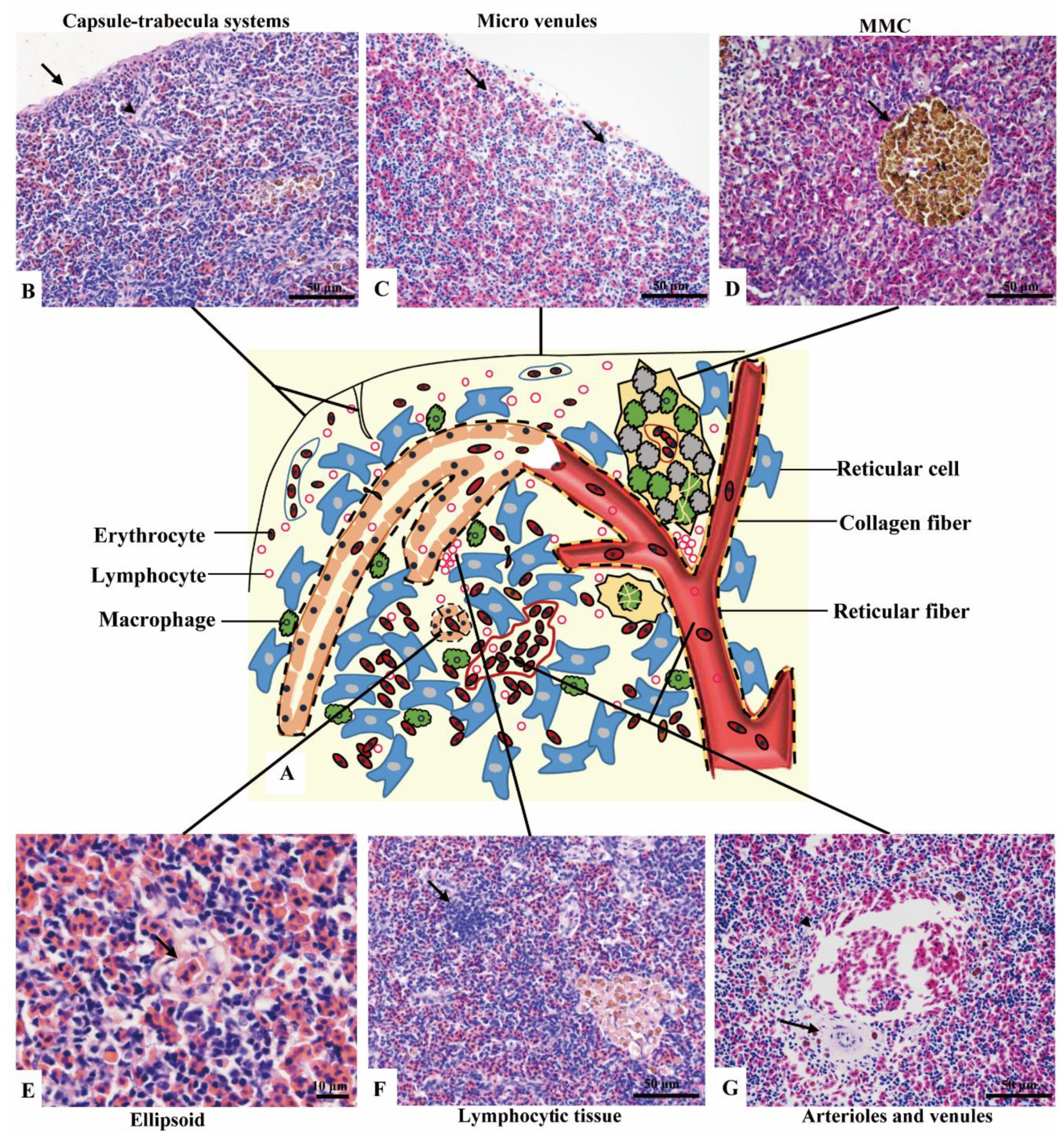
3.2.1. Subregions and Components Visualized by H&E Staining
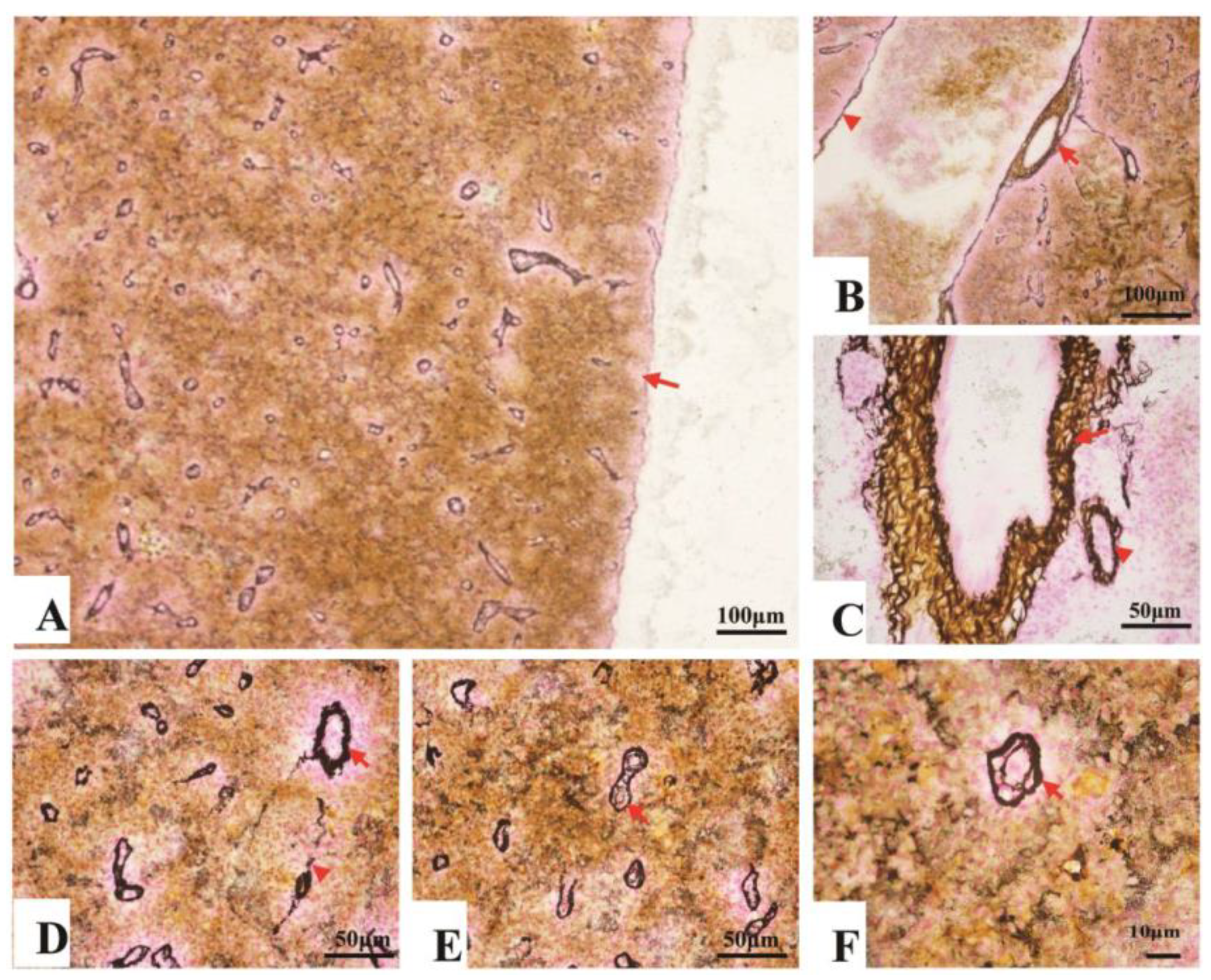
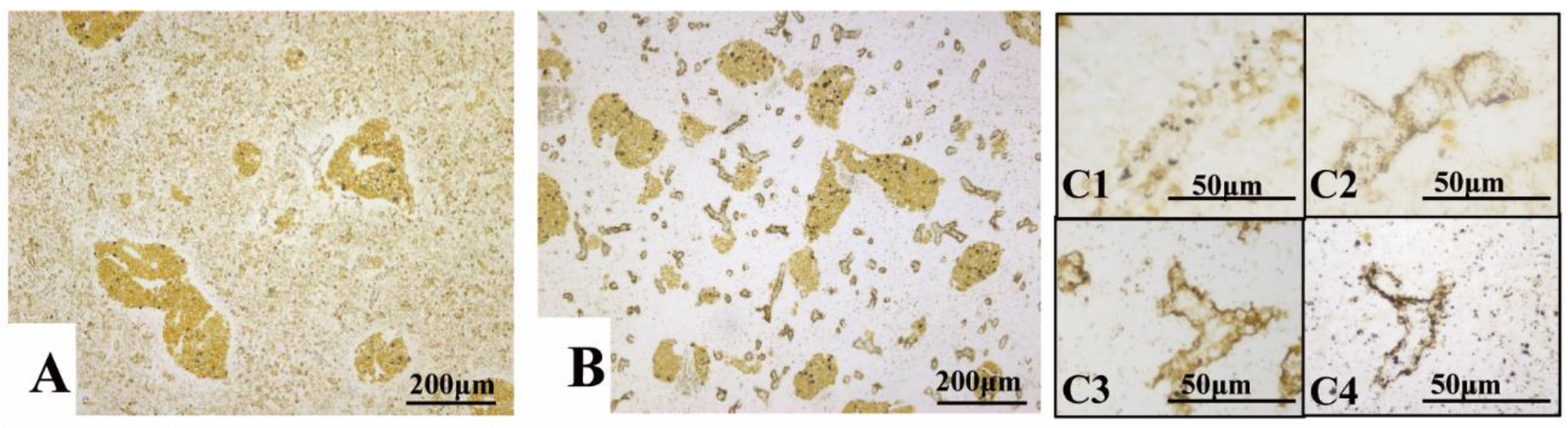
3.2.2. Fibrous Skeleton Visualized by Jame’s Staining
3.3. Ultramicroscopic Morphology of Spleen
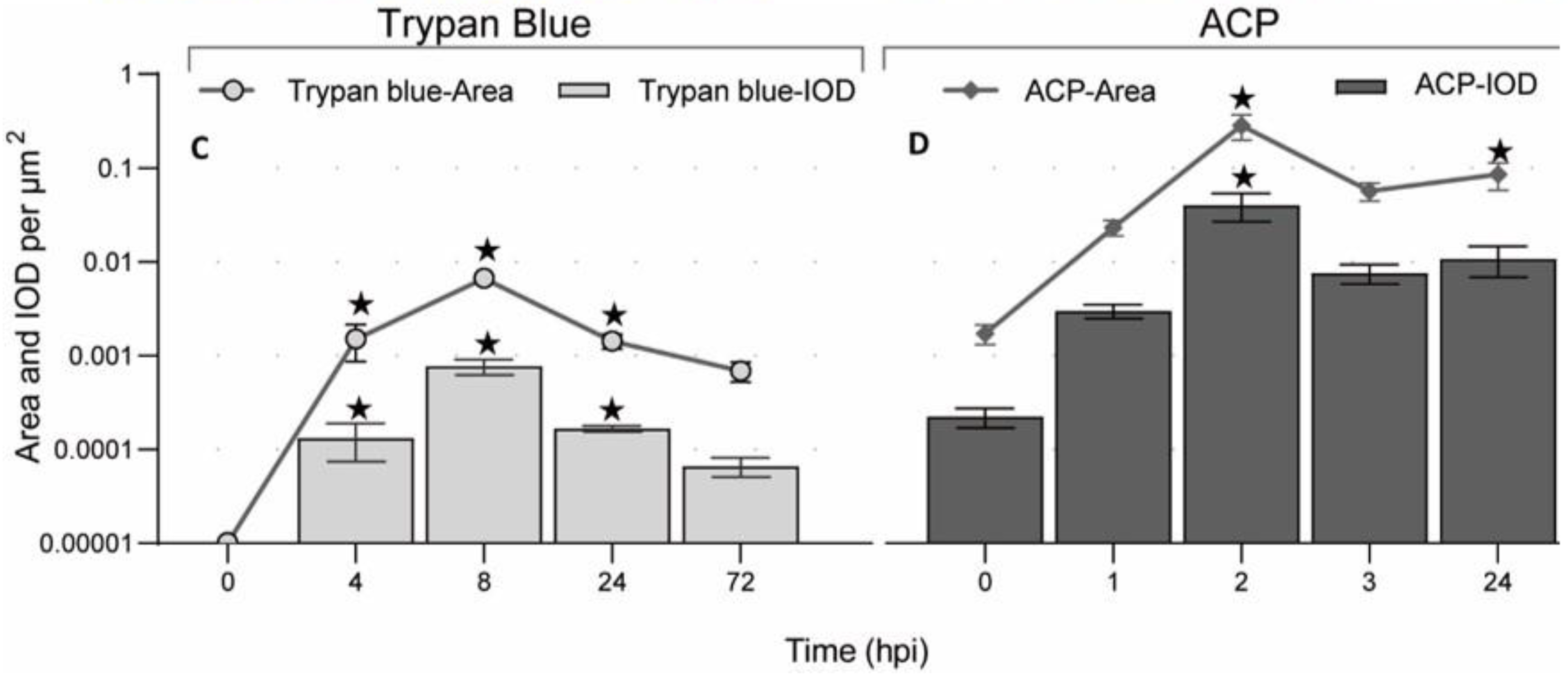
3.4. BSB Visualized by Vital Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fazio, F.; Saoca, C.; Costa, G.; Zumbo, A.; Piccione, G.; Parrino, V. Flow cytometry and automatic blood cell analysis in striped bass Morone saxatilis (Walbaum, 1792): A new hematological approach. Aquaculture 2019, 513, 734398. [Google Scholar] [CrossRef]
- He, Y.; Ding, Z.; Marana, M.H.; Dalsgaard, I.; Rzgar, J.; Heidi, M.; Asma, K.M.; Per, K.W.; Kurt, B. Immersion vaccines against Yersinia ruckeri infection in rainbow trout: Comparative effects of strain differences. J. Fish Dis. 2021. [Google Scholar] [CrossRef]
- Hoare, R.; Leigh, W.; Limakom, T.; Wongwaradechkul, R.; Metselaar, M.; Shinn, A.P.; Ngo, T.P.; Thompson, K.D.; Adams, A. Oral vaccination of Nile tilapia (Oreochromis niloticus) against francisellosis elevates specific antibody titres in serum and mucus. Fish Shellfish. Immunol. 2021, 113, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Ngu, R.; Royer, B.; Giovanella, L.; Bigorgne, C.; Simo, R.; Carroll, P.; Russ, G. A multicentre validation study for the EU-TIRADS using histological diagnosis as a gold standard. Clin. Endocrinol. 2019, 91, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, H. A text and atlas of normal tissues in teleosts and their responses in disease. Syst. Pathol. Fish 2006, 10–23. [Google Scholar] [CrossRef]
- Weiss, L.; Geduldig, U.; Weidanz, W. Mechanisms of splenic control of murine malaria: Reticular cell activation and the development of a blood-spleen barrier. Am. J. Anat. 1986, 176, 251–285. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Yang, S.; Sun, X.; Tarique, I.; Yang, P.; Chen, Q. Morphological characterization of postembryonic development of blood–spleen barrier in duck. Poult. Sci. 2020, 99, 3823–3830. [Google Scholar] [CrossRef] [PubMed]
- Del Portillo, H.A.; Ferrer, M.; Brugat, T.; Martin-Jaular, L.; Langhorne, J.; Lacerda, M.V. The role of the spleen in malaria. Cell. Microbiol. 2012, 14, 343–355. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Yang, P.; Zhang, L.; Liu, Y.; Ullah, S.; Wu, L.; Waqas, Y.; Le, Y.; Chen, W. Identification and structural composition of the blood–spleen barrier in chickens. Vet. J. 2015, 204, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, E.; Wang, T.; Zhang, Q.; Yang, P.; Ahmed, N.; Zhao, Q.; Chen, Q. The novel histological evidence of the blood-spleen barrier in duck (Anas platyrhynchos). Histol. Histopathol. 2018, 34, 33–45. [Google Scholar]
- Bao, H.J.; Li, M.Y.; Wang, J.; Qin, J.H.; Xu, C.S.; Hei, N.N.; Yang, P.; Gandahi, J.; Chen, Q.S. Architecture of the blood-spleen barrier in the soft-shelled turtle, Pelodiseus sinensis. Anat. Rec. 2009, 292, 1079–1087. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Abd-Elsalam, R.M.; Younis, N.A. Streptococcosis, Lactococcosis and Enterococcosis are potential threats facing cultured Nile tilapia (Oreochomis niloticus) production. Aquacult. Res. 2020, 51, 4183–4195. [Google Scholar] [CrossRef]
- Aich, N.; Paul, A.; Choudhury, T.G.; Saha, H. Tilapia Lake Virus (TiLV) disease: Current status of understanding. Aquac. Fish. 2021. [Google Scholar] [CrossRef]
- Itsaro, A.; Suanyuk, N.; Tantikitti, C. Multiplex PCR for simultaneous detection of Streptococcus agalactiae, Streptococcus iniae and Lactococcus garvieae: A case of S. agalactiae infection in cultured Nile tilapia (Oreochromis niloticus) and red tilapia (Oreochromis niloticus x Oreochromis mossambicus). Songklanakarin J. Sci. Technol. 2012, 34, 495–500. [Google Scholar]
- Fathi, M.; Dickson, C.; Dickson, M.; Leschen, W.; Baily, J.; Muir, F.; Ulrich, K.; Weidmann, M. Identification of Tilapia Lake Virus in Egypt in Nile tilapia affected by ‘summer mortality’syndrome. Aquaculture 2017, 473, 430–432. [Google Scholar] [CrossRef]
- Kitiyodom, S.; Trullàs, C.; Rodkhum, C.; Thompson, K.D.; Katagiri, T.; Temisak, S.; Namdee, K.; Yata, T.; Pirarat, N. Modulation of the mucosal immune response of red tilapia (Oreochromis sp.) against columnaris disease using a biomimetic-mucoadhesive nanovaccine. Fish Shellfish Immunol. 2021, 112, 81–91. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, K.-y.; Xiao, D.; Chen, D.-f.; Huang, L.; Liu, T.; Wang, J.; Geng, Y.; Wang, E.-l.; Yang, Q. A recombinant truncated surface immunogenic protein (tSip) plus adjuvant FIA confers active protection against Group B streptococcus infection in tilapia. Vaccine 2014, 32, 7025–7032. [Google Scholar] [CrossRef]
- Pierezan, F.; Yun, S.; Piewbang, C.; Surachetpong, W.; Soto, E. Pathogenesis and immune response of Nile tilapia (Oreochromis niloticus) exposed to Tilapia lake virus by intragastric route. Fish Shellfish Immunol. 2020, 107, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Iregui, C.; Comas, J.; Vásquez, G.; Verjan, N. Experimental early pathogenesis of Streptococcus agalactiae infection in red tilapia Oreochromis spp. J. Fish Dis. 2016, 39, 205–215. [Google Scholar] [CrossRef]
- Hancock, R.E.; Nijnik, A.; Philpott, D.J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 2012, 10, 243–254. [Google Scholar] [CrossRef]
- Parrino, V.; Cappello, T.; Costa, G.; Cannavà, C.; Sanfilippo, M.; Fazio, F.; Fasulo, S. Comparative study of haematology of two teleost fish (Mugil cephalus and Carassius auratus) from different environments and feeding habits. Eur. Zool. J. 2018, 85, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Acar, Ü.; Kesbiç, O.S.; Yılmaz, S.; İnanan, B.E.; Zemheri-Navruz, F.; Terzi, F.; Fazio, F.; Parrino, V. Effects of Essential Oil Derived from the Bitter Orange (Citrus aurantium) on Growth Performance, Histology and Gene Expression Levels in Common Carp Juveniles (Cyprinus carpio). Animals 2021, 11, 1431. [Google Scholar] [CrossRef]
- Michalczyk-Wetula, D.; Salwiński, A.; Popik, M.; Jakubowska, M.; Płonka, P.M. Splenic melanosis during normal murine C57BL/6 hair cycle and after chemotherapy. Acta Biochim. Pol. 2013, 60. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, J. Application and effect of modified James staining. J. Shanghai Jiaotong Univ. Med Sci. 2009, 29, 359–360. [Google Scholar]
- Polliack, A.; Lampen, N.; Clarkson, B.; De Harven, E.; Bentwich, Z.; Siegal, F.; Kunkel, H. Identification of human B and T lymphocytes by scanning electron microscopy. J. Exp. Med. 1973, 138, 607–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribe, C.; Folch, H.; Enríquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486. [Google Scholar] [CrossRef] [Green Version]
- Wiens, G.D.; Marancik, D.P.; Zwollo, P.; Kaattari, S.L. Reduction of rainbow trout spleen size by splenectomy does not alter resistance against bacterial cold water disease. Dev. Comp. Immunol. 2015, 49, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef]
- Furukawa, T.; Nakamura, O.; Suzuki, Y.; Atsuta, S.; Nakamura, H.; Watanabe, T. Entrapment and transport of foreign material in the spleen and kidney of Japanese conger Conger myriaster. Fish. Sci. 2002, 68, 1219–1225. [Google Scholar] [CrossRef]
- He, Y.; Dai, X.; Fan, W.; Wang, J.; Qin, C.; Chen, J.; Cao, X. Location of blood-spleen barrier of Pelteobagrus vachelli by Trypan blue staining. S. China Fish. Sci. 2021, 17, 44–50. [Google Scholar]
- Kendall, M.; Hawkins, W. Hepatic morphology and acid phosphatase localization in the channel catfish (Ictalurus punctatus). J. Fish. Board Can. 1975, 32, 1459–1464. [Google Scholar] [CrossRef]
- Vidhya, V.; Nair, C.R. Pyrethroid insecticide, Lambda-Cyhalothrin impact on tissue acid phosphatase (ACP) activity of the fish, Etroplus Suratensis. Pramana Res. J. 2019, 9, 1749–1756. [Google Scholar]
- Weiss, L. Barrier cells in the spleen. Immunol. Today 1991, 12, 24–29. [Google Scholar] [CrossRef]



| Analysis | A Standard Area (μm2) | Area of Fibers (μm2) | Percentage |
|---|---|---|---|
| Collagen fibers | 275,800 | 28,876.85 ± 7385.15 | 7.7–14.3% |
| Reticular fibers | 41,821.99 ± 6948.70 | 11.4–18.8% | |
| Reticular fibers in ellipsoids | 18,002.24 ± 3640.57 | 5.2–8.8% | |
| Reticular fibers in other areas | 23,819.75 ± 4204.20 | 5.7–9.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wang, E.; Wang, K.; Wang, J.; Fan, W.; Chen, D.; Yang, Q. Morphology of the Spleen in Oreochromis niloticus: Splenic Subregions and the Blood-Spleen Barrier. Animals 2021, 11, 2934. https://doi.org/10.3390/ani11102934
He Y, Wang E, Wang K, Wang J, Fan W, Chen D, Yang Q. Morphology of the Spleen in Oreochromis niloticus: Splenic Subregions and the Blood-Spleen Barrier. Animals. 2021; 11(10):2934. https://doi.org/10.3390/ani11102934
Chicago/Turabian StyleHe, Yang, Erlong Wang, Kaiyu Wang, Jun Wang, Wei Fan, Defang Chen, and Qian Yang. 2021. "Morphology of the Spleen in Oreochromis niloticus: Splenic Subregions and the Blood-Spleen Barrier" Animals 11, no. 10: 2934. https://doi.org/10.3390/ani11102934
APA StyleHe, Y., Wang, E., Wang, K., Wang, J., Fan, W., Chen, D., & Yang, Q. (2021). Morphology of the Spleen in Oreochromis niloticus: Splenic Subregions and the Blood-Spleen Barrier. Animals, 11(10), 2934. https://doi.org/10.3390/ani11102934







