Protein and Energy Requirements for Maintenance and Growth in Juvenile Meagre Argyrosomus regius (Asso, 1801) (Sciaenidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Growth
3.2. Energy and Protein Intake
3.3. Body Composition
3.4. Retention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatzifotis, S.; Panagiotidou, M.; Papaioannou, N.; Pavlidis, M.; Nengas, I.; Mylonas, C.C. Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 2010, 307, 65–70. [Google Scholar] [CrossRef]
- El-Shebly, A.A.; El-Kady, M.A.H.; Hussin, A.B.; Hossain, M.Y. Preliminary observations on the pond culture of meagre, Argyrosomus regius (Asso, 1801) (Sciaenidae) in Egypt. J. Fish. Aquat. Sci. 2007, 2, 345–352. [Google Scholar] [CrossRef]
- Estévez, A.; Treviño, L.; Kotzamanis, Y.; Karacostas, I.; Tort, L.; Gisbert, E. Effects of different levels of plant proteins on the ongrowing of meagre (Argyrosomus regius) juveniles at low temperatures. Aquac. Nutr. 2011, 17, e572–e582. [Google Scholar] [CrossRef]
- Velazco-Vargas, J.; Martínez-Llorens, S.; Cerda, M.J.; Tomás-Vidal, A. Evaluation of soybean meal as protein source for Argyrosomus regius (Asso, 1801) (Sciaenidae). Int. J. Fish. Aquac. 2013, 5, 35–44. [Google Scholar]
- Poli, B.M.; Parisi, G.; Zampacavallo, G.; Iurzan, F.; Mecatti, M.; Lupi, P.; Bonelli, A. Preliminary results on quality and quality changes in reared meagre (Argyrosomus regius): Body and fillet traits and freshness changes in refrigerated commercial-size fish. Aquac. Int. 2003, 11, 301–311. [Google Scholar] [CrossRef]
- Jiménez, M.T.; Pastor, E.; Grau, A.; Alconchel, J.I.; Sánchez, R.; Cárdenas, S. Revisión del cultivo de esciénidos en el mundo, con especial atención a la corvina Argyrosomus regius (Asso, 1801). Bol. Inst. Esp. Oceanogr. 2005, 21, 169–175. [Google Scholar]
- Martínez-Llorens, S.; Espert, J. Growth and nutrient efficiency of meagre (Argyrosomus regius, Asso 1801) fed extruded diets with different protein and lipid levels. Int. J. Fish. Aquac. 2011, 3, 195–203. [Google Scholar]
- Roo, J.; Hernández-Cruz, C.M.; Borrero, C.; Schuchardt, D.; Fernández-Palacios, H. Effect of larval density and feeding sequence on meagre (Argyrosomus regius; Asso, 1801) larval rearing. Aquaculture 2010, 302, 82–88. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Panagiotidou, M.; Divanach, P. Effect of protein and lipid dietary levels on the growth of juvenile meagre (Argyrosomus regius). Aquac. Int. 2012, 20, 91–98. [Google Scholar] [CrossRef]
- Alvarez-González, C.A.; Civera-Cerecedo, R.; Ortiz-Galindo, J.L.; Dumas, S.; Moreno-Legorreta, M.; Grayeb-Del Alamo, T. Effect of dietary protein level on growth and body composition of juvenile spotted sand bass, Paralabrax maculatofasciatus, fed practical diets. Aquaculture 2001, 194, 151–159. [Google Scholar] [CrossRef]
- Chong, A.S.C.; Ishak, S.D.; Osman, Z.; Hashim, R. Effect of dietary protein level on the reproductive performance of female swordtails Xiphophorus helleri (Poeciliidae). Aquaculture 2004, 234, 381–392. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M.; Kawanna, M. Effects of dietary protein and energy levels on spawning performance of Nile tilapia (Oreochromis niloticus) broodstock in a recycling system. Aquaculture 2008, 280, 179–184. [Google Scholar] [CrossRef]
- Lee, S.M.; Im, G.J.; Jong, Y.L. Effects of digestible protein and lipid levels in practical diets on growth, protein utilization and body composition of juvenile rockfish (Sebastes schlegeli). Aquaculture 2002, 211, 227–239. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Wang, L.L.; Shao, Q.; Xu, Z.; Xu, J. Dietary Protein Requirement of Juvenile Black Sea Bream, Sparus macrocephalus. J. World Aquac. Soc. 2010, 41, 151–164. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Lall, S.P.; Anderson, D.M. Dietary protein requirement of juvenile American eel (Anguilla rostrata) fed practical diets. Aquaculture 2000, 186, 145–155. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Seiliez, I. Protein and amino acid nutrition and metabolism in fish: Current knowledge and future needs. Aquac. Res. 2010, 41, 322–332. [Google Scholar] [CrossRef]
- Gunasekera, R.M.; De Silva, S.S.; Collins, R.A.; Gooley, G.; Ingram, B.A. Effect of dietary protein level on growth and food utilization in juvenile Murray cod Maccullochella peelii peelii (Mitchell). Aquac. Res. 2000, 31, 181–187. [Google Scholar] [CrossRef]
- Booth, M.A.; Allan, G.L.; Pirozzi, I. Estimation of digestible protein and energy requirements of yellowtail kingfish Seriola lalandi using a factorial approach. Aquaculture 2010, 307, 247–259. [Google Scholar] [CrossRef]
- Jauralde, I.; Martínez-Llorens, S.; Tomás, A.; Jover, M. Protein deposition and energy recovery in gilthead sea bream (Sparus aurata): Evaluation of nutritional requirements. Aquaculture 2016. [Google Scholar] [CrossRef]
- Lupatsch, I.; Kissil, G.W.; Sklan, D.; Pfeffer, E. Energy and protein requirements for maintenance and growth in gilthead seabream (Sparus aurata L.). Aquac. Nutr. 1998, 4, 165–173. [Google Scholar] [CrossRef]
- Lupatsch, I.; Kissil, G.W.; Sklan, D. Comparison of energy and protein efficiency among three fish species gilthead sea bream (Sparus aurata), European sea bass (Dicentrarchus labrax) and white grouper (Epinephelus aeneus): Energy expenditure for protein and lipid deposition. Aquaculture 2003, 225, 175–189. [Google Scholar] [CrossRef]
- Lupatsch, I.; Kissil, G.W.; Sklan, D.; Pfeffer, E. Effects of varying dietary protein and energy supply on growth, body composition and protein utilization in gilthead seabream (Sparus aurata L.). Aquac. Nutr. 2001, 7, 71–80. [Google Scholar] [CrossRef]
- Peres, H.; Oliva-Teles, A. Protein and energy metabolism of European seabass (Dicentrarchus labrax) juveniles and estimation of maintenance requirements. Fish Physiol. Biochem. 2005, 31, 23–31. [Google Scholar] [CrossRef]
- Lupatsch, I.; Kissil, G.W. Feed formulations based on energy and protein demands in white grouper (Epinephelus aeneus). Aquaculture 2005, 248, 83–95. [Google Scholar] [CrossRef]
- Pirozzi, I.; Booth, M.A.; Allan, G.L. Protein and energy utilization and the requirements for maintenance in juvenile mulloway (Argyrosomus japonicus). Fish Physiol. Biochem. 2010, 36, 109–121. [Google Scholar] [CrossRef] [PubMed]
- McGoogan, B.B.; Gatlin, D.M. Metabolic requirements of red drum, Sciaenops ocellatus, for protein and energy based on weight gain and body composition. J. Nutr. 1998, 128, 123–129. [Google Scholar] [CrossRef][Green Version]
- Glencross, B.D. Reduced water oxygen levels affect maximal feed intake, but not protein or energy utilization efficiency of rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Glencross, B.; Hawkins, W.; Evans, D.; Rutherford, N.; Dods, K.; McCafferty, P.; Sipsas, S. Evaluation of the influence of drying process on the nutritional value of lupin protein concentrates when fed to rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 265, 218–229. [Google Scholar] [CrossRef]
- Rodehutscord, M.; Pfeffer, E. Maintenance requirement for digestible energy and efficiency of utilisation of digestible energy for retention in rainbow trout, Oncorhynchus mykiss. Aquaculture 1999, 179, 95–107. [Google Scholar] [CrossRef]
- Booth, M.A.; Allan, G.L. Utilization of digestible nitrogen and energy from four agricultural ingredients by juvenile silver perch Bidyanus bidyanus. Aquac. Nutr. 2003, 9, 317–326. [Google Scholar] [CrossRef]
- Hatlen, B.; Helland, S.J.; Grisdale-Helland, B. Energy and nitrogen partitioning in 250 g Atlantic cod (Gadus morhua L.) given graded levels of feed with different protein and lipid content. Aquaculture 2007, 270, 167–177. [Google Scholar] [CrossRef]
- Glencross, B.D. A factorial growth and feed utilization model for barrmundi, Lates calcarifer based on Austratian production conditions. Aquac. Nutr. 2008, 14, 360–373. [Google Scholar] [CrossRef]
- Helland, S.J.; Hatlen, B.; Grisdale-Helland, B. Energy, protein and amino acid requirements for maintenance and efficiency of utilization for growth of Atlantic salmon post-smolts determined using increasing ration levels. Aquaculture 2010, 305, 150–158. [Google Scholar] [CrossRef]
- Fournier, V.; Gouillou-Coustans, M.F.; Métailler, R.; Vachot, C.; Guedes, M.J.; Tulli, F.; Oliva-Teles, A.; Tibaldit, E.; Kaushik, S.J. Protein and arginine requirements for maintenance and nitrogen gain in four teleosts. Br. J. Nutr. 2002, 87, 459–469. [Google Scholar] [CrossRef]
- Bureau, D.P.; Hua, K.; Cho, C.Y. Effect of feeding level on growth and nutrient deposition in rainbow trout (Oncorhynchus mykiss Walbaum) growing from 150 to 600 g. Aquac. Res. 2006. [Google Scholar] [CrossRef]
- Atkinson, J.L.; Hilton, J.W.; Slinger, S.J. Evaluation of acid-insoluble ash as an indicator of feed digestibility in rainbow trout (Salmo gairdneri). Can. J. Fish. Aquat. Sci. 1984. [Google Scholar] [CrossRef]
- Snedecor, G.; Cochran, W. Statistical Methods, 8th ed.; Iowa State University Press: Iowa, IA, USA, 1989; ISBN 0813815614. [Google Scholar]
- Ministerio de la Presidencia. Real Decreto 53/2013, de 1 de febrero, por el que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docenci; BOE. 8 de febrero de 2013, Num. 34, sección I; BOE: Madrid, Spain, 2013; pp. 11370–11421. [Google Scholar]
- Watanabe, K.; Ura, K.; Yada, T.; Kiron, V.; Satoh, S.; Watanabe, T. Energy and protein requirements of yellowtail for maximum growth and maintenance of body weight. Fish. Sci. 2000, 66, 1053–1061. [Google Scholar] [CrossRef][Green Version]
- Dumas, A.; France, J.; Bureau, D.P. Evidence of three growth stanzas in rainbow trout (Oncorhynchus mykiss) across life stages and adaptation of the thermal-unit growth coefficient. Aquaculture 2007, 267, 139–146. [Google Scholar] [CrossRef]
- Jauralde, I.; Martínez-Llorens, S.; Tomás, A.; Ballestrazzi, R.; Jover, M. A proposal for modelling the thermal-unit growth coefficient and feed conversion ratio as functions of feeding rate for gilthead sea bream (Sparus aurata, L.) in summer conditions. Aquac. Res. 2013, 44, 242–253. [Google Scholar] [CrossRef]
- Mayer, P.; Estruch, V.D.; Jover, M. A two-stage growth model for gilthead sea bream (Sparus aurata) based on the thermal growth coefficient. Aquaculture 2012, 358–359, 6–13. [Google Scholar] [CrossRef]
- Panettieri, V.; Chatzifotis, S.; Messina, C.M.; Olivotto, I.; Manuguerra, S.; Randazzo, B.; Ariano, A.; Bovera, F.; Santulli, A.; Severino, L.; et al. Honey bee pollen in meagre (Argyrosomus regius) juvenile diets: Effects on growth, diet digestibility, intestinal traits, and biochemical markers related to health and stress. Animals 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Knibb, W. Genetic improvement of marine fish—Which method for industry? Aquac. Res. 2000, 31, 11–23. [Google Scholar] [CrossRef]
- Watanabe, K.; Hara, Y.; Ura, K.; Yada, T.; Kiron, V.; Satoh, S.; Watanabe, T. Energy and protein requirements for maximum growth and maintenance of bodyweight of yellowtail. Fish. Sci. 2000, 66, 884–893. [Google Scholar] [CrossRef]
- Lupatsch, I.; Kissil, G.W.; Sklan, D. Optimization of feeding regimes for European sea bass Dicentrarchus labrax: A factorial approach. Aquaculture 2001, 202, 289–302. [Google Scholar] [CrossRef]
- Arshad Hossain, M.; Almatar, S.M.; James, C.M. Optimum dietary protein level for juvenile silver pomfret, Pampus argenteus (Euphrasen). J. World Aquac. Soc. 2010. [Google Scholar] [CrossRef]
- Sandberg, F.B.; Emmans, G.C.; Kyriazakis, I. Partitioning of limiting protein and energy in the growing pig: Description of the problem, possible rules and their qualitative evaluation. Br. J. Nutr. 2005, 93, 213–224. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Lozano, N.B.; Martínez-Llorens, S.; Tomás-Vidal, A.; Cerdá, M.J. Effect of high-level fish meal replacement by pea and rice concentrate protein on growth, nutrient utilization and fillet quality in gilthead seabream (Sparus aurata, L.). Aquaculture 2009, 298, 83–89. [Google Scholar] [CrossRef]
- Sánchez-Lozano, N.B.; Martínez-Llorens, S.; Tomás-Vidal, A.; Jover Cerdá, M. Amino acid retention of gilthead sea bream (Sparus aurata, L.) fed with pea protein concentrate. Aquac. Nutr. 2011, 17, e604–e614. [Google Scholar] [CrossRef]
- Azevedo, P.A.; Cho, C.Y.; Leeson, S.; Bureau, D.P. Effects of feeding level and water temperature on growth, nutrient and energy utilization and waste outputs of rainbow trout (Oncorhynchus mykiss). In Proceedings of the Aquatic Living Resources; 1998. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0990744098890050 (accessed on 4 January 2021).
- Hillestad, M.; Johnsen, F. High-energy/low-protein diets for Atlantic salmon: Effects on growth, nutrient retention and slaughter quality. Aquaculture 1994, 124, 109–116. [Google Scholar] [CrossRef]
- Shearer, K.D. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 1994, 119, 63–88. [Google Scholar] [CrossRef]
| Parameters | Trial A | Trial B |
|---|---|---|
| Initial weight | 53 g | 188 g |
| Number of fish | 169 | 77 |
| Experimental system | 12 cages of 1000 liters in 4 tanks of 4000 L, recirculating saltwater system | 8 cages of 1500 liters in 4 tanks of 4000 L, recirculating saltwater system |
| Feeding rate | 0, 0.75, 1.5, 2.5, 3.5, and 4.5% | 0, 0.5, 1.5, and 2.5% |
| Feeding | 1–2 times daily (hand feeding) | 1–2 times daily (hand feeding) |
| Replicates | 2 | 2 |
| Duration | 53 days | 53 days |
| Commercial Diet | |
|---|---|
| Dry matter (%) | 93 |
| Crude protein (%) | 46.06 |
| Crude lipid (%) | 19.46 |
| Ash (%) | 6.35 |
| Crude fibre (%) | 1.5 |
| NFE 1 (%) | 26.63 |
| GE 2 (MJ kg−1) | 24.26 |
| CP/GE (g MJ−1) | 18.98 |
| CDMs 3(%) | 64.6 |
| CDP 4(%) | 84.85 |
| CDE 5 (%) | 84.94 |
| DP 6 (g kg−1) | 39.08 |
| DE 7 (MJ kg−1) | 20.60 |
| Trial A | Trial B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feeding Rate (%) | Feeding Rate (%) | |||||||||||||
| 0 | 0.75 | 1.5 | 2.5 | 3.5 | 4.5 | SEM | 0 | 0.5 | 1.5 | 2.5 | SEM | |||
| Performance Indices | ||||||||||||||
| IW 1 | 55 | 52 | 52 | 53 | 54 | 54 | 2 | 140 a | 209 b | 209 b | 197 b | 9 | ||
| FW 2 | 38 a | 57 b | 70 bc | 77 bc | 88 c | 81 c | 4 | 111 a | 248 b | 295 c | 339 c | 7.92 | ||
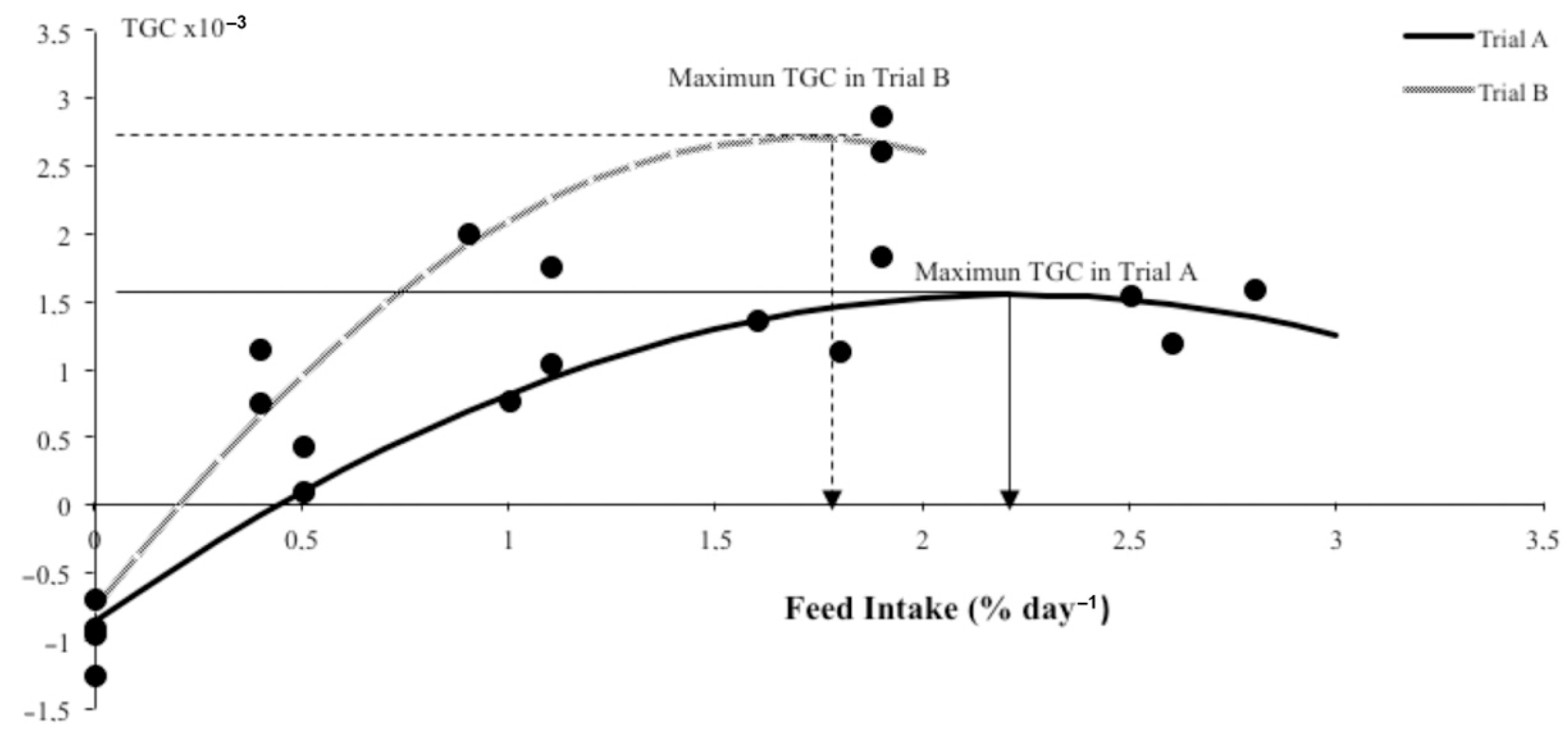
| TGC 3 × 10−3 | −0.93 a | 0.27 b | 0.91 c | 1.25 c | 1.69 c | 1.4 c | 0.15 | −0.98 a | 0.95 b | 1.88 c | 2.74 d | 0.15 | ||
| FI 4 | 0 a | 0.50 b | 1.05 c | 1.72 d | 2.22 e | 2.75 f | 0.10 | 0 a | 0.38 b | 1.03 c | 1.88 d | 0.06 | ||
| DPI 5 | 0 a | 1.65 b | 3.53 c | 5.72 d | 7.25 e | 8.79 f | 0.40 | 0 a | 1.59 b | 4.46 c | 8.07 c | 0.24 | ||
| PR 6 | −0.61 a | 0.68 b | 1.26 c | 1.62 cd | 1.81 d | 1.61 cd | 0.11 | −0.80 a | 1.08 b | 1.89 b | 2.33 b | 0.19 | ||
| DEI 7 | 0 a | 91.5 b | 193.9 c | 311.5 d | 393.0 e | 481.7 f | 22.1 | 0 a | 78.4 b | 217.1 c | 395.8 d | 10.0 | ||
| ER 8 | −18.9 a | 34.8 b | 62.8 c | 77.9 cd | 90.8 d | 81.2 cd | 5.0 | −29.9 a | 44.3 b | 92.1 c | 119.9 c | 10.4 | ||
| Carcass Composition | ||||||||||||||
| Initial | Initial | |||||||||||||
| Moisture | 80.06 | 82.83 a | 77.50 b | 75.52 c | 75.23 c | 74.06 c | 74.35 c | 0.40 | 79.35 | 83.54 a | 76.10 b | 74.29 b | 71.59 b | 1.09 |
| Protein | 11.91 | 11.76 a | 14.82 b | 15.77 c | 16.02 d | 16.30 e | 16.10 de | 0.05 | 13.24 | 11.76 a | 15.57 b | 16.24 b | 16.36 b | 0.57 |
| Lipid | 1.95 | 1.96 a | 4.21 b | 5.46 c | 5.80 c | 6.60 c | 6.24 c | 0.34 | 1.96 | 0.76 a | 3.89 b | 6.42 b | 7.69 b | 0.76 |
| Ash | 4.34 | 4.63 a | 3.38 b | 3.29 b | 3.09 b | 3.33 b | 3.29 b | 0.22 | 4.68 | 4.39 a | 4.03 b | 3.13 b | 3.88 b | 0.22 |
| GE 9 | 3.62 | 3.59 a | 5.22 b | 5.94 c | 6.13 c | 6.52 c | 6.33 c | 0.14 | 3.94 | 3.11 a | 5.27 b | 6.43 b | 6.97 b | 0.40 |
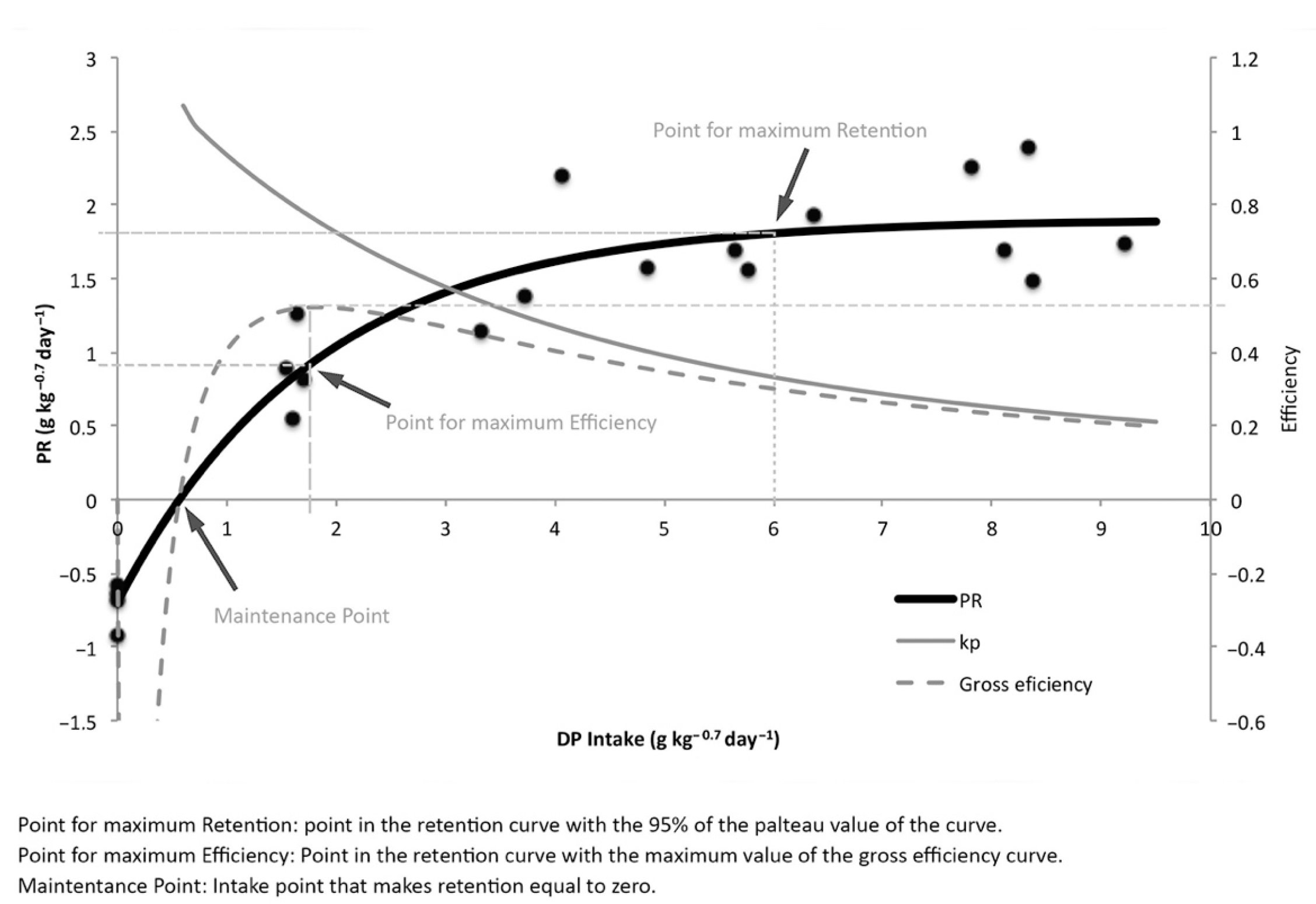
| Protein Retention Key Points | ||||
| DPI | PR | Gross Efficiency | Net Efficiency | |
| Maintenance | 0.57 | 0 | 0 | - |
| Max. Retention | 6.0 | 1.81 | 0.30 | 0.33 |
| Max. Efficiency | 1.8 | 0.94 | 0.52 | 0.76 |
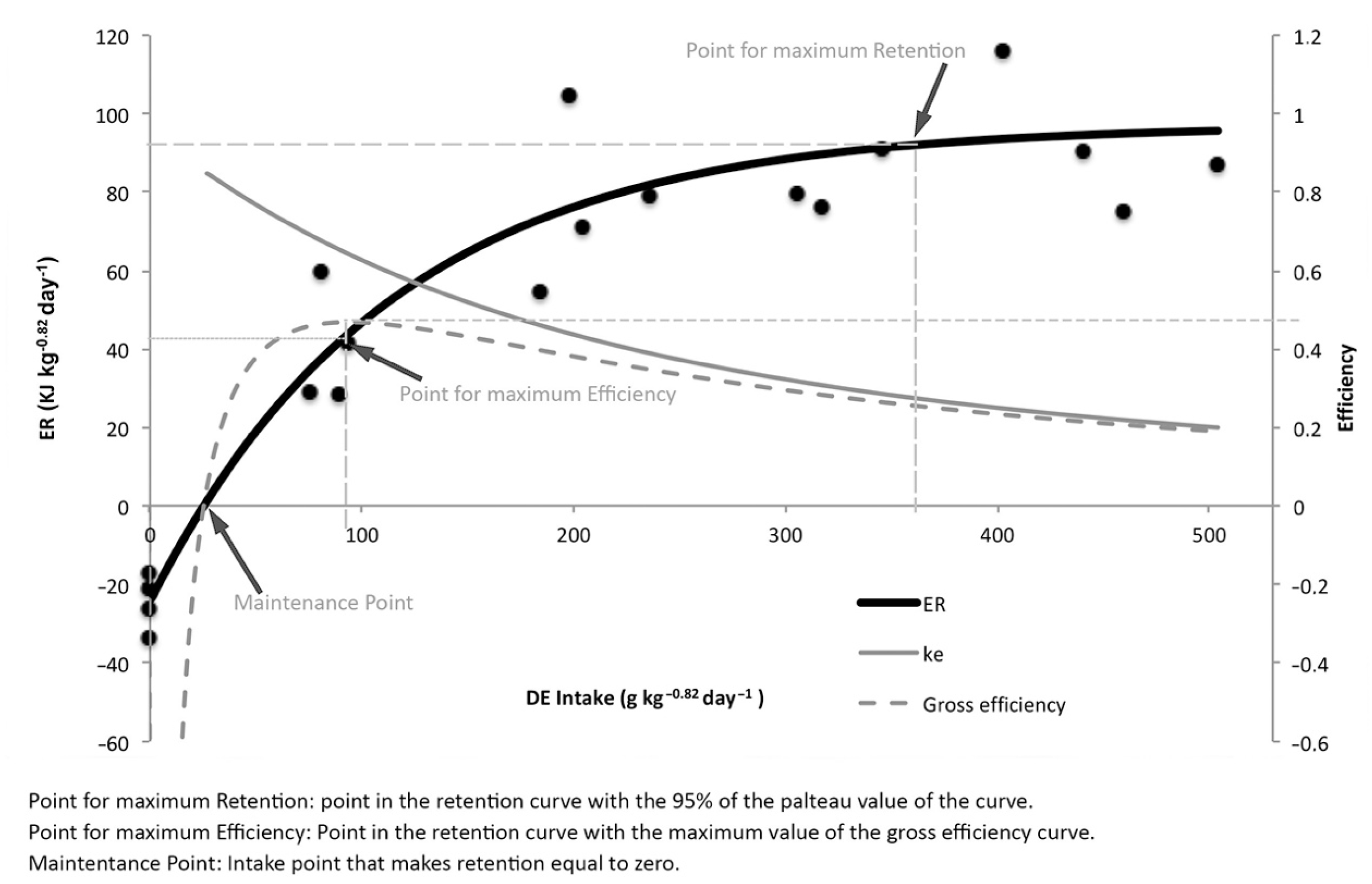
| Energy Retention Key Points | ||||
| DEI | ER | Gross Efficiency | Net Efficiency | |
| Maintenance | 25.4 | 0 | 0 | - |
| Max. Retention | 365 | 92.2 | 0.25 | 0.27 |
| Max. Efficiency | 93 | 43.5 | 0.47 | 0.64 |
| Species | Maintenance Protein Requirements | Maintenance Energy Requirements | Ta (°C) | Study |
|---|---|---|---|---|
| Meagre (Argyrosomus regius) | 0.57 g DP kg−0.7 fish−1 day−1 | 25.4 kJ DE kg−0.82 fish−1 day−1 | 19 | Present study |
| Gilthead sea bream (Sparus aurata) | 0.86 g DP kg−0.7 fish−1 day−1 | 55.8 kJ DE kg−0.83 fish−1 day−1 | 23–24 | [20] |
| Gilthead sea bream (Sparus aurata) | 1.39 g DP kg−0.7 fish−1 day−1 | 59.84 kJ DE kg−0.82 fish−1 day−1 | 21–25 | [19] |
| European sea bass (Dicentrarchus labrax) | 0.66 g DP kg−0.69 fish−1 day−1 | 43.6 kJ DE kg−0.79 fish−1 day−1 | 19–26 | [22] |
| European sea bass (Dicentrarchus labrax) | 0.87 g DP kg−0.7 fish−1 day−1 | 50.9 kJ DE kg−0.8 fish−1 day−1 | 25 | [23] |
| White grouper (Epinephelus aeneus) | 34.05 kJ DE kg−0.8 fish−1 day−1 | 19–27 | [21] | |
| Mulloway (Argyrosomus japonicus) | 0.47 g DP kg−0.7 fish−1 day−1 | 44.21–49.59 kJ DE kg−0.8 fish−1 day−1 | 20–26 | [25] |
| Red drum (Sciaenops ocellatus) | 0.5–2.2 g DP kg fish−1 day−1 | 58 to 97 kJ DE kg fish−1 day−1 | 25 | [26] |
| Yellowtail (Seriola quinqueradiata) | 2.7–3.1 g DP kg fish−1 day−1 | 62.7 kJ DE kg fish−1 day−1 | 22–27 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jauralde, I.; Velazco-Vargas, J.; Tomás-Vidal, A.; Jover Cerdá, M.; Martínez-Llorens, S. Protein and Energy Requirements for Maintenance and Growth in Juvenile Meagre Argyrosomus regius (Asso, 1801) (Sciaenidae). Animals 2021, 11, 77. https://doi.org/10.3390/ani11010077
Jauralde I, Velazco-Vargas J, Tomás-Vidal A, Jover Cerdá M, Martínez-Llorens S. Protein and Energy Requirements for Maintenance and Growth in Juvenile Meagre Argyrosomus regius (Asso, 1801) (Sciaenidae). Animals. 2021; 11(1):77. https://doi.org/10.3390/ani11010077
Chicago/Turabian StyleJauralde, Ignacio, Jorge Velazco-Vargas, Ana Tomás-Vidal, Miguel Jover Cerdá, and Silvia Martínez-Llorens. 2021. "Protein and Energy Requirements for Maintenance and Growth in Juvenile Meagre Argyrosomus regius (Asso, 1801) (Sciaenidae)" Animals 11, no. 1: 77. https://doi.org/10.3390/ani11010077
APA StyleJauralde, I., Velazco-Vargas, J., Tomás-Vidal, A., Jover Cerdá, M., & Martínez-Llorens, S. (2021). Protein and Energy Requirements for Maintenance and Growth in Juvenile Meagre Argyrosomus regius (Asso, 1801) (Sciaenidae). Animals, 11(1), 77. https://doi.org/10.3390/ani11010077






