The Digestive Function of Pseudoplatystoma punctifer Early Juveniles Is Differentially Modulated by Dietary Protein, Lipid and Carbohydrate Content and Their Ratios
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing and Feeding Protocol
2.2. Growth and Survival Measurements
2.3. Proximate Composition and Fatty Acid Analyses
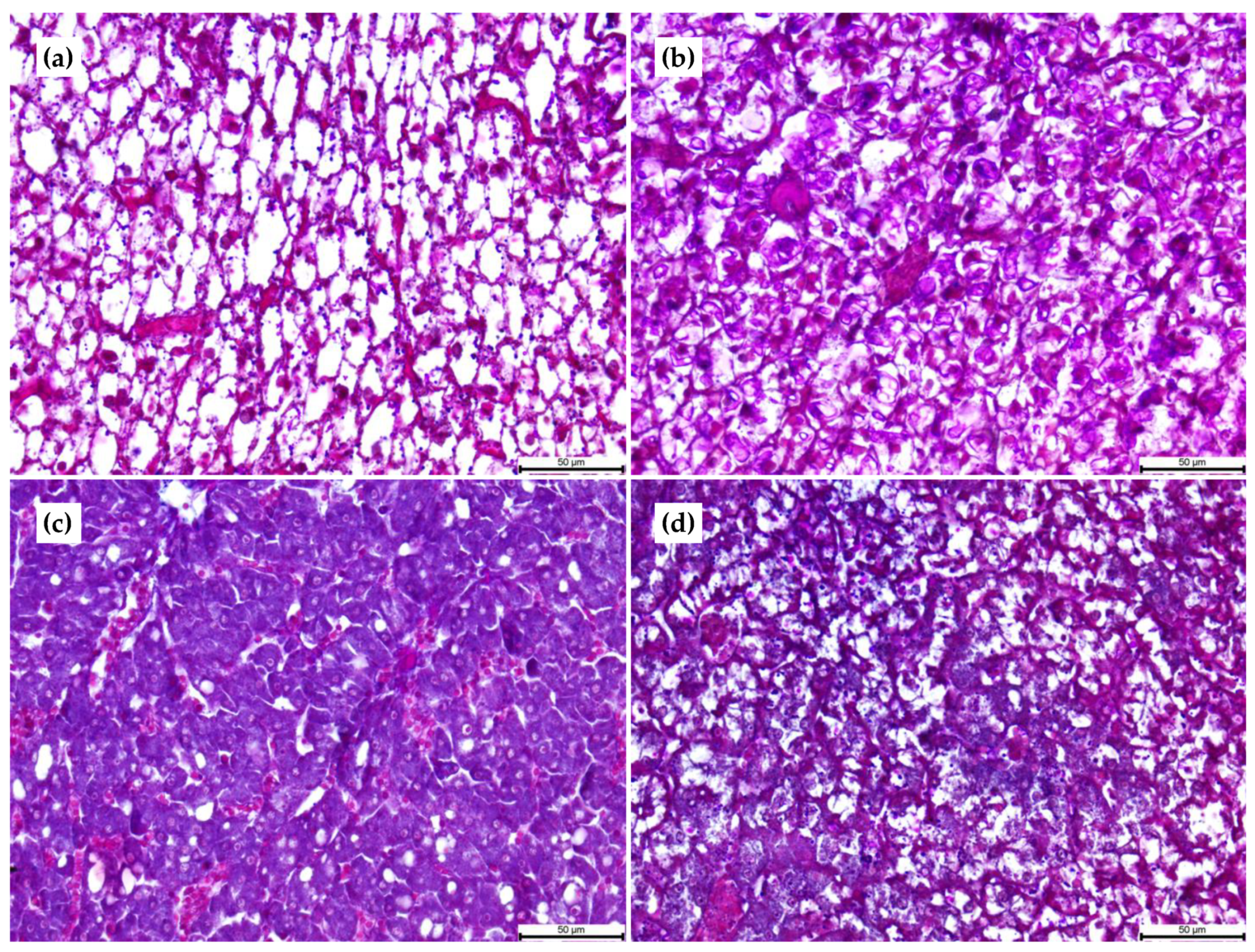
2.4. Histological Analyses
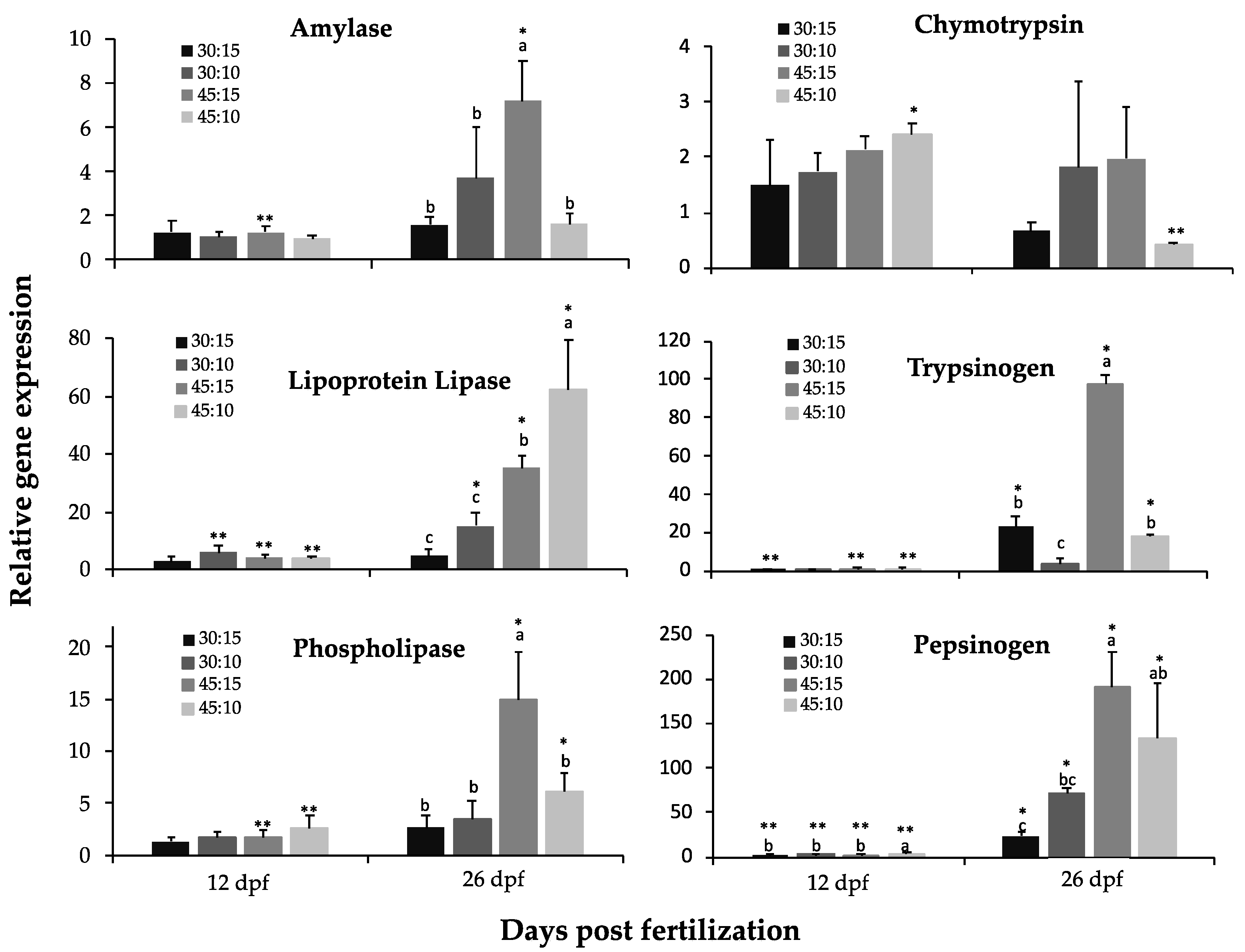
2.5. RNA Extraction and Gene Expression Analyses
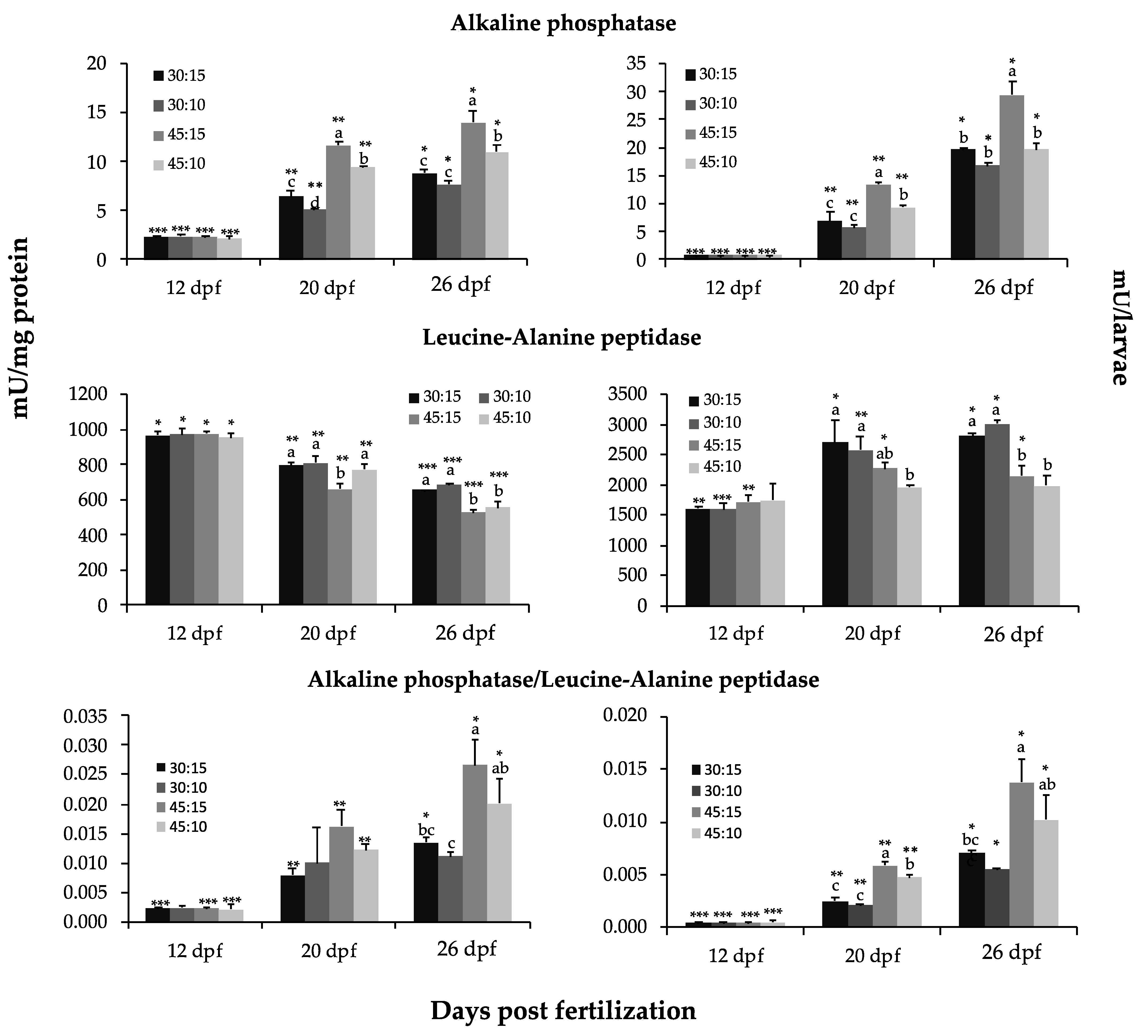
2.6. Digestive Enzyme Activity Assays
2.7. Statistics
3. Results
3.1. Growth and Survival
3.2. Proximate Composition and Fatty Acid of Diets and P. punctifer Early Juveniles
3.3. Histological Analyses
3.4. Gene Expression Analyses
3.5. Digestive Enzyme Activity Analyses
4. Discussion
4.1. The Influence of Dietary Protein:Lipid:Carbohydrate Content and Ratios in P. punctifer Performance
4.2. The Influence of Dietary Fatty Acid Composition in P. punctifer Body Fatty Acid Composition
4.3. The Influence of Dietary Composition in the Digestive Function of P. punctifer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winfree, R.A.; Stickney, R.R. Effects of dietary protein and energy on growth, feed conversion efficiency and body composition of Tilapia aurea. J. Nutr. 1981, 111, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.-F.M.; Teshima, S. Protein and energy requirements of Nile tilapia, Oreochromis niloticus, fry. Aquaculture 1992, 103, 55–63. [Google Scholar] [CrossRef]
- Alam, M.S.; Liang, X.-F.; Liu, L.; He, S.; Kuang, Y.; Hoseinifar, S.H.; Dawar, F.U. Growth and metabolic response of chinese perch to different dietary protein-to-energy ratios in artificial diets. Int. J. Mol. Sci. 2019, 20, 5983. [Google Scholar] [CrossRef] [PubMed]
- Boonanuntanasarn, S.; Kumkhong, S.; Yoohat, K.; Plagnes-Juan, E.; Burel, C.; Marandel, L.; Panserat, S. Molecular responses of Nile tilapia (Oreochromis niloticus) to different levels of dietary carbohydrates. Aquaculture 2018, 482, 117–123. [Google Scholar] [CrossRef]
- Hemre, G.-I.; Sandnes, K.; Lie, Ø.; Torrissen, O.; Waagbø, R. Carbohydrate nutrition in Atlantic salmon, Salmo salar L.: Growth and feed utilization. Aquac. Res. 1995, 26, 149–154. [Google Scholar] [CrossRef]
- Jafri, A.K.E. Effect of dietary carbohydrate-to-lipid ratio on growth and body composition of walking catfish (Clarias batrachus). Aquaculture 1998, 161, 159–168. [Google Scholar] [CrossRef]
- Torstensen, B.E.; Lie, Ø.; Frøyland, L. Lipid metabolism and tissue composition in Atlantic salmon (Salmo salar L.)—Effects of capelin oil, palm oil, and oleic acid-enriched sunflower oil as dietary lipid sources. Lipids 2000, 35, 653–664. [Google Scholar] [CrossRef]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 143, 89–96. [Google Scholar] [CrossRef]
- Francis, D.S.; Turchini, G.M.; Jones, P.L.; De Silva, S.S. Effects of fish oil substitution with a mix blend vegetable oil on nutrient digestibility in Murray cod, Maccullochella peelii peelii. Aquaculture 2007, 269, 447–455. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Y.-J.; Tian, L.-X.; Mai, K.-S.; Liang, G.-Y.; Yang, H.-J.; Huai, M.-Y.; Luo, W.-J. Effect of dietary carbohydrate-to-lipid ratios on growth performance, body composition, nutrient utilization and hepatic enzymes activities of herbivorous grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2010, 16, 327–333. [Google Scholar] [CrossRef]
- Castro, C.; Corraze, G.; Basto, A.; Larroquet, L.; Panserat, S.; Oliva-Teles, A. Dietary lipid and carbohydrate interactions: Implications on lipid and glucose absorption, transport in gilthead sea bream (Sparus aurata) juveniles. Lipids 2016, 51, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, W.; Jin, J.; Zhu, X.; Yang, Y.; Han, D.; Liu, H.; Xie, S. Effects of dietary carbohydrate and lipid concentrations on growth performance, feed utilization, glucose, and lipid metabolism in two strains of gibel carp. Front. Vet. Sci. 2019, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Taj, S.; Irm, M.; Jin, M.; Yuan, Y.; Andriamialinirina, H.J.T.; Zhou, Q. Effects of dietary carbohydrate to lipid ratios on growth performance, muscle fatty acid composition, and intermediary metabolism in juvenile black seabream (Acanthopagrus schlegelii). Front. Physiol. 2020, 11, 507. [Google Scholar] [CrossRef]
- Halver, J.E.; Hardy, R.W. Fish Nutrition, 3rd ed.; Academic Press: San Diego, CA, USA, 2003; ISBN 978-0-12-319652-1. [Google Scholar]
- García-Meilán, I.; Ordóñez-Grande, B.; Gallardo, M.A. Meal timing affects protein-sparing effect by carbohydrates in sea bream: Effects on digestive and absorptive processes. Aquaculture 2014, 434, 121–128. [Google Scholar] [CrossRef]
- Wilson, R.P. Utilization of dietary carbohydrate by fish. Aquaculture 1994, 124, 67–80. [Google Scholar] [CrossRef]
- Brauge, C.; Medale, F.; Corraze, G. Effect of dietary carbohydrate levels on growth, body composition and glycaemia in rainbow trout, Oncorhynchus mykiss, reared in seawater. Aquaculture 1994, 123, 109–120. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.-J.; Tian, L.-X.; Du, Z.-Y.; Wang, J.-T.; Wang, S.; Xiao, W.P. Effects of dietary carbohydrate level on growth and body composition of juvenile tilapia, Oreochromis niloticus × O. aureus. Aquac. Res. 2005, 36, 1408–1413. [Google Scholar] [CrossRef]
- Buitrago–Suárez, U.A.; Burr, B.M. Taxonomy of the catfish genus Pseudoplatystoma Bleeker (Siluriformes: Pimelodidae) with recognition of eight species. Zootaxa 2007, 1512, 1–38. [Google Scholar] [CrossRef]
- Garcia, A.; Tello, S.; Vargas, G.; Duponchelle, F. Patterns of commercial fish landings in the Loreto region (Peruvian Amazon) between 1984 and 2006. Fish Physiol. Biochem. 2009, 35, 53–67. [Google Scholar] [CrossRef]
- Pinaya, W.H.D.; Lobon-Cervia, F.J.; Pita, P.; de Souza, R.B.; Freire, J.; Isaac, V.J. Multispecies fisheries in the lower Amazon river and its relationship with the regional and global climate variability. PLoS ONE 2016, 11, e0157050. [Google Scholar] [CrossRef]
- Baras, E.; del Aguila, D.V.S.; Naranjos, G.V.M.; Dugué, R.; Koo, F.C.; Duponchelle, F.; Renno, J.-F.; Garcia-Dávila, C.; Nuñez, J. How many meals a day to minimize cannibalism when rearing larvae of the Amazonian catfish Pseudoplatystoma punctifer? The cannibal’s point of view. Aquat. Living Resour. 2011, 24, 379–390. [Google Scholar] [CrossRef]
- Gisbert, E.; Moreira, C.; Castro-Ruiz, D.; Öztürk, S.; Fernández, C.; Gilles, S.; Nuñez, J.; Duponchelle, F.; Tello, S.; Renno, J.F.; et al. Histological development of the digestive system of the Amazonian pimelodid catfish Pseudoplatystoma punctifer. Animal 2014, 8, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ruiz, D.; Mozanzadeh, M.T.; Fernández-Méndez, C.; Andree, K.B.; García-Dávila, C.; Cahu, C.; Gisbert, E.; Darias, M.J. Ontogeny of the digestive enzyme activity of the Amazonian pimelodid catfish Pseudoplatystoma punctifer (Castelnau, 1855). Aquaculture 2019, 504, 210–218. [Google Scholar] [CrossRef]
- Darias, M.J.; Castro-Ruiz, D.; Estivals, G.; Quazuguel, P.; Fernández-Méndez, C.; Núñez-Rodríguez, J.; Clota, F.; Gilles, S.; García-Dávila, C.; Gisbert, E.; et al. Influence of dietary protein and lipid levels on growth performance and the incidence of cannibalism in Pseudoplatystoma punctifer (Castelnau, 1855) larvae and early juveniles. J. Appl. Ichthyol. 2015, 31, 74–82. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis; Pergamon: Oxford, UK, 1982. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Pearse, A.G.E. Histochemistry, Theoretical and Applied; Analytic Technology; Churchill Livingstone: New York, NY, USA, 1985; Volume 2. [Google Scholar]
- Darias, M.J.; Gómez, M.A.; Tello, S.; Gisbert, E. Growth, survival and the histology of the digestive tract of juvenile Osteoglossum bicirrhosum (Cuvier, 1829) fed three diets containing different protein and lipid levels. J. Appl. Ichthyol. 2015, 31, 67–73. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Cahu, C.L.; Infante, J.L.Z. Early weaning of sea bass (Dicentrarchus labrax) larvae with a compound diet: Effect on digestive enzymes. Comp. Biochem. Physiol. A Physiol. 1994, 109, 213–222. [Google Scholar] [CrossRef]
- Gisbert, E.; Nolasco, H.; Solovyev, M. Towards the standardization of brush border purification and intestinal alkaline phosphatase quantification in fish with notes on other digestive enzymes. Aquaculture 2018, 487, 102–108. [Google Scholar] [CrossRef]
- Crane, R.K.; Boge, G.; Rigal, A. Isolation of brush border membranes in vesicular form from the intestinal spiral valve of the small dogfish (Scyliorhinus canicula). Biochim. Biophys. Acta BBA-Biomembr. 1979, 554, 264–267. [Google Scholar] [CrossRef]
- Bessey, O.A.; Lowry, O.H.; Brock, M.J. A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J. Biol. Chem. 1946, 164, 321–329. [Google Scholar] [CrossRef]
- Nicholson, J.A.; Kim, Y.S. A one-step l-amino acid oxidase assay for intestinal peptide hydrolase activity. Anal. Biochem. 1975, 63, 110–117. [Google Scholar] [CrossRef]
- Métais, P.; Bieth, J. Détermination de l’alpha-amylase par une microtechnique. Ann. Biol. Clin. 1968, 26, 133–142. [Google Scholar]
- Iijima, N.; Tanaka, S.; Ota, Y. Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiol. Biochem. 1998, 18, 59–69. [Google Scholar] [CrossRef]
- Holm, H.; Hanssen, L.E.; Krogdahl, Å.; Florholmen, J. High and low inhibitor soybean meals affect human duodenal proteinase activity differently: In vivo comparison with bovine serum albumin. J. Nutr. 1988, 118, 515–520. [Google Scholar] [CrossRef]
- Worthington Biochemical Corporation. Worthington Enzyme Manual: Enzymes, Enzyme Reagents, Related Biochemicals; Worthington Biochemical Corp.: Freehold, NJ, USA, 1991. [Google Scholar]
- Solovyev, M.; Gisbert, E. Influence of time, storage temperature and freeze/thaw cycles on the activity of digestive enzymes from gilthead sea bream (Sparus aurata). Fish Physiol. Biochem. 2016, 42, 1383–1394. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, F.; Xie, S.; Zhu, X.; Lei, W.; Shen, J. Effect of high dietary starch levels on the growth performance, blood chemistry and body composition of gibel carp (Carassius auratus var. gibelio). Aquac. Res. 2009, 40, 1011–1018. [Google Scholar] [CrossRef]
- Moreira, I.S.; Peres, H.; Couto, A.; Enes, P.; Oliva-Teles, A. Temperature and dietary carbohydrate level effects on performance and metabolic utilisation of diets in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2008, 274, 153–160. [Google Scholar] [CrossRef]
- Panserat, S.; Skiba-Cassy, S.; Seiliez, I.; Lansard, M.; Plagnes-Juan, E.; Vachot, C.; Aguirre, P.; Larroquet, L.; Chavernac, G.; Medale, F.; et al. Metformin improves postprandial glucose homeostasis in rainbow trout fed dietary carbohydrates: A link with the induction of hepatic lipogenic capacities? Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R707–R715. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Moon, T.W.; Aguirre, P.; Skiba-Cassy, S.; Panserat, S. Effects of insulin infusion on glucose homeostasis and glucose metabolism in rainbow trout fed a high-carbohydrate diet. J. Exp. Biol. 2010, 213, 4151–4157. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Jantrarotai, W.; Sitasit, P.; Rajchapakdee, S. The optimum carbohydrate to lipid ratio in hybrid Clarias catfish (Clarias macrocephalus × C. gariepinus) diets containing raw broken rice. Aquaculture 1994, 127, 61–68. [Google Scholar] [CrossRef]
- Panserat, S.; Médale, F.; Blin, C.; Brèque, J.; Vachot, C.; Plagnes-Juan, E.; Gomes, E.; Krishnamoorthy, R.; Kaushik, S. Hepatic glucokinase is induced by dietary carbohydrates in rainbow trout, gilthead seabream, and common carp. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1164–R1170. [Google Scholar] [CrossRef]
- Figueiredo-Silva, A.C.; Saravanan, S.; Schrama, J.W.; Panserat, S.; Kaushik, S.; Geurden, I. A comparative study of the metabolic response in rainbow trout and Nile tilapia to changes in dietary macronutrient composition. Br. J. Nutr. 2013, 109, 816–826. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Bordinhon, A.M.; Davis, D.A.; Zhang, W.; Zhu, X. Protein: Energy ratio in practical diets for Nile tilapia Oreochromis niloticus. Aquac. Int. 2013, 21, 1109–1119. [Google Scholar] [CrossRef]
- Honorato, C.A.; Almeida, L.C.; Nunes, C.D.S.; Carneiro, D.J.; Moraes, G. Effects of processing on physical characteristics of diets with distinct levels of carbohydrates and lipids: The outcomes on the growth of pacu (Piaractus mesopotamicus). Aquac. Nutr. 2010, 16, 91–99. [Google Scholar] [CrossRef]
- Shyong, W.-J.; Huang, C.-H.; Chen, H.-C. Effects of dietary protein concentration on growth and muscle composition of juvenile Zacco barbata. Aquaculture 1998, 167, 35–42. [Google Scholar] [CrossRef]
- Yang, S.-D.; Liou, C.-H.; Liu, F.-G. Effects of dietary protein level on growth performance, carcass composition and ammonia excretion in juvenile silver perch (Bidyanus bidyanus). Aquaculture 2002, 213, 363–372. [Google Scholar] [CrossRef]
- Kim, K.W.; Wang, X.J.; Bai, S.C. Optimum dietary protein level for maximum growth of juvenile olive flounder Paralichthys olivaceus (Temminck et Schlegel). Aquac. Res. 2002, 33, 673–679. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.J.; Mai, K.S.; Tian, L.X.; Liu, D.H.; Tan, X.Y. Optimal dietary protein requirement of grouper Epinephelus coioides juveniles fed isoenergetic diets in floating net cages. Aquac. Nutr. 2004, 10, 247–252. [Google Scholar] [CrossRef]
- Islam, M.S.; Tanaka, M. Optimization of dietary protein requirement for pond-reared mahseer Tor putitora Hamilton (Cypriniformes: Cyprinidae). Aquac. Res. 2004, 35, 1270–1276. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Wang, L.; Shao, Q.; Xu, Z.; Xu, J. Dietary protein requirement of juvenile black sea bream, Sparus macrocephalus. J. World Aquac. Soc. 2010, 41, 151–164. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Tian, L.-X.; Liang, G.-Y.; Liu, Y.-J. Effect of dietary energy to protein ratios on growth performance and feed efficiency of juvenile grass carp (Ctenopharyngodon idella). Open Fish Sci. J. 2009, 2, 25–31. [Google Scholar] [CrossRef]
- Garling, D.L.; Wilson, R.P. Optimum dietary protein to energy ratio for channel catfish fingerlings, Ictalurus punctatus. J. Nutr. 1976, 106, 1368–1375. [Google Scholar] [CrossRef]
- Catacutan, M.R.; Coloso, R.M. Effect of dietary protein to energy ratios on growth, survival, and body composition of juvenile Asian seabass, Lates calcarifer. Aquaculture 1995, 131, 125–133. [Google Scholar] [CrossRef]
- Henken, A.M.; Machiels, M.A.M.; Dekker, W.; Hogendoorn, H. The effect of dietary protein and energy content on growth rate and feed utilization of the African catfish Clarias gariepinus (Burchell 1822). Aquaculture 1986, 58, 55–74. [Google Scholar] [CrossRef]
- Couto, A.; Enes, P.; Peres, H.; Oliva-Teles, A. Temperature and dietary starch level affected protein but not starch digestibility in gilthead sea bream juveniles. Fish Physiol. Biochem. 2012, 38, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ge, X.; Liu, B.; Xie, J.; Chen, R.; Ren, M. Effect of high dietary carbohydrate on the growth performance, blood chemistry, hepatic enzyme activities and growth hormone gene expression of wuchang bream (Megalobrama amblycephala) at two temperatures. Asian-Australas. J. Anim. Sci. 2015, 28, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Brodtkorb, T.; Rosenlund, G.; Lie, Ø. Effects of dietary levels of 20:5n-3 and 22:6n-3 on tissue lipid composition in juvenile Atlantic salmon, Salmo salar, with emphasis on brain and eye. Aquac. Nutr. 1997, 3, 175–187. [Google Scholar] [CrossRef]
- Sargent, J.R.; McEvoy, L.A.; Bell, J.G. Requirements, presentation and sources of polyunsaturated fatty acids in marine fish larval feeds. Aquaculture 1997, 155, 117–127. [Google Scholar] [CrossRef]
- Bell, J.G.; McEvoy, J.; Webster, J.L.; McGhee, F.; Millar, R.M.; Sargent, J.R. Flesh lipid and carotenoid composition of Scottish farmed Atlantic salmon (Salmo salar). J. Agric. Food Chem. 1998, 46, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Verreth, J.; Coppoolse, J.; Segner, H. The effect of low HUFA- and high HUFA-enriched Artemia, fed at different feeding levels, on growth, survival, tissue fatty acids and liver histology of Clarias gariepinus larvae. Aquaculture 1994, 126, 137–150. [Google Scholar] [CrossRef]
- Işik, O.; Sarihan, E.; Kuşvuran, E.; Gül, Ö.; Erbatur, O. Comparison of the fatty acid composition of the freshwater fish larvae Tilapia zillii, the rotifer Brachionus calyciflorus, and the microalgae Scenedesmus abundans, Monoraphidium minitum and Chlorella vulgaris in the algae-rotifer-fish larvae food chains. Aquaculture 1999, 174, 299–311. [Google Scholar] [CrossRef]
- Sillero, J.Z.; Ramos, L.R.V.; Romano, L.A.; Monserrat, J.M.; Tesser, M.B. Effect of dietary dextrin levels on the growth performance, blood chemistry, body composition, hepatic triglicerides and glycogen of Lebranche mullet juveniles (Mugil liza Valenciennes 1836, Mugilidae). J. Appl. Ichthyol. 2013, 29, 1342–1347. [Google Scholar] [CrossRef]
- He, A.-Y.; Ning, L.-J.; Chen, L.-Q.; Chen, Y.-L.; Xing, Q.; Li, J.-M.; Qiao, F.; Li, D.-L.; Zhang, M.-L.; Du, Z.-Y. Systemic adaptation of lipid metabolism in response to low- and high-fat diet in Nile tilapia (Oreochromis niloticus). Physiol. Rep. 2015, 3, e12485. [Google Scholar] [CrossRef]
- Henderson, J.R.; Daniel, P.M.; Fraser, P.A. The pancreas as a single organ: The influence of the endocrine upon the exocrine part of the gland. Gut 1981, 22, 158–167. [Google Scholar] [CrossRef]
- Yost, T.J.; Jensen, D.R.; Haugen, B.R.; Eckel, R.H. Effect of dietary macronutrient composition on tissue-specific lipoprotein lipase activity and insulin action in normal-weight subjects. Am. J. Clin. Nutr. 1998, 68, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Boualga, A.; Bouchenak, M.; Belleville, J. Low-protein diet prevents tissue lipoprotein lipase activity increase in growing rats. Br. J. Nutr. 2000, 84, 663–671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ginsberg, H.N. Lipoprotein physiology in nondiabetic and diabetic states: Relationship to atherogenesis. Diabetes Care 1991, 14, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Boucher, P.; Ducluzeau, P.-H.; Davelu, P.; Andreelli, F.; Vallier, P.; Riou, J.-P.; Laville, M.; Vidal, H. Expression and regulation by insulin of low-density lipoprotein receptor-related protein mRNA in human skeletal muscle. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2002, 1588, 226–231. [Google Scholar] [CrossRef]
- Gutniak, M.; Grill, V.; Efendlć, S. Effect of composition of mixed meals—low-versus high-carbohydrate content—on insulin, glucagon, and somatostatin release in healthy humans and in patients with NIDDM. Diabetes Care 1986, 9, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.L.Z.; Gisbert, E.; Sarasquete, C.; Navarro, I.; Gutiérrez, J.; Cahu, C. Ontogeny and physiology of the digestive system of marine fish larvae. In Feeding and Digestive Functions in Fishes; Cyrino, J.E.P., Bureau, D.P., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 281–348. ISBN 978-1-57808-575-6. [Google Scholar]
- Gisbert, E.; Ortiz-Delgado, J.B.; Sarasquete, C. Nutritional cellular biomarkers in early life stages of fish. Histol. Histopathol. 2008, 23, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Sarasquete, C.; Gisbert, E.; Ribeiro, L.; Vieira, L.; Dinis, M. Glyconjugates in epidermal, branchial and digestive mucous cells and gastric glands of gilthead sea bream, Sparus aurata, Senegal sole, Solea senegalensis and Siberian sturgeon, Acipenser baeri development. Eur. J. Histochem. 2001, 45, 267–278. [Google Scholar] [CrossRef]
- Péres, A.; Infante, J.L.Z.; Cahu, C. Dietary regulation of activities and mRNA levels of trypsin and amylase in sea bass (Dicentrarchus labrax) larvae. Fish Physiol. Biochem. 1998, 19, 145–152. [Google Scholar] [CrossRef]
- Martínez, I.; Moyano, F.J.; Fernández-Díaz, C.; Yúfera, M. Digestive enzyme activity during larval development of the Senegal sole (Solea senegalensis). Fish Physiol. Biochem. 1999, 21, 317–323. [Google Scholar] [CrossRef]
- Cahu, C.; Infante, J.Z.; Takeuchi, T. Nutritional components affecting skeletal development in fish larvae. Aquaculture 2003, 227, 245–258. [Google Scholar] [CrossRef]
- Bell, J.G.; Sargent, J.R. Arachidonic acid in aquaculture feeds: Current status and future opportunities. Aquaculture 2003, 218, 491–499. [Google Scholar] [CrossRef]
- Gisbert, E.; Villeneuve, L.; Zambonino-Infante, J.L.; Quazuguel, P.; Cahu, C.L. Dietary phospholipids are more efficient than neutral lipids for long-chain polyunsaturated fatty acid supply in European sea bass Dicentrarchus labrax larval development. Lipids 2005, 40, 609. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, M.D. Utilization of yolk fatty acids by goldfish embryos and larvae. Fish Physiol. Biochem. 1996, 15, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dakka, N.; Puigserver, A.; Wicker, C. Regulation by a protein-free carbohydrate-rich diet of rat pancreatic mRNAs encoding trypsin and elastase isoenzymes. Biochem. J. 1990, 268, 471–474. [Google Scholar] [CrossRef]
- Lemieux, H.; Blier, P.; Dutil, J.-D. Do digestive enzymes set a physiological limit on growth rate and food conversion efficiency in the Atlantic cod (Gadus morhua)? Fish Physiol. Biochem. 1999, 20, 293–303. [Google Scholar] [CrossRef]
- Koven, W.; Rojas-García, C.; Finn, R.; Tandler, A.; Rønnestad, I. Stimulatory effect of ingested protein and/or free amino acids on the secretion of the gastro-endocrine hormone cholecystokinin and on tryptic activity, in early-feeding herring larvae, Clupea harengus. Mar. Biol. 2002, 140, 1241–1247. [Google Scholar] [CrossRef]
- Infante, J.L.Z.; Cahu, C.L. Dietary modulation of some digestive enzymes and Metabolic processes in developing marine fish: Applications to diet formulation. Aquaculture 2007, 268, 98–105. [Google Scholar] [CrossRef]

| Ingredients 1 (in % DM) | Dietary Treatments | |||
|---|---|---|---|---|
| 30:15 | 30:10 | 45:15 | 45:10 | |
| Fishmeal | 36 | 36 | 53 | 53 |
| Hydrolyzed fishmeal (CPSP) | 9 | 9 | 14 | 14 |
| Lipids | 14 | 8 | 12 | 7 |
| Marine lecithin | 3 | 8 | 3 | 7 |
| Soybean lecithin | 11 | 0 | 9 | 0 |
| Gelatin | 15 | 15 | 15 | 15 |
| Wheat starch | 20 | 26 | 0 | 5 |
| Vitamin mix 2 (x4) | 2 | 2 | 2 | 2 |
| Mineral mix 3 | 3 | 3 | 3 | 3 |
| Betain | 1 | 1 | 1 | 1 |
| Parameters | Dietary Treatments | |||
|---|---|---|---|---|
| 30:15 | 30:10 | 45:15 | 45:10 | |
| Total length (mm) | ||||
| 12 dpf | 11.92 ± 0.50 | 12.17 ± 0.47 | 12.19 ± 0.54 | 11.90 ± 0.70 |
| 20 dpf | 22.45 ± 0.41 b | 20.54 ± 1.08 b | 25.78 ± 1.24 a | 21.09 ± 0.94 b |
| 26 dpf | 38.35 ± 1.48 b | 33.02 ± 0.77 c | 46.90 ± 0.98 a | 37.03 ± 0.94 b |
| Wet weight (mg) | ||||
| 12 dpf | 7.32 ± 0.74 | 7.19 ± 0.20 | 8.09 ± 0.35 | 7.49 ± 1.71 |
| 20 dpf | 68.04 ± 4.90 b | 55.31 ± 7.97 b | 97.91 ± 19.48 a | 60.63 ± 0.86 b |
| 26 dpf | 264.44 ± 19.80 c | 220.85 ± 8.40 d | 563.71 ± 8.75 a | 306.76 ± 11.87 b |
| Survival (%) | ||||
| 12 dpf | 93.88 ± 6.77 | 85.62 ± 7.14 | 78.58 ± 5.88 | 85.21 ± 9.10 |
| 26 dpf | 17.68 ± 1.52 c | 12.66 ± 2.29 d | 36.32 ± 1.23 a | 23.44 ± 0.59 b |
| Analyses of the Diets (% DM) | Dietary Treatments | |||
|---|---|---|---|---|
| 30:15 | 30:10 | 45:15 | 45:10 | |
| Proteins | 30.07 ± 2.44 b | 30.90 ± 4.01 b | 43.13 ± 2.65 a | 42.86 ± 2.03 a |
| Lipids | 12.50 ± 1.21 a | 7.43 ± 0.27 b | 12.46 ± 0.51 a | 10.36 ± 0.69 b |
| Carbohydrates | 24.87 ± 1.22 b | 31.31 ± 2.26 a | 2.34 ± 0.17 d | 7.58 ± 0.60 c |
| Moisture | 18.89 ± 0.16 b | 22.35 ± 0.02 a | 17.48 ± 0.04 c | 15.25 ± 0.07 d |
| Energy (KJ) | 1387.50 ± 24.89 | 1316.13 ± 43.67 | 1203.68 ± 81.53 | 1291.56 ± 54.47 |
| E:P ratio (kcal g−1 protein) | 11.07 ± 1.10 a | 10.19 ± 0.73 a | 6.66 ± 0.04 b | 7.20 ± 0.04 b |
| Proximate Composition (%) | Dietary Treatments | |||
|---|---|---|---|---|
| 30:15 | 30:10 | 45:15 | 45:10 | |
| Proteins | 52.70 ± 4.97 | 52.78 ± 1.79 | 47.06 ± 3.76 | 56.55 ± 3.65 |
| Lipids | 13.21 ± 0.23 a | 13.02 ± 0.87 a | 9.07 ± 0.45 b | 11.21 ± 0.59 a |
| Carbohydrates | 5.72 ± 0.02 a | 5.95 ± 0.22 a | 1.66 ± 0.12 c | 3.31 ± 0.33 b |
| Ashes | 1.65 ± 0.01 | 1.38 ± 0.03 | 1.80 ± 0.37 | 1.47 ± 0.09 |
| Total Lipids and Fatty Acids | Dietary Treatments | |||
|---|---|---|---|---|
| 30:15 | 30:10 | 45:15 | 45:10 | |
| Total lipid (mg g−1 DW) | 124.99 ± 12.07 a | 74.31 ± 2.75 b | 124.58 ± 5.07 a | 103.62 ± 11.64 ab |
| Total fatty acid (mg g−1 DW) | 71.18 ± 7.85 a | 38.91 ± 6.76 b | 66.81 ± 3.38 a | 52.16 ± 2.50 ab |
| 14:0 | 0.82 ± 0.04 c | 1.72 ± 0.00 a | 1.04 ± 0.00 c | 1.55 ± 0.25ab |
| 16:0 | 19.71 ± 1.86 | 20.99 ± 1.54 | 19.21 ± 1.21 | 19.94 ± 1.48 |
| 18:0 | 3.62 ± 0.52 | 3.37 ± 0.33 | 3.09 ± 0.18 | 3.15 ± 0.40 |
| Total saturated | 24.15 ± 1.30 | 26.08 ± 1.21 | 23.34 ± 1.02 | 24.64 ± 1.33 |
| 16:1 | 1.72 ± 0.79 | 3.60 ± 1.74 | 2.26 ± 0.90 | 3.09 ± 1.26 |
| 18:1n-9 (OA) | 19.15 ± 2.90 | 16.71 ± 0.99 | 19.41 ± 1.26 | 19.16 ± 0.70 |
| 20:1 | 2.78 ± 0.04 c | 6.61 ± 0.45 a | 4.42 ± 0.46 b | 7.06 ± 0.62 a |
| Total monounsaturated | 23.66 ± 2.1 6 b | 26.91 ± 0.30 ab | 26.09 ± 0.82 ab | 29.31 ± 0.06 a |
| 18:2n-6 (LA) | 33.88 ± 0.38 a | 2.33 ± 0.17 c | 26.99 ± 0.99 b | 2.69 ± 0.46 c |
| 18:3n-6 | 0.00 ± 0.00 | 0.21 ± 0.08 | 0.00 ± 0.00 | 0.08 ± 0.11 |
| 20:4n-6 (ARA) | 0.16 ± 0.22 | 1.10 ± 0.35 | 0.16 ± 0.22 | 0.26 ± 0.37 |
| 22:5n-6 | 0.00 ± 0.00 | 0.10 ± 0.15 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Total n-6 PUFA | 34.03 ± 0.60 a | 3.75 ± 0.45 c | 27.15 ± 1.21 b | 3.02 ± 0.02 c |
| 18:3n-3 (LLA) | 2.80 ± 0.37 a | 0.73 ± 0.02 b | 2.39 ± 0.16 a | 0.83 ± 0.06 b |
| 18:4n-3 | 0.29 ± 0.28 | 0.00 ± 0.00 | 0.31 ± 0.44 | 0.44 ± 0.62 |
| 20:4n-3 | 0.13 ± 0.18 | 0.30 ± 0.01 | 0.25 ± 0.05 | 0.37 ± 0.01 |
| 20:5n-3 (EPA) | 6.11 ± 0.34 c | 16.44 ± 0.21 a | 9.12 ± 0.62 b | 16.82 ± 0.69 a |
| 21:5n-3 | 0.00 ± 0.00 | 0.06 ± 0.09 | 0.05 ± 0.06 | 0.14 ± 0.01 |
| 22:5n-3 (DPA) | 0.30 ± 0.16 | 0.81 ± 0.20 | 0.48 ± 0.11 | 0.87 ± 0.24 |
| 22:6n-3 (DHA) | 7.98 ± 0.20 b | 24.00 ± 0.91 a | 10.36 ± 0.98 b | 22.43 ± 1.14 a |
| Total n-3 PUFA | 17.62 ± 0.45 c | 42.34 ± 0.59 a | 22.96 ± 0.91 b | 41.91 ± 0.92 a |
| Total PUFA | 51.65 ± 1.04 a | 46.09 ± 1.04 b | 50.11 ± 0.31 a | 44.93 ± 0.90 b |
| (n-3)/(n-6) | 0.52 ± 0.00 c | 11.37 ± 1.21 b | 0.85 ± 0.07c | 13.86 ± 0.41a |
| DHA/EPA | 1.31 ± 0.04 ab | 1.46 ± 0.07 a | 1.14 ± 0.03 b | 1.33 ± 0.01 ab |
| ARA/DHA | 0.02 ± 0.03 | 0.05 ± 0.01 | 0.02 ± 0.02 | 0.01 ± 0.02 |
| ARA/EPA | 0.03 ± 0.04 | 0.07 ± 0.02 | 0.02 ± 0.03 | 0.02 ± 0.02 |
| LA/PUFA | 0.66 ± 0.01 a | 0.05 ± 0.00c | 0.54 ± 0.02 b | 0.06 ± 0.01 c |
| LLA/PUFA | 0.05 ± 0.01 a | 0.02 ± 0.00b | 0.05 ± 0.00 a | 0.02 ± 0.00 b |
| OA/PUFA | 0.37 ± 0.06 | 0.36 ± 0.01 | 0.39 ± 0.03 | 0.43 ± 0.01 |
| PUFA/saturated | 2.14 ± 0.07 | 1.77 ± 0.12 | 2.15 ± 0.08 | 1.83 ± 0.14 |
| Total Lipids and Fatty Acids | Dietary Treatments | |||
|---|---|---|---|---|
| 30:15 | 30:10 | 45:15 | 45:10 | |
| Total lipid (mg g−1 DW) | 132.11 ± 2.34 a | 130.23 ± 8.69 a | 90.74 ± 4.51 b | 112.07 ± 5.94 a |
| Total fatty acid (mg g−1 DW) | 88.99 ± 4.62 a | 79.86 ± 2.03 a | 59.33 ± 3.15 b | 65.94 ± 2.40 ab |
| 14:0 | 0.47 ± 0.07 b | 1.07 ± 0.07 a b | 0.70 ± 0.13 ab | 1.10 ± 0.21 a |
| 16:0 | 23.34 ± 0.41 | 23.96 ± 0.78 | 23.09 ± 0.37 | 23.83 ± 1.73 |
| 18:0 | 8.06 ± 0.04 | 8.60 ± 0.36 | 7.79 ± 0.63 | 8.28 ± 0.63 |
| Total saturated | 31.88 ± 0.53 | 33.63 ± 1.24 | 31.58 ± 0.38 | 33.21 ± 2.61 |
| 16:1 | 1.49 ± 0.12 b | 1.92 ± 0.09 a | 1.47 ± 0.15 b | 1.89 ± 0.09 a |
| 18:1n-9 (OA) | 14.21 ± 0.21 | 13.75 ± 0.10 | 14.36 ± 0.14 | 13.80 ± 0.30 |
| 20:1 | 4.27 ± 0.10 | 4.92 ± 0.51 | 4.00 ± 0.25 | 5.00 ± 0.33 |
| Total monounsaturated | 19.98 ± 0.15 | 20.59 ± 0.48 | 19.83 ± 0.22 | 20.69 ± 0.83 |
| 18:2n-6 (LA) | 9.86 ± 0.38 b | 3.10 ± 0.28 c | 12.81 ± 0.93 a | 3.00 ± 0.45 c |
| 18:3n-6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 20:4n-6 (ARA) | 1.35 ± 0.05 | 1.37 ± 0.04 | 1.25 ± 0.05 | 1.43 ± 0.12 |
| 22:5n-6 | 0.27 ± 0.01 | 0.26 ± 0.04 | 0.25 ± 0.03 | 0.28 ± 0.04 |
| Total n-6 PUFA | 11.48 ± 0.34 b | 4.72 ± 0.32 c | 14.31 ± 0.94 a | 4.79 ± 0.31 c |
| 18:3n-3 (LLA) | 0.99 ± 0.12 a | 0.70 ± 0.02 b | 1.19 ± 0.12 a | 0.68 ± 0.07 b |
| 18:4n-3 | 0.59 ± 0.04 b | 0.70 ± 0.05 a | 0.56 ± 0.01 b | 0.69 ± 0.02 a |
| 20:4n-3 | 0.57 ± 0.00 b | 0.67 ± 0.00 a | 0.55 ± 0.00 b | 0.67 ± 0.02 a |
| 20:5n-3 (EPA) | 8.44 ± 0.14 b | 9.88 ± 0.14 a b | 7.57 ± 0.11 b | 10.25 ± 0.73 a |
| 21:5n-3 | 0.29 ± 0.00 | 0.51 ± 0.02 | 0.27 ± 0.00 | 0.45 ± 0.21 |
| 22:5n-3 (DPA) | 2.08 ± 0.04 a b | 2.27 ± 0.09 a | 1.90 ± 0.06 b | 2.37 ± 0.34 a |
| 22:6n-3 (DHA) | 18.97 ± 0.06 b | 21.19 ± 0.43 ab | 17.22 ± 1.05 b | 21.57 ± 0.98 a |
| Total n-3 PUFA | 31.94 ± 0.33 b | 35.92 ± 0.59 a | 29.25 ± 1.02 b | 36.68 ± 1.71 a |
| Total PUFA | 43.42 ± 0.01 ab | 40.64 ± 0.26 b | 43.89 ± 0.68 a | 41.47 ± 1.56 b |
| (n-3)/(n-6) | 0.56 ± 0.02 c | 7.61 ± 0.27 a | 1.28 ± 0.11 b | 7.68 ± 0.74 a |
| DHA/EPA | 2.53 ± 0.08 a | 2.50 ± 0.01 a | 2.52 ± 0.15 a | 2.11 ± 0.07 b |
| ARA/DHA | 0.10 ± 0.01 a | 0.06 ± 0.00 b | 0.07 ± 0.00 b | 0.07 ± 0.00 b |
| ARA/EPA | 0.25 ± 0.02 a | 0.15 ± 0.01 b | 0.19 ± 0.01 a b | 0.14 ± 0.00 b |
| LA/PUFA | 0.60 ± 0.01 a | 0.14 ± 0.01 c | 0.40 ± 0.02 b | 0.07 ± 0.01d |
| LLA/PUFA | 0.04 ± 0.00 a | 0.02 ± 0.00 c | 0.03 ± 0.00 b | 0.02 ± 0.00 c |
| OA/PUFA | 0.33 ± 0.00 | 0.31 ± 0.00 | 0.32 ± 0.01 | 0.33 ± 0.01 |
| PUFA/saturated | 1.43 ± 0.02 | 1.25 ± 0.05 | 1.40 ± 0.04 | 1.24 ± 0.14 |
| Dietary Treatments | Hepatocyte Number (in 100 µm2) | S Hepatic Lipid Vacuoles (µm2) |
|---|---|---|
| 30:15 | 30 ± 0.9 b | 140.99 ± 15.68 a |
| 30:10 | 19 ± 1.2 c | 117.89 ± 4.35 a |
| 45:15 | 54 ± 3.2 a | 19.97 ± 1.81 c |
| 45:10 | 28 ± 2.1 b | 26.53 ± 2.34 b |
| Dietary Treatments | N° Enterocytes (in 100 µm) | Height Enterocytes (µm) | N° Folds (in 100 µm2) | Length Folds (µm) | N° Lipid Droplets (in 100 µm) | S. Lipid Droplets (µm2) | N° Goblet Cells (in 100 µm) |
|---|---|---|---|---|---|---|---|
| Anterior intestine | |||||||
| 30:15 | 30.0 ± 1.4 ax | 16.9 ± 1.2 by | 1.0 ± 0.0 ay | 154.8 ± 122.6 | 0.0 ± 0.0 y | 0.0 ± 0.0 | 3.8 ± 1.5 b |
| 30:10 | 20.7 ± 1.2 bx | 15.6 ± 1.2 by | 0.0 ± 0.0 b | 0.0 ± 0.0 y | 0.0 ± 0.0 y | 0.0 ± 0.0 | 3.9 ± 0.9 bx |
| 45:15 | 26.0 ± 2.0 ax | 14.4 ± 0.9 bz | 1.0 ± 0.0 a | 151.7 ± 54.9 x | 0.0 ± 0.0 y | 0.0 ± 0.0 y | 11.0 ± 2.0 ax |
| 45:10 | 18.0 ± 2.0 bx | 22.4 ± 1.6ax | 1.0 ± 0.0 ay | 282.8 ± 148.1 | 0.0 ± 0.0 y | 0.0 ± 0.0 y | 1.0 ± 0.9 by |
| Middle intestine | |||||||
| 30:15 | 22.3 ± 1.5 ay | 23.0 ± 4.1 axy | 2.2 ± 0.4 bx | 75.4 ± 21.3 a | 0.0 ± 0.0 y | 0.0 ± 0.0 | 3.4 ± 1.9 |
| 30:10 | 16.3 ± 1.5 by | 19.6 ± 1.5 ax | 3.8 ± 0.3 a | 10.9 ± 15.4 cy | 0.0 ± 0.0 y | 0.0 ± 0.0 | 5.3 ± 0.9 x |
| 45:15 | 22.3 ± 1.5 ay | 19.8 ± 0.9 ay | 1.3 ± 0.6 b | 37.3 ± 0.1 bcy | 0.0 ± 0.0 y | 0.0 ± 0.0 y | 3.3 ± 1.3 y |
| 45:10 | 18.0 ± 1.4 bx | 11.5 ± 0.8 bz | 2.0 ± 0.0 bx | 56.71 ± 0.9 ab | 0.0 ± 0.0 y | 0.0 ± 0.0 y | 4.1 ± 1.4 x |
| Posterior intestine | |||||||
| 30:15 | 11.3 ± 1.5 bz | 27.8 ± 5.3 bx | 0.7 ± 0.3 y | 166.21 ± 9.1 ab | 7.5 ± 1.9 bx | 148.6 ± 107.5 | 3.0 ± 1.6 |
| 30:10 | 13.0 ± 0.7 bz | 19.7 ± 0.5 cx | 0.5 ± 0.7 | 111.5 ± 27.7 bx | 7.0 ± 1.1 bx | 48.4 ± 68.4 | 1.2 ± 0.5 y |
| 45:15 | 18.3 ± 0.5 az | 37.8 ± 4.5 ax | 1.0 ± 0.0 | 227.9 ± 40.0 ax | 11.5 ± 0.5 ax | 301.5 ± 122.5 x | 1.8 ± 1.3 y |
| 45:10 | 11.7 ± 0.6 by | 19.6 ± 1.1 bcy | 0.7 ± 0.6 y | 216.6 ± 52.3 ax | 11.3 ± 1.2 ax | 362.4 ± 141.4 x | 0.3 ± 0.5 y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Ruiz, D.; Andree, K.B.; Solovyev, M.M.; Fernández-Méndez, C.; García-Dávila, C.; Cahu, C.; Gisbert, E.; Darias, M.J. The Digestive Function of Pseudoplatystoma punctifer Early Juveniles Is Differentially Modulated by Dietary Protein, Lipid and Carbohydrate Content and Their Ratios. Animals 2021, 11, 369. https://doi.org/10.3390/ani11020369
Castro-Ruiz D, Andree KB, Solovyev MM, Fernández-Méndez C, García-Dávila C, Cahu C, Gisbert E, Darias MJ. The Digestive Function of Pseudoplatystoma punctifer Early Juveniles Is Differentially Modulated by Dietary Protein, Lipid and Carbohydrate Content and Their Ratios. Animals. 2021; 11(2):369. https://doi.org/10.3390/ani11020369
Chicago/Turabian StyleCastro-Ruiz, Diana, Karl B. Andree, Mikhail M. Solovyev, Christian Fernández-Méndez, Carmen García-Dávila, Chantal Cahu, Enric Gisbert, and Maria J. Darias. 2021. "The Digestive Function of Pseudoplatystoma punctifer Early Juveniles Is Differentially Modulated by Dietary Protein, Lipid and Carbohydrate Content and Their Ratios" Animals 11, no. 2: 369. https://doi.org/10.3390/ani11020369
APA StyleCastro-Ruiz, D., Andree, K. B., Solovyev, M. M., Fernández-Méndez, C., García-Dávila, C., Cahu, C., Gisbert, E., & Darias, M. J. (2021). The Digestive Function of Pseudoplatystoma punctifer Early Juveniles Is Differentially Modulated by Dietary Protein, Lipid and Carbohydrate Content and Their Ratios. Animals, 11(2), 369. https://doi.org/10.3390/ani11020369








