Wild-Type Zebrafish (Danio rerio) Larvae as a Vertebrate Model for Diabetes and Comorbidities: A Review
Abstract
Simple Summary
Abstract
1. Introduction
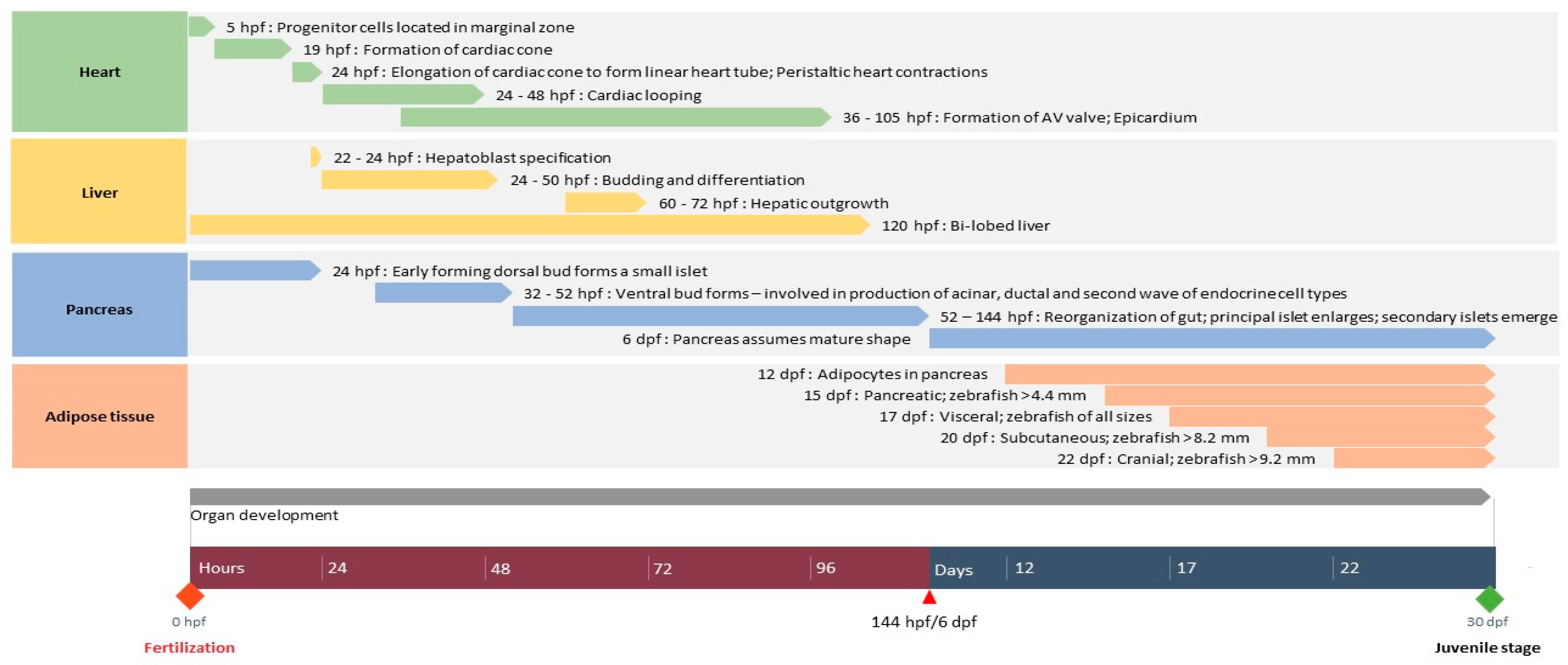
2. Development of Organs Associated with Diabetes in Zebrafish
2.1. Heart
2.1.1. Structure
2.1.2. Development
2.1.3. Link Between Heart and Diabetes
2.2. Liver
2.2.1. Structure
2.2.2. Development
2.2.3. Link between Liver and Diabetes
2.3. Pancreas
2.3.1. Structure
2.3.2. Development
2.3.3. Link between Pancreas and Diabetes
2.4. Adipose Tissue
2.4.1. Structure
2.4.2. Development
2.4.3. Link between Adipose Tissue and Diabetes
2.5. Other Organ Systems Involved in Diabetes
3. Wild-Type Zebrafish Larvae as Models for Diabetes and Comorbidities
3.1. Diabetes: Causes and Comorbidities
3.2. Assessment of Pancreatic Function in Wild-Type Zebrafish Larvae
3.3. Monitoring Glucose Uptake in Wild-Type Zebrafish Larvae
3.4. Methods to Assess Glucose Metabolism in Wild-Type Zebrafish Larvae
3.5. Induction and Monitoring Oxidative Stress in Wild-Type Zebrafish Larvae
3.6. Induction and Monitoring Inflammation in Wild-Type Zebrafish Larvae
3.7. Modelling Obesity in Wild-Type Zebrafish Larvae
3.8. Wound Healing in Wild-Type Zebrafish Larvae
3.9. Non-Alcoholic Fatty Liver Disease
4. Advantages, Disadvantages, Shortcomings, and Suggestions
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besancon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Colagiuri, S.; Chan, J.; Gregg, E.; Ke, C.; Lim, L.-L.; Yang, X. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; pp. 1–170. [Google Scholar]
- International Diabetes Federation. IDF DIABETES ATLAS 9th Edition 2019. Available online: https://diabetesatlas.org/en/ (accessed on 22 December 2020).
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. (Lausanne) 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Blaslov, K.; Naranda, F.S.; Kruljac, I.; Renar, I.P. Treatment approach to type 2 diabetes: Past, present and future. World J. Diabetes 2018, 9, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Tripathi, D.N.; Kumar, A.; Singh, S. Oxidative stress and inflammation in diabetic complications. Int. J. Endocrinol 2014, 2014, 679754. [Google Scholar] [CrossRef] [PubMed]
- Pickering, R.J.; Rosado, C.J.; Sharma, A.; Buksh, S.; Tate, M.; de Haan, J.B. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin. Transl. Immunol. 2018, 7, e1016. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schurmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szucs, G.; Attieh, Z.; Murlasits, Z.; Torok, S.; Posa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 9051426. [Google Scholar] [CrossRef]
- Van de Venter, M.; Boukes, G.; Venables, L.; Dambuza, N.; Koekemoer, T. The Need for an Integrated, Multi-target In vitro Anti-diabetic Screening Platform. In Drug Discovery from Herbs: Approaches and Applications; Daya Publishing House: New Delhi, India, 2017. [Google Scholar]
- Sneddon, L.U.; Halsey, L.G.; Bury, N.R. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 2017, 220 Pt 17, 3007–3016. [Google Scholar] [CrossRef]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M.; et al. The use of zebrafish (Danio rerio) as biomedical models. Anim Front. 2019, 9, 68–77. [Google Scholar] [CrossRef]
- Nishimura, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit Anom (Kyoto) 2016, 56, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Alderton, W.K. Zebrafish in pharmaceutical industry research: Finding the best fit. Drug Discov. Today Dis. Models 2013, 10, e43–e50. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Strahle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Conteh, A.M.; Sorrell, C.A.; Mirmira, A.; Tersey, S.A.; Mirmira, R.G.; Linnemann, A.K.; Anderson, R.M. An In Vivo Zebrafish Model for Interrogating ROS-Mediated Pancreatic beta-Cell Injury, Response, and Prevention. Oxid. Med. Cell. Longev. 2018, 2018, 1324739. [Google Scholar] [CrossRef]
- Kinkel, M.D.; Prince, V.E. On the diabetic menu: Zebrafish as a model for pancreas development and function. Bioessays 2009, 31, 139–152. [Google Scholar] [CrossRef]
- Jorgens, K.; Hillebrands, J.L.; Hammes, H.P.; Kroll, J. Zebrafish: A model for understanding diabetic complications. Exp. Clin. Endocrinol. Diabetes 2012, 120, 186–187. [Google Scholar] [CrossRef]
- Seth, A.; Stemple, D.L.; Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis Model. Mech 2013, 6, 1080–1088. [Google Scholar] [CrossRef]
- Tabassum, N.; Tai, H.; Jung, D.W.; Williams, D.R. Fishing for Nature’s Hits: Establishment of the Zebrafish as a Model for Screening Antidiabetic Natural Products. Evid. Based Complement. Altern. Med. 2015, 2015, 287847. [Google Scholar] [CrossRef]
- Schlegel, A.; Gut, P. Metabolic insights from zebrafish genetics, physiology, and chemical biology. Cell. Mol. Life Sci. 2015, 72, 2249–2260. [Google Scholar] [CrossRef]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef] [PubMed]
- Heckler, K.; Kroll, J. Zebrafish as a Model for the Study of Microvascular Complications of Diabetes and Their Mechanisms. Int. J. Mol. Sci. 2017, 18, 2002. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Benson, S.A. Inconsistent ethical regulation of larval zebrafish in research. J. Fish. Biol. 2020, 97, 324–327. [Google Scholar] [CrossRef]
- Hernandez, R.E.; Galitan, L.; Cameron, J.; Goodwin, N.; Ramakrishnan, L. Delay of Initial Feeding of Zebrafish Larvae Until 8 Days Postfertilization Has No Impact on Survival or Growth Through the Juvenile Stage. Zebrafish 2018, 15, 515–518. [Google Scholar] [CrossRef]
- The Zebrafish Information Network. ZFIN: Wild-Type Lines: Summary Listing. Available online: https://zfin.org/action/feature/wildtype-list (accessed on 22 December 2020).
- Sarmah, S.; Marrs, J.A. Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function. Int. J. Mol. Sci. 2016, 17, 2123. [Google Scholar] [CrossRef]
- Brown, D.R.; Samsa, L.A.; Qian, L.; Liu, J. Advances in the Study of Heart Development and Disease Using Zebrafish. J. Cardiovasc. Dev. Dis. 2016, 3, 13. [Google Scholar] [CrossRef]
- Staudt, D.; Stainier, D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu. Rev. Genet. 2012, 46, 397–418. [Google Scholar] [CrossRef]
- Glickman, N.S.; Yelon, D. Cardiac development in zebrafish coordination of form and function. Semin. Cell Dev. Biol. 2002, 13, 507–513. [Google Scholar] [CrossRef]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef]
- Ahuja, N.; Ostwald, P.; Bark, D.; Garrity, D. Biomechanical Cues Direct Valvulogenesis. J. Cardiovasc. Dev. Dis. 2020, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Lieberth, J.; Tillman, S.; Natalizio, J.; Bloomekatz, J. Using Zebrafish to Analyze the Genetic and Environmental Etiologies of Congenital Heart Defects. Adv. Exp. Med. Biol. 2020, 1236, 189–223. [Google Scholar] [PubMed]
- Scherz, P.J.; Huisken, J.; Sahai-Hernandez, P.; Stainier, D.Y. High-speed imaging of developing heart valves reveals interplay of morphogenesis and function. Development 2008, 135, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, B.; Tschoepe, D. Heart in diabetes: Not only a macrovascular disease. Diabetes Care 2011, 34 (Suppl. 2), S138–S144. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.; Vitale, C.; Seferovic, P. Heart Failure in Patients with Diabetes Mellitus. Card. Fail. Rev. 2017, 3, 52. [Google Scholar] [CrossRef]
- Wang, S.; Miller, S.R.; Ober, E.A.; Sadler, K.C. Making It New Again: Insight into Liver Development, Regeneration, and Disease from Zebrafish Research. Curr. Top. Dev. Biol. 2017, 124, 161–195. [Google Scholar]
- Wilkins, B.J.; Pack, M. Zebrafish models of human liver development and disease. Compr. Physiol. 2013, 3, 1213–1230. [Google Scholar]
- Goessling, W.; Sadler, K.C. Zebrafish: An important tool for liver disease research. Gastroenterology 2015, 149, 1361–1377. [Google Scholar] [CrossRef]
- Wu, J.; Lu, C.; Ge, S.; Mei, J.; Li, X.; Guo, W. Igf2bp1 is required for hepatic outgrowth during early liver development in zebrafish. Gene 2020, 744, 144632. [Google Scholar] [CrossRef]
- Field, H.A.; Ober, E.A.; Roeser, T.; Stainier, D.Y. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev. Biol. 2003, 253, 279–290. [Google Scholar] [CrossRef]
- Tao, T.; Peng, J. Liver development in zebrafish (Danio rerio). J. Genet. Genom. 2009, 36, 325–334. [Google Scholar] [CrossRef]
- Yi, X.; Yu, J.; Ma, C.; Li, L.; Luo, L.; Li, H.; Ruan, H.; Huang, H. Yap1/Taz are essential for the liver development in zebrafish. Biochem. Biophys. Res. Commun. 2018, 503, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef] [PubMed]
- Tolman, K.G.; Fonseca, V.; Dalpiaz, A.; Tan, M.H. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 2007, 30, 734–743. [Google Scholar] [CrossRef]
- Gnugge, L.; Meyer, D.; Driever, W. Pancreas Development in Zebrafish. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 76, pp. 531–551. [Google Scholar]
- Li, Z.; Wen, C.; Peng, J.; Korzh, V.; Gong, Z. Generation of living color transgenic zebrafish to trace somatostatin-expressing cells and endocrine pancreas organization. Differentiation 2009, 77, 128–134. [Google Scholar] [CrossRef]
- Prince, V.E.; Anderson, R.M.; Dalgin, G. Zebrafish Pancreas Development and Regeneration: Fishing for Diabetes Therapies. Curr. Top. Dev. Biol. 2017, 124, 235–276. [Google Scholar]
- Field, H.A.; Dong, P.D.S.; Beis, D.; Stainier, D.Y.R. Formation of the digestive system in zebrafish. ii. pancreas morphogenesis. Dev. Biol. 2003, 261, 197–208. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. Endocrinology. In The Physiology of Fishes; Evans, D.E., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 469–502. [Google Scholar]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef]
- Minchin, J.E.; Rawls, J.F. In vivo analysis of white adipose tissue in zebrafish. Methods Cell Biol. 2011, 105, 63–86. [Google Scholar]
- Imrie, D.; Sadler, K.C. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev. Dyn. 2010, 239, 3013–3023. [Google Scholar] [CrossRef]
- Elemans, L.M.H.; Cervera, I.P.; Riley, S.E.; Wafer, R.; Fong, R.; Tandon, P.; Minchin, J.E.N. Quantitative analyses of adiposity dynamics in zebrafish. Adipocyte 2019, 8, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, Z.; Lu, Q.; Chang, C.; Zhou, Z. Beyond Genetics: What Causes Type 1 Diabetes. Clin. Rev. Allergy Immunol. 2017, 52, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Hollander, P.A.; Kushner, P. Type 2 Diabetes Comorbidities and Treatment Challenges Rationale for DPP-4 Inhibitors. Postgrad. Med. 2010, 122, 71–80. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Nunes, S.; Rolo, A.P.; Reis, F.; Palmeira, C.M. Therapeutic Options Targeting Oxidative Stress, Mitochondrial Dysfunction and Inflammation to Hinder the Progression of Vascular Complications of Diabetes. Front. Physiol. 2019, 9, 1857. [Google Scholar] [CrossRef] [PubMed]
- Khneizer, G.; Rizvi, S.; Gawrieh, S. Non-alcoholic Fatty Liver Disease and Diabetes Mellitus. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2020; pp. 1–24. [Google Scholar]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Ji, M.G.; Kim, K.; Kim, U.J.; Kang, T.H. Synergistic Potentials of Coffee on Injured Pancreatic Islets and Insulin Action via KATP Channel Blocking in Zebrafish. J. Agric. Food Chem. 2015, 63, 5612–5621. [Google Scholar] [CrossRef]
- Lee, J.; Jung, D.W.; Kim, W.H.; Um, J.I.; Yim, S.H.; Oh, W.K.; Williams, D.R. Development of a highly visual, simple, and rapid test for the discovery of novel insulin mimetics in living vertebrates. ACS Chem. Biol. 2013, 8, 1803–1814. [Google Scholar] [CrossRef]
- Park, J.; Um, J.I.; Jo, A.; Lee, J.; Jung, D.W.; Williams, D.R.; Park, S.B. Impact of molecular charge on GLUT-specific cellular uptake of glucose bioprobes and in vivo application of the glucose bioprobe, GB2-Cy3. Chem. Commun. 2014, 50, 9251–9254. [Google Scholar] [CrossRef]
- Gut, P.; Baeza-Raja, B.; Andersson, O.; Hasenkamp, L.; Hsiao, J.; Hesselson, D.; Akassoglou, K.; Verdin, E.; Hirschey, M.D.; Stainier, D.Y. Whole-organism screening for gluconeogenesis identifies activators of fasting metabolism. Nat. Chem. Biol. 2013, 9, 97–104. [Google Scholar] [CrossRef]
- Jurczyk, A.; Roy, N.; Bajwa, R.; Gut, P.; Lipson, K.; Yang, C.; Covassin, L.; Racki, W.J.; Rossini, A.A.; Phillips, N.; et al. Dynamic glucoregulation and mammalian-like responses to metabolic and developmental disruption in zebrafish. Gen. Comp. Endocrinol. 2011, 170, 334–345. [Google Scholar] [CrossRef]
- Elo, B.; Villano, C.M.; Govorko, D.; White, L.A. Larval zebrafish as a model for glucose metabolism: Expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J. Mol. Endocrinol. 2007, 38, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar]
- Vargas, F.; Rivas, C.; Medrano, M. Interaction of emodin, aloe-emodin, and rhein with human serum albumin: A fluorescence spectroscopic study. Toxicol. Mech. Methods 2004, 14, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, S.J.; Hwang, J.H.; Lee, H.Y.; Ryu, M.J.; Park, J.; Kim, S.J.; Jo, Y.S.; Kim, Y.K.; Lee, C.H.; et al. Antidiabetic and antiobesity effects of Ampkinone (6f), a novel small molecule activator of AMP-activated protein kinase. J. Med. Chem. 2010, 53, 7405–7413. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Kang, M.-C.; Lee, J.-H.; Kang, N.; Lee, W.; Oh, J.-Y.; Yang, H.-W.; Lee, J.-S.; Jeon, Y.-J. Protective effect of marine brown algal polyphenols against oxidative stressed zebrafish with high glucose. RSC Adv. 2015, 5, 25738–25746. [Google Scholar] [CrossRef]
- Walker, S.L.; Ariga, J.; Mathias, J.R.; Coothankandaswamy, V.; Xie, X.; Distel, M.; Koster, R.W.; Parsons, M.J.; Bhalla, K.N.; Saxena, M.T.; et al. Automated reporter quantification in vivo: High-throughput screening method for reporter-based assays in zebrafish. PLoS ONE 2012, 7, e29916. [Google Scholar] [CrossRef]
- Félix, L.M.; Vidal, A.M.; Serafim, C.; Valentim, A.M.; Antunes, L.M.; Campos, S.; Matos, M.; Monteiro, S.M.; Coimbra, A.M. Ketamine-induced oxidative stress at different developmental stages of zebrafish (Danio rerio) embryos. RSC Adv. 2016, 6, 61254–61266. [Google Scholar] [CrossRef]
- Sapp, V.; Gaffney, L.; EauClaire, S.F.; Matthews, R.P. Fructose leads to hepatic steatosis in zebrafish that is reversed by mechanistic target of rapamycin (mTOR) inhibition. Hepatology 2014, 60, 1581–1592. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Chen, R.D.; Lee, J.R.; Liu, S.T.; Lee, S.J.; Hwang, P.P. Specific expression and regulation of glucose transporters in zebrafish ionocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R275–R290. [Google Scholar] [CrossRef]
- Jimenez-Amilburu, V.; Jong-Raadsen, S.; Bakkers, J.; Spaink, H.P.; Marin-Juez, R. GLUT12 deficiency during early development results in heart failure and a diabetic phenotype in zebrafish. J. Endocrinol. 2015, 224, 1–15. [Google Scholar] [CrossRef]
- Okamoto, H.; Cavino, K.; Na, E.; Krumm, E.; Kim, S.Y.; Cheng, X.; Murphy, A.J.; Yancopoulos, G.D.; Gromada, J. Glucagon receptor inhibition normalizes blood glucose in severe insulin-resistant mice. Proc. Natl. Acad. Sci. USA 2017, 114, 2753–2758. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wang, M.Y.; Yu, X.X.; Unger, R.H. Glucagon is the key factor in the development of diabetes. Diabetologia 2016, 59, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Mourabit, S.; Fitzgerald, J.A.; Ellis, R.P.; Takesono, A.; Porteus, C.S.; Trznadel, M.; Metz, J.; Winter, M.J.; Kudoh, T.; Tyler, C.R. New insights into organ-specific oxidative stress mechanisms using a novel biosensor zebrafish. Environ. Int. 2019, 133 Pt A, 105138. [Google Scholar] [CrossRef] [PubMed]
- Gyimah, E.; Dong, X.; Qiu, W.; Zhang, Z.; Xu, H. Sublethal concentrations of triclosan elicited oxidative stress, DNA damage, and histological alterations in the liver and brain of adult zebrafish. Environ. Sci. Pollut. Res. Int. 2020, 27, 17329–17338. [Google Scholar] [CrossRef] [PubMed]
- Sobhi, W.; Khenchouche, A. Involvement of Oxidative Stress in Type 1 Diabetes. Am. J. Biomed. Sci. Res. 2020, 6, 538–543. [Google Scholar] [CrossRef]
- Velki, M.; Lackmann, C.; Barranco, A.; Ereño Artabe, A.; Rainieri, S.; Hollert, H.; Seiler, T.-B. Pesticides diazinon and diuron increase glutathione levels and affect multixenobiotic resistance activity and biomarker responses in zebrafish (Danio rerio) embryos and larvae. Environ. Sci. Eur. 2019, 31. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Flores, J.M.M.; Gonzalez, A.M.N. Anti-inflammatory effect of procumbenoside B from Justicia spicigera on lipopolysaccharide-stimulated RAW 264.7 macrophages and zebrafish model. Pharmacogn. Res. 2018, 10, 218–224. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Tingaud-Sequeira, A.; Ouadah, N.; Babin, P.J. Zebrafish obesogenic test: A tool for screening molecules that target adiposity. J. Lipid Res. 2011, 52, 1765–1772. [Google Scholar] [CrossRef]
- den Broeder, M.J.; Moester, M.J.B.; Kamstra, J.H.; Cenijn, P.H.; Davidoiu, V.; Kamminga, L.M.; Ariese, F.; de Boer, J.F.; Legler, J. Altered Adipogenesis in Zebrafish Larvae Following High Fat Diet and Chemical Exposure Is Visualised by Stimulated Raman Scattering Microscopy. Int. J. Mol. Sci. 2017, 18, 894. [Google Scholar] [CrossRef]
- Faillaci, F.; Milosa, F.; Critelli, R.M.; Turola, E.; Schepis, F.; Villa, E. Obese zebrafish: A small fish for a major human health condition. Anim. Model. Exp. Med. 2018, 1, 255–265. [Google Scholar] [CrossRef]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Shimada, Y.; Wang, Z.; Umemoto, N.; Kuroyanagi, J.; Nishimura, N.; Tanaka, T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meguro, S.; Hasumura, T.; Hase, T. Body fat accumulation in zebrafish is induced by a diet rich in fat and reduced by supplementation with green tea extract. PLoS ONE 2015, 10, e0120142. [Google Scholar] [CrossRef] [PubMed]
- Forn-Cuni, G.; Varela, M.; Fernandez-Rodriguez, C.M.; Figueras, A.; Novoa, B. Liver immune responses to inflammatory stimuli in a diet-induced obesity model of zebrafish. J. Endocrinol. 2015, 224, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Turola, E.; Petta, S.; Vanni, E.; Milosa, F.; Valenti, L.; Critelli, R.; Miele, L.; Maccio, L.; Calvaruso, V.; Fracanzani, A.L.; et al. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis. Dis. Model. Mech. 2015, 8, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Rajapriya, R.; Veena, V.; Kumaresan, G. High Cholesterol Diet Induces Obesity in Zebrafish. Int. J. Adv. Sci. Eng. 2016, 2, 197–201. [Google Scholar]
- Zang, L.; Shimada, Y.; Nishimura, N. Development of a Novel Zebrafish Model for Type 2 Diabetes Mellitus. Sci. Rep. 2017, 7, 1461. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, K.; Schuster, S.; Meusel, A.; Garten, A.; Riemer, T.; Schleinitz, D.; Kiess, W.; Korner, A. Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol. 2017, 17, 4. [Google Scholar] [CrossRef]
- Clifton, J.D.; Lucumi, E.; Myers, M.C.; Napper, A.; Hama, K.; Farber, S.A.; Smith, A.B., 3rd; Huryn, D.M.; Diamond, S.L.; Pack, M. Identification of novel inhibitors of dietary lipid absorption using zebrafish. PLoS ONE 2010, 5, e12386. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Y.Q.; Guo, S.Y.; Li, C.Q. Rapid analysis of hypolipidemic drugs in a live zebrafish assay. J. Pharm. Toxicol. Methods 2015, 72, 47–52. [Google Scholar] [CrossRef]
- Schlegel, A.; Stainer, D.Y. Microsomal triglyceride transfer protein is required for yolk lipid utilization and absorption of dietary lipids in zebrafish larvae. Biochemistry 2006, 45, 15179–15787. [Google Scholar] [CrossRef]
- Den Dekker, A.; Davis, F.M.; Kunkel, S.L.; Gallagher, K.A. Targeting epigenetic mechanisms in diabetic wound healing. Transl. Res. 2019, 204, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.A.; Lattimer, S.A.; Sima, A.A.F. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N. Engl. J. Med. 1987, 316, 599–606. [Google Scholar] [PubMed]
- Lee, T.; Saltsman, K.A.; Ohashi, H.; King, G.L. Activation of protein kinase C by elevation of glucose concentration: Proposal for a mechanism in the development of diabetic vascular complications. Proc. Natl. Acad. Sci. USA 1989, 86, 5141–5145. [Google Scholar] [CrossRef]
- Lioupis, C. Effects of diabetes mellitus on wound healing: An update. J. Wound Care 2005, 14, 84–86. [Google Scholar] [CrossRef]
- Sarras, M.P., Jr. Genetic and chemically-induced Zebrafish models for the study of diabetes mellitus. MOJ Anat. Physiol. 2018, 5, 319–321. [Google Scholar] [CrossRef]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult zebrafish as a model system for cutaneous wound-healing research. J. Investig. Derm. 2013, 133, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Derm. 2018, 138, 2095.e1–2105.e1. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.S.; Sarras, M.P., Jr.; Intine, R.V. Limb regeneration is impaired in an adult zebrafish model of diabetes mellitus. Wound Repair Regen 2010, 18, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Intine, R.V.; Olsen, A.S.; Sarras, M.P., Jr. A zebrafish model of diabetes mellitus and metabolic memory. J. Vis. Exp. 2013, e50232. [Google Scholar] [CrossRef]
- Sarras, M.P., Jr.; Leontovich, A.A.; Olsen, A.S.; Intine, R.V. Impaired tissue regeneration corresponds with altered expression of developmental genes that persists in the metabolic memory state of diabetic zebrafish. Wound Repair Regen. 2013, 21, 320–328. [Google Scholar] [CrossRef]
- Pang, S.; Gao, Y.; Wang, F.; Wang, Y.; Cao, M.; Zhang, W.; Liang, Y.; Song, M.; Jiang, G. Toxicity of silver nanoparticles on wound healing: A case study of zebrafish fin regeneration model. Sci. Total Environ. 2020, 717, 137178. [Google Scholar] [CrossRef] [PubMed]
- Dane, P.J.; Tucker, J.B. Modulation of epidermal cell shaping and extracellular matrix during caudal fin morphogenesis in the zebra fish Brachydanio rerio. J. Embryol. Exp. Morph. 1985, 87, 145–161. [Google Scholar] [PubMed]
- Mateus, R.; Pereira, T.; Sousa, S.; de Lima, J.E.; Pascoal, S.; Saude, L.; Jacinto, A. In vivo cell and tissue dynamics underlying zebrafish fin fold regeneration. PLoS ONE 2012, 7, e51766. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef]
- Yoo, S.K.; Deng, Q.; Cavnar, P.J.; Wu, Y.I.; Hahn, K.M.; Huttenlocher, A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev. Cell 2010, 18, 226–236. [Google Scholar] [CrossRef]
- LeBert, D.C.; Squirrell, J.M.; Rindy, J.; Broadbridge, E.; Lui, Y.; Zakrzewska, A.; Eliceiri, K.W.; Meijer, A.H.; Huttenlocher, A. Matrix metalloproteinase 9 modulates collagen matrices and wound repair. Development 2015, 142, 2136–2146. [Google Scholar] [CrossRef]
- Miskolci, V.; Squirrell, J.; Rindy, J.; Vincent, W.; Sauer, J.D.; Gibson, A.; Eliceiri, K.W.; Huttenlocher, A. Distinct inflammatory and wound healing responses to complex caudal fin injuries of larval zebrafish. Elife 2019, 8, e45976. [Google Scholar] [CrossRef]
- Beckman, S. Analyzing Wound Healing in Zebrafish Embryos; BioTek Instruments, Inc.: Winooski, VT, USA, 2019; pp. 1–6. [Google Scholar]
- Martin, P.; Feng, Y. Wound healing in zebrafish. Nature 2009, 459, 921–923. [Google Scholar] [CrossRef]
- Yoo, S.K.; Lam, P.Y.; Eichelberg, M.R.; Zasadil, L.; Bement, W.M.; Huttenlocher, A. The role of microtubules in neutrophil polarity and migration in live zebrafish. J. Cell Sci. 2012, 125 Pt 23, 5702–5710. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Y.M.; Zhang, J.P. Comparative Study of Different Diets-Induced NAFLD Models of Zebrafish. Front. Endocrinol. (Lausanne) 2018, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.; Wang, B.; Ma, K.; Deng, Y.; Li, M.; Yang, L.; Yang, Y.; Zhao, J.; Cheng, L.; Zhou, Q.; et al. Lipid Modulating Anti-oxidant Stress Activity of Gastrodin on Nonalcoholic Fatty Liver Disease Larval Zebrafish Model. Int. J. Mol. Sci. 2019, 20, 1984. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yin, H.; Li, M.; Deng, Y.; Ahmad, O.; Qin, G.; He, Q.; Li, J.; Gao, K.; Zhu, J.; et al. A Comprehensive Study of High Cholesterol Diet-Induced Larval Zebrafish Model: A Short-Time In Vivo Screening Method for Non-Alcoholic Fatty Liver Disease Drugs. Int. J. Biol. Sci. 2019, 15, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Dellambra, E.; Odorisio, T.; D’Arcangelo, D.; Failla, C.M.; Facchiano, A. Non-animal models in dermatological research. ALTEX 2019, 36, 177–202. [Google Scholar] [CrossRef]
- Aman, A.J.; Parichy, D.M. Zebrafish Integumentary System. In The Zebrafish in Biomedical Research; Academic Press: Cambridge, MA, USA, 2020; pp. 91–96. [Google Scholar]
| Target | Larval Age | Treatment or Positive Controls | Detection | References |
|---|---|---|---|---|
| Pancreatic function | 5 dpf | Alloxan to destroy beta cells | Fluorescent staining with 2-NBDG to measure islet size | [63] |
| Glucose uptake | 3 dpf | Insulin mimetics, e.g., emodin * | 2-NBDG or GB2-Cy3 fluorescence in the eye or flourimetry on homogenized larvae | [64,65] |
| Glucose production (gluconeogenesis) | From 4 dpf when yolk depletes | Gluconeogenesis stimulated with glucocorticoids, cAMP, isoprenaline | Glucose oxidase assay with sensitive fluorescence detection | [66,67] |
| PEPCK expression levels (gluconeogenesis) | 4 dpf | PEPCK levels increased with glucocorticoids and cAMP | Quantitative RT-PCR | [68] |
| Target | Larval Age | Treatment or Positive Controls | Detection | References |
|---|---|---|---|---|
| ROS levels | 4–106 hpf | Metronidazole (inducer of ROS) or high glucose/fructose concentration or H2O2 Resveratrol or N-acetyl-l-cysteine as inhibitors | Fluorimetry or fluorescence microscopy after staining with CellROX Green or DCFH-DA Immunofluorescent staining of insulin or glucagon can be used to locate pancreas | [18,72,73] |
| NOS levels | 4 dpf | As above | As above, but staining with DAF-FM DA | [72] |
| Expression levels of genes involved in inflammation and oxidative stress (including iNOS, COX-2 and others) | 5–7 dpf | High glucose/fructose concentrations | Quantitative RT-PCR | [72,74,75] |
| Target | Larval Age | Treatment or Positive Controls | Detection | References |
|---|---|---|---|---|
| Inhibition of dietary lipid absorption | 4–6 dpf | Ezetimibe used as inhibitor | Fluorescently stained with PED-6, NBD-cholesterol, BODIPY-C5, BODIPY-C16, and BODIPY-C12 | [96,98] |
| Intestinal lipid absorption, hyperlipidemia, lipid accumulation in vasculature and whole-larval TAG | 5–6 dpf | Fed with 1% egg yolkEzetimibe, lovastatin and simvastatin used as inhibitors | Oil Red O staining | [96,97] |
| Lipid Green staining | [87] | |||
| 6 dpf | Heavy whipping cream | Oil Red O staining | [98] | |
| Size dependent (standard length of 7.5–9 mm) | Hard-boiled chicken egg yolk (55% total fat content)Rosiglitazone, phenylephrine and tributyltin chloride used as inhibitors | Nile Red staining | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van de Venter, M.; Didloff, J.; Reddy, S.; Swanepoel, B.; Govender, S.; Dambuza, N.S.; Williams, S.; Koekemoer, T.C.; Venables, L. Wild-Type Zebrafish (Danio rerio) Larvae as a Vertebrate Model for Diabetes and Comorbidities: A Review. Animals 2021, 11, 54. https://doi.org/10.3390/ani11010054
van de Venter M, Didloff J, Reddy S, Swanepoel B, Govender S, Dambuza NS, Williams S, Koekemoer TC, Venables L. Wild-Type Zebrafish (Danio rerio) Larvae as a Vertebrate Model for Diabetes and Comorbidities: A Review. Animals. 2021; 11(1):54. https://doi.org/10.3390/ani11010054
Chicago/Turabian Stylevan de Venter, Maryna, Jenske Didloff, Shanika Reddy, Bresler Swanepoel, Sharlene Govender, Ntokozo Shirley Dambuza, Saralene Williams, Trevor Craig Koekemoer, and Luanne Venables. 2021. "Wild-Type Zebrafish (Danio rerio) Larvae as a Vertebrate Model for Diabetes and Comorbidities: A Review" Animals 11, no. 1: 54. https://doi.org/10.3390/ani11010054
APA Stylevan de Venter, M., Didloff, J., Reddy, S., Swanepoel, B., Govender, S., Dambuza, N. S., Williams, S., Koekemoer, T. C., & Venables, L. (2021). Wild-Type Zebrafish (Danio rerio) Larvae as a Vertebrate Model for Diabetes and Comorbidities: A Review. Animals, 11(1), 54. https://doi.org/10.3390/ani11010054






