Fat Encapsulation Reduces Diarrhea in Piglets Partially by Repairing the Intestinal Barrier and Improving Fatty Acid Transport
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets
2.2. Animals and Experimental Design
2.3. Data Collection
2.4. Chemical Analyses
2.5. RNA Extraction and Relative Qualification of mRNA
2.6. Western Blotting Analysis
2.7. Statistical Analysis
3. Results
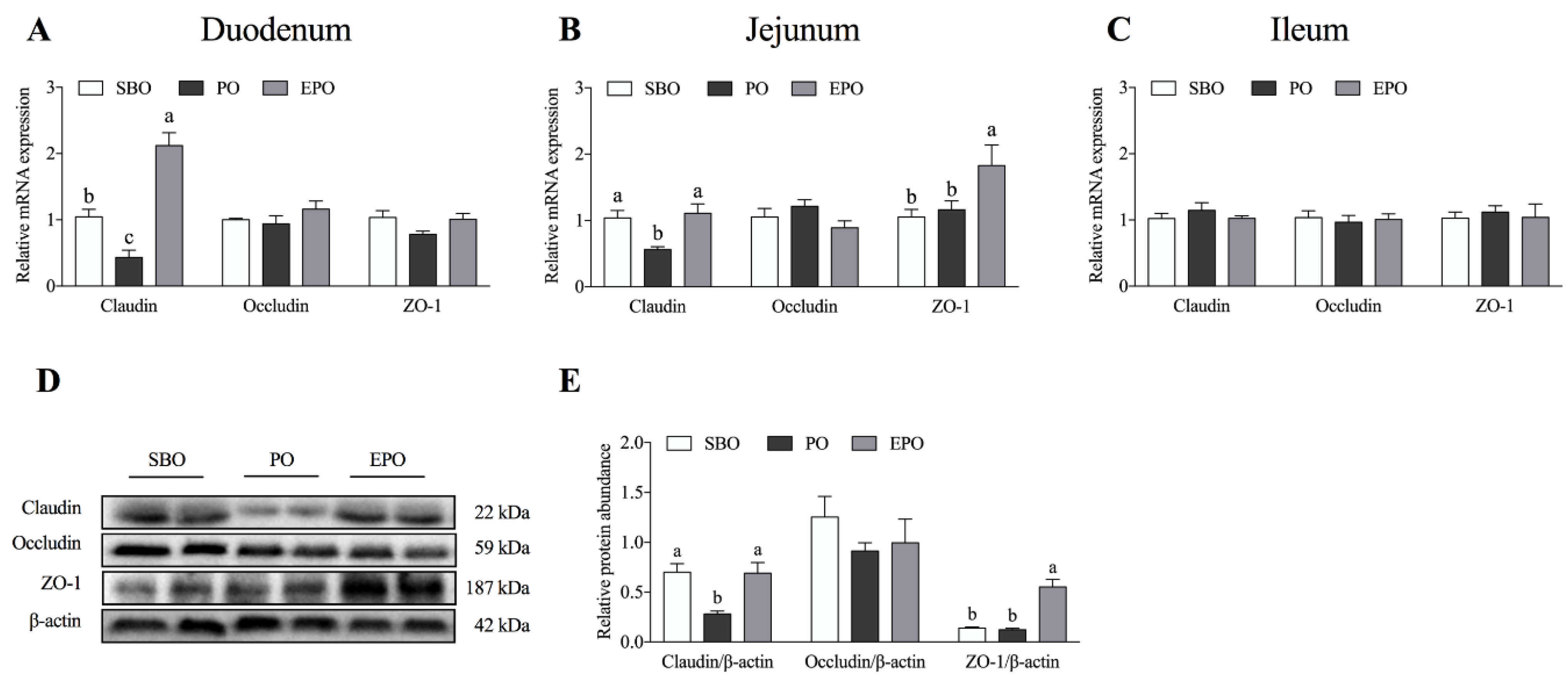
3.1. mRNA Expression and Protein Abundance of Tight Junction Proteins in the Intestine
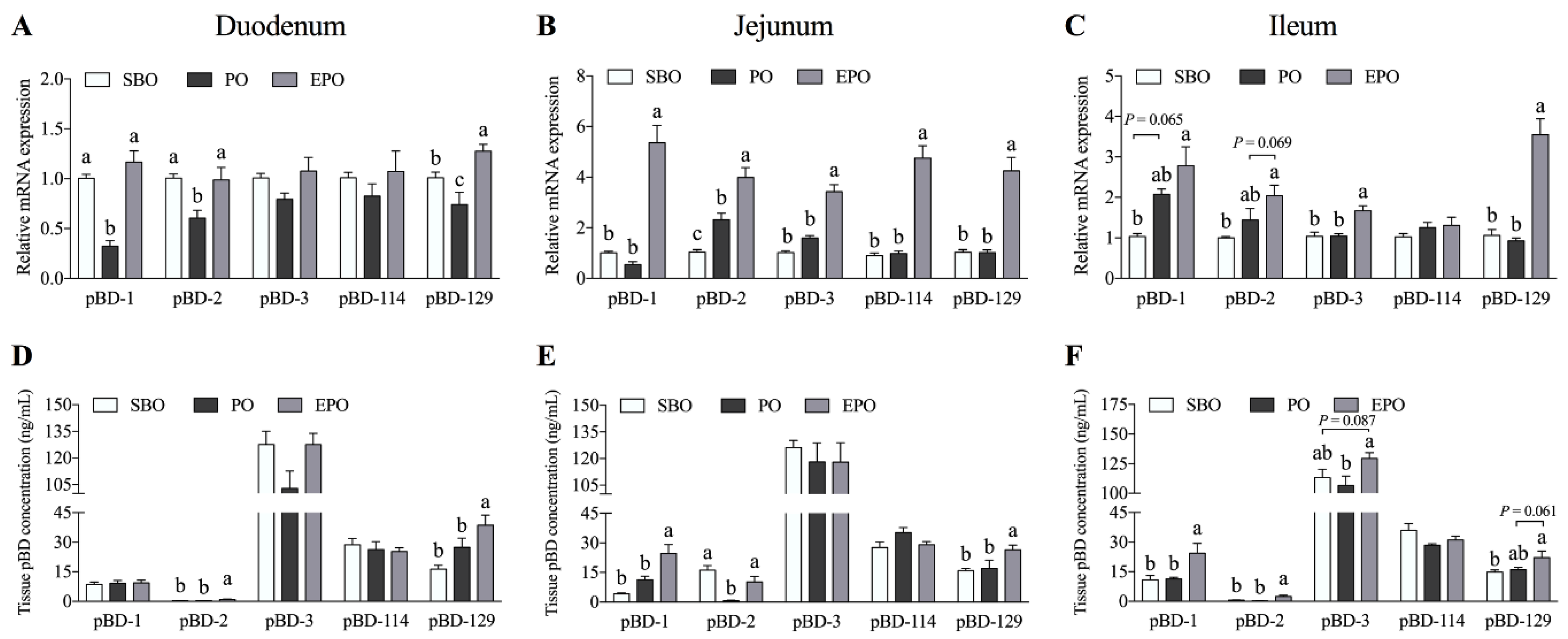
3.2. Secretion of β-defensin in the Small Intestine of Piglets.
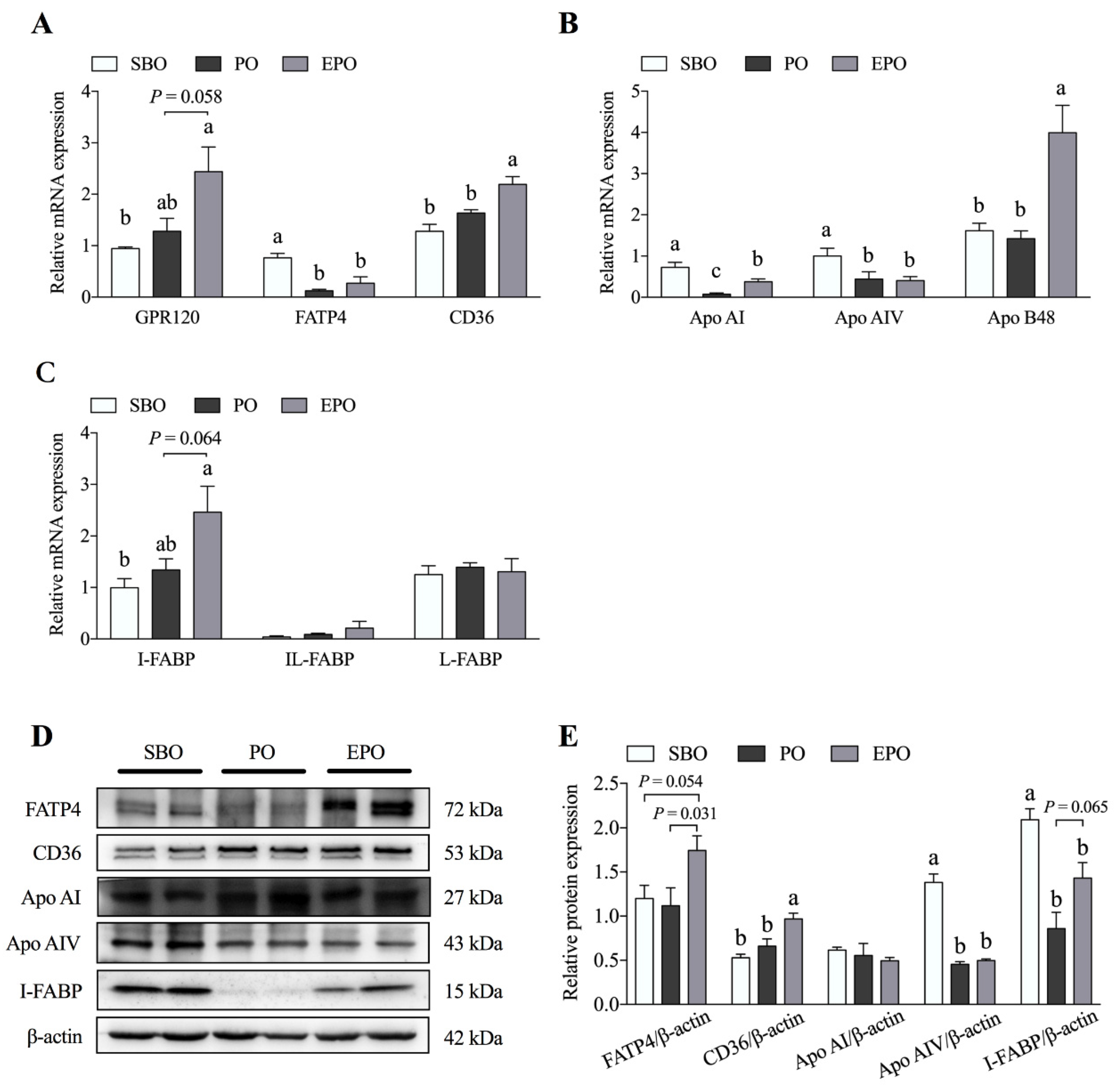
3.3. Fatty Acid Transport System in the Jejunum Tissues
3.4. AMPK/mTORC1 and AMPK/Sirt1/NF-κB Pathways in the Jejunum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P.; Boudry, G.; Favier, C.; Le Floc’H, N.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Wang, X.; Andersson, R. Role of intestinal permeability in monitoring mucosal barrier function. History, methodology, and significance of pathophysiology. Dig. Surg. 1998, 15, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Blikslager, A.T.; Moeser, A.J.; Gookin, J.L.; Jones, S.L.; Odle, J. Restoration of Barrier Function in Injured Intestinal Mucosa. Physiol. Rev. 2007, 87, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Steubesand, N.; Kiehne, K.; Brunke, G.; Pahl, R.; Reiss, K.; Herzig, K.-H.; Schubert, S.; Schreiber, S.; Fölsch, U.R.; Rosenstiel, P. The expression of the β-defensins hBD-2 and hBD-3 is differentially regulated by NF-κB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. BMC. Immunol. 2009, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, B.C.; Lim, K.J.; Paik, J.H.; Kwon, Y.K.; Shin, S.W.; Kim, S.C.; Jung, T.Y.; Kwon, T.K.; Cho, J.W.; Baek, W.K. Up-regulation of human β-defensin 2 by interleukin-1β in A549 cells: Involvement of PI3K, PKC, p38 MAPK, JNK, and NF-κB. Biochem. Biophys. Res. Commun. 2004, 320, 1026–1033. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K. SIRT1 longevity factor suppresses NF-κB-driven immune responses: Regulation of aging via NF-κB acetylation? Bioessays 2008, 30, 939–942. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Huuskonen, J.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K. Interaction of aging-associated signaling cascades: Inhibition of NF-κB signaling by longevity factors FoxOs and SIRT1. Cell. Mol. life. Sci. 2008, 65, 1049–1058. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N.; Lin, S.-C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.-P.; Hulea, L.; Larsson, O.; Pollak, M.; St-Pierre, J.; Topisirovic, I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 2015, 14, 473–480. [Google Scholar] [CrossRef] [Green Version]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Ren, C.; Wang, Y.; Lin, X.; Song, H.; Zhou, Q.; Xu, W.; Shi, K.; Chen, J.; Song, J.; Chen, F.; et al. A Combination of Formic Acid and Monolaurin Attenuates Enterotoxigenic Escherichia coli Induced Intestinal Inflammation in Piglets by Inhibiting the NF-κB/MAPK Pathways with Modulation of Gut Microbiota. J. Agric. Food. Chem. 2020, 68, 4155–4165. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Arlington, VA, USA, 2006. [Google Scholar]
- Thiex, N.J.; Anderson, S.; Gildemeister, B. Crude fat, hexanes extraction, in feed, cereal grain, and forage (Randall/Soxtec/submersion method): Collaborative study. J. AOAC Int. 2003, 86, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.-W.; Cao, Y.; Kaplan, J.; Zhang, M.; Li, W.; Conroy, M.; Walker, W.A.; Shi, H.N. Duodenal Helminth Infection Alters Barrier Function of the Colonic Epithelium via Adaptive Immune Activation. Infect. Immun. 2011, 79, 2285–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nusrat, A.; Turner, J.R.; Madara, J.L. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: Nutrients, cytokines, and immune cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G851–G857. [Google Scholar] [CrossRef] [Green Version]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta BBA Biomembr. 2009, 1788, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs1. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res. 2008, 52, 885–897. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty Acids and Immune Function: Relevance to Inflammatory Bowel Diseases. Int. Rev. Immunol. 2009, 28, 506–534. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668, S50–S58. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Clausen, M.R. Short-Chain Fatty Acids in the Human Colon: Relation to Gastrointestinal Health and Disease. Scand. J. Gastroenterol. 1996, 31, 132–148. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Mariadason, J.M.; Barkla, D.H.; Gibson, P.R. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am. J. Physiol. Liver Physiol. 1997, 272, G705–G712. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Suzuki, Y.; Saito, Y. Butyrate reduces colonic paracellular permeability by enhancing PPARγ activation. Biochem. Biophys. Res. Commun. 2002, 293, 827–831. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions1. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Guillot, E.; Vaugelade, P.; Lemarchal, P.; Rerat, A. Intestinal absorption and liver uptake of medium-chain fatty acids in non-anaesthetized pigs. Br. J. Nutr. 1993, 69, 431–442. [Google Scholar] [CrossRef]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, S.K.; Odle, J. Nutritional Factors Influencing Intestinal Health of the Neonate. Adv. Nutr. 2012, 3, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Hontecillas, R.; Wannemeulher, M.J.; Zimmerman, D.R.; Hutto, D.L.; Wilson, J.H.; Ahn, D.U.; Bassaganya-Riera, J. Nutritional Regulation of Porcine Bacterial-Induced Colitis by Conjugated Linoleic Acid. J. Nutr. 2002, 132, 2019–2027. [Google Scholar] [CrossRef]
- Shirkey, T.W.; Siggers, R.H.; Goldade, B.G.; Marshall, J.K.; Drew, M.D.; Laarveld, B.; Van Kessel, A.G. Effects of Commensal Bacteria on Intestinal Morphology and Expression of Proinflammatory Cytokines in the Gnotobiotic Pig. Exp. Biol. Med. 2006, 231, 1333–1345. [Google Scholar] [CrossRef]
- Beguin, P.; Errachid, A.; Larondelle, Y.; Schneider, Y.-J. Effect of polyunsaturated fatty acids on tight junctions in a model of the human intestinal epithelium under normal and inflammatory conditions. Food Funct. 2013, 4, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish Oil Enhances Intestinal Integrity and Inhibits TLR4 and NOD2 Signaling Pathways in Weaned Pigs after LPS Challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Zhang, S.; Kim, S.W.; Ren, C.; Tian, M.; Cheng, L.; Song, J.; Chen, J.; Chen, F.; Guan, W. Fat encapsulation enhances dietary nutrients utilization and growth performance of nursery pigs. J. Anim. Sci. 2018, 96, 3337–3347. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Sunkara, L.T.; Jiang, W.; Bible, M.; Carter, S.; Ma, X.; Qiao, S.; Zhang, G. Induction of Porcine Host Defense Peptide Gene Expression by Short-Chain Fatty Acids and Their Analogs. PLoS ONE 2013, 8, e72922. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, N.; Xiong, J.; Wei, H.; Jiang, S.; Peng, J. Caprylic acid and nonanoic acid upregulate endogenous host defense peptides to enhance intestinal epithelial immunological barrier function via histone deacetylase inhibition. Int. Immunopharmacol. 2018, 65, 303–311. [Google Scholar] [CrossRef]

| Genes | Accession | Direction | Sequences (5′–3′) | Size |
|---|---|---|---|---|
| Apo AI | NM_214398.1 | Forward | CGTGTATGTGGATGCGATCAAA | 104 |
| Reverse | CCCAGTTGTCCAGGAGTTTCAG | |||
| Apo AIV | AJ222966.1 | Forward | TCAACACCCAGGTTCAGCAG | 121 |
| Reverse | AACTCATCCGCATAGGGTGC | |||
| Apo B48 | AH001207.2 | Forward | GAGGTAACTGTGCCTGCATA | 436 |
| Reverse | CTGCACTAAGGTCACGATGT | |||
| GPR120 | HQ662564.1 | Forward | CTCTTCCTGCTCATGATCTCCTTC | 178 |
| Reverse | GTGACATGTTGTAGAGAATGGGGTT | |||
| FATP4 | XM_003353676.1 | Forward | AGCCGCATCCTGTCCTTT | 213 |
| Reverse | GACATCCTTGGCGATCTTTT | |||
| CD36 | DQ192230.1 | Forward | GGACTCAT TGCTGGTGCTGT | 169 |
| Reverse | GTCTGTAAACTTCCGTGCCTGT | |||
| I-FABP | NM_001031780.1 | Forward | GAGCTTGGTGTCACTTTTAACTAC | 192 |
| Reverse | CCTCTTGGCTTCTACTCCTTCATA | |||
| IL-FABP | NM_214215.2 | Forward | GGAGGTCTCCACTGTCGG | 115 |
| Reverse | CTGTTACACCAGGTTTATTTG | |||
| L-FABP | NM_001004046.2 | Forward | GAAGGGGAAGGACATCA | 138 |
| Reverse | CAGTCAGGGTCTCCATCTCACA | |||
| Claudin | NM_001244539.1 | Forward | TCTTTCTTATTTCAGGTCTGGCTATCT | 247 |
| Reverse | CCACTGGAAGGCGAAGGTTTT | |||
| Occludin | NM_001163647.2 | Forward | ACTGGCGGCGAGTCCTGCGACGAGC | 244 |
| Reverse | TATTGTATTCATCAGCAGCAGCCAT | |||
| ZO-1 | XM_021098856.1 | Forward | AAAGCCCTAAGTTCAATCACAATCT | 253 |
| Reverse | TCCTCATCTTCATCATCTTCTACAG | |||
| pBD-1 | NM_213838.1 | Forward | TGCCACAGGTGCCGATCT | 81 |
| Reverse | CTGTTAGCTGCTTAAGGAATAAAGGC | |||
| pBD-2 | NM_214442.2 | Forward | CCAGAGAGGTCCGACACTACA | 168 |
| Reverse | GGTCCCTTCAATCCTTGTAGGTGAA | |||
| pBD-3 | XM_021074698.1 | Forward | ACCAAGCACGCCTTCCTATC | 236 |
| Reverse | GCATTTTCGGCCACTCACAG | |||
| pBD-114 | NM_001129973.1 | Forward | TGTACCTTGGTGGATCCTGAACGA | 240 |
| Reverse | CGCCCTCTGAATGCAGCATATCTT | |||
| pBD-129 | NM_001129975.1 | Forward | CAAAGACCACTGTGCCGTGAATGA | 131 |
| Reverse | TTGATGCTGGCGAAAGGGTTGGTA | |||
| β-actin | 397563 | Forward | TGCGGGACATCAAGGAGAAG | 176 |
| Reverse | AGTTGAAGGTGGTCTCGTGG |
| Antibodies | Code No. | Company | kDa |
|---|---|---|---|
| Apo AI | ab64308 | Abcam | 27 |
| Apo AIV | ab239480 | Abcam | 43 |
| FATP4 | ab200353 | Abcam | 72 |
| CD36 | D161529 | Sangon | 53 |
| I-FABP | ab60272 | Abcam | 15 |
| Claudin | ab15098 | Abcam | 23 |
| Occludin | ab31721 | Abcam | 59 |
| ZO-1 | ab96587 | Abcam | 187 |
| AMPK | 2532S | Cell Signaling Technology | 62 |
| p-AMPK | 2535S | Cell Signaling Technology | 62 |
| NF-κB | 10745-1-AP | Proteintech Group | 65 |
| p-NF-κB | 3033S | Cell Signaling Technology | 65 |
| mTOR | 2983S | Cell Signaling Technology | 289 |
| p-mTOR | 5536S | Cell Signaling Technology | 289 |
| p70 S6K | 9202S | Cell Signaling Technology | 70, 85 |
| p-p70 S6K | 9234S | Cell Signaling Technology | 70, 85 |
| 4EBP1 | ab2606 | Abcam | 20–21 |
| p-4EBP1 | 9451S | Cell Signaling Technology | 15–20 |
| β-actin | bs-0061R | Bioss | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Chen, J.; Wu, Z.; Song, H.; Yang, F.; Cui, C.; Chen, F.; Zhang, S.; Guan, W. Fat Encapsulation Reduces Diarrhea in Piglets Partially by Repairing the Intestinal Barrier and Improving Fatty Acid Transport. Animals 2021, 11, 28. https://doi.org/10.3390/ani11010028
Tian M, Chen J, Wu Z, Song H, Yang F, Cui C, Chen F, Zhang S, Guan W. Fat Encapsulation Reduces Diarrhea in Piglets Partially by Repairing the Intestinal Barrier and Improving Fatty Acid Transport. Animals. 2021; 11(1):28. https://doi.org/10.3390/ani11010028
Chicago/Turabian StyleTian, Min, Jiaming Chen, Zhihui Wu, Hanqing Song, Fei Yang, Chang Cui, Fang Chen, Shihai Zhang, and Wutai Guan. 2021. "Fat Encapsulation Reduces Diarrhea in Piglets Partially by Repairing the Intestinal Barrier and Improving Fatty Acid Transport" Animals 11, no. 1: 28. https://doi.org/10.3390/ani11010028
APA StyleTian, M., Chen, J., Wu, Z., Song, H., Yang, F., Cui, C., Chen, F., Zhang, S., & Guan, W. (2021). Fat Encapsulation Reduces Diarrhea in Piglets Partially by Repairing the Intestinal Barrier and Improving Fatty Acid Transport. Animals, 11(1), 28. https://doi.org/10.3390/ani11010028









