Accumulation of C-CTX1 in Muscle Tissue of Goldfish (Carassius auratus) by Dietary Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Fish Species
2.2. Maintenance of Goldfish
2.3. Experimental Model
2.4. Food Preparation
2.5. Toxic Dietary Exposure
2.6. Extraction of CTX from Experimental Fish Flesh
2.7. Determination
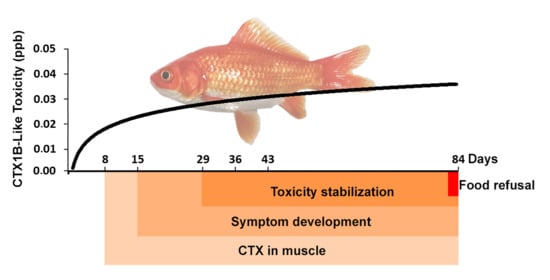
3. Results
3.1. Symptomatology and Fish Behavior
3.2. CTX Accumulation Profile in Goldfish Muscle
4. Discussion
4.1. Behavioral Disturbances and Signs of Intoxication
4.2. CTX Accumulation in Muscle Tissue
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholson, G.M.; Lewis, R.J. Ciguatoxins: Cyclic Polyether Modulators of Voltage-gated Iion Channel Function. Mar. Drugs 2006, 4, 82–118. [Google Scholar] [CrossRef]
- Lewis, R.J.; Inserra, M.; Vetter, I.; Holland, W.C.; Hardison, D.R.; Tester, P.A.; Litaker, R.W. Rapid Extraction and Identification of Maitotoxin and Ciguatoxin-Like Toxins from Caribbean and Pacific Gambierdiscus Using a New Functional Bioassay. PLoS ONE 2016, 11, e0160006. [Google Scholar] [CrossRef] [PubMed]
- FAO. Report of the Expert Meeting on Ciguatera Poisoning, Rome, Italy, 19–23 November 2018. Food Safety and Quality Series 9; FAO: Rome, Italy, 2020. [Google Scholar]
- Soliño, L.; Costa, P.R. Differential toxin profiles of ciguatoxins in marine organisms: Chemistry, fate and global distribution. Off. J. Int. Soc. Toxinol. 2018. [Google Scholar] [CrossRef]
- Perez-Arellano, J.L.; Luzardo, O.P.; Perez Brito, A.; Hernandez Cabrera, M.; Zumbado, M.; Carranza, C.; Angel-Moreno, A.; Dickey, R.W.; Boada, L.D. Ciguatera fish poisoning, Canary Islands. Emerg. Infect. Dis. 2005, 11, 1981–1982. [Google Scholar] [CrossRef] [PubMed]
- Boada, L.D.; Zumbado, M.; Luzardo, O.P.; Almeida-Gonzalez, M.; Plakas, S.M.; Granade, H.R.; Abraham, A.; Jester, E.L.; Dickey, R.W. Ciguatera fish poisoning on the West Africa Coast: An emerging risk in the Canary Islands (Spain). Off. J. Int. Soc. Toxinol. 2010, 56, 1516–1519. [Google Scholar] [CrossRef]
- Otero, P.; Perez, S.; Alfonso, A.; Vale, C.; Rodriguez, P.; Gouveia, N.N.; Gouveia, N.; Delgado, J.; Vale, P.; Hirama, M.; et al. First toxin profile of ciguateric fish in Madeira Arquipelago (Europe). Anal. Chem. 2010, 82, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Suárez, F.C.; Ramírez, A.S.; Acosta, F. Ciguatera, an emerging human poisoning in Europe. J. Aquac. Mar. Biol. 2015, 3, 53. [Google Scholar]
- Sanchez-Henao, J.A.; García-Álvarez, N.; Fernández, A.; Saavedra, P.; Silva Sergent, F.; Padilla, D.; Acosta-Hernández, B.; Martel Suárez, M.; Diogène, J.; Real, F. Predictive score and probability of CTX-like toxicity in fish samples from the official control of ciguatera in the Canary Islands. Sci. Total Environ. 2019, 673, 576–584. [Google Scholar] [CrossRef]
- Kibler, S.R.; Davenport, E.D.; Tester, P.A.; Hardison, D.R.; Holland, W.C.; Litaker, R.W. Gambierdiscus and Fukuyoa species in the greater Caribbean: Regional growth projections for ciguatera-associated dinoflagellates. Ecol. Model. 2017, 360, 204–218. [Google Scholar] [CrossRef]
- Banner, A.H.; Helfrich, P.; Piyakarnchana, T. Retention of Ciguatera Toxin by the Red Snapper. Lutjanus Bohar. 1966, 1966, 297. [Google Scholar]
- Dickey, R.W.; Plakas, S.M. Ciguatera: A public health perspective. Off. J. Int. Soc. Toxinol. 2010, 56, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Holmes, M.J. Origin and transfer of toxins involved in ciguatera. Comp. Biochem. Physiol. C Comp. Pharm. Toxicol. 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gamb. Toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- Satake, M.; Morohashi, A.; Oguri, H.; Oishi, T.; Hirama, M.; Harada, N.; Yasumoto, T. The Absolute Configuration of Ciguatoxin. J. Am. Chem. Soc. 1997, 119, 11325–11326. [Google Scholar] [CrossRef]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Darius, H.T.; Ponton, D.; Revel, T.; Cruchet, P.; Ung, A.; Tchou Fouc, M.; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Off. J. Int. Soc. Toxinol. 2007, 50, 612–626. [Google Scholar] [CrossRef]
- Gaboriau, M.; Ponton, D.; Darius, H.T.; Chinain, M. Ciguatera fish toxicity in French Polynesia: Size does not always matter. Off. J. Int. Soc. Toxinol. 2014, 84, 41–50. [Google Scholar] [CrossRef]
- Bottein, M.Y.; Wang, Z.; Ramsdell, J.S. Toxicokinetics of the ciguatoxin P-CTX-1 in rats after intraperitoneal or oral administration. Toxicology 2011, 284, 1–6. [Google Scholar] [CrossRef]
- Dechraoui, M.Y.; Wacksman, J.J.; Ramsdell, J.S. Species selective resistance of cardiac muscle voltage gated sodium channels: Characterization of brevetoxin and ciguatoxin binding sites in rats and fish. Off. J. Int. Soc. Toxinol. 2006, 48, 702–712. [Google Scholar] [CrossRef]
- Soliño, L.; Costa, P.R. Global impact of ciguatoxins and ciguatera fish poisoning on fish, fisheries and consumers. Environ. Res. 2020, 182, 109111. [Google Scholar] [CrossRef]
- Benoit, E.; Legrand, A.M.; Dubois, J.M. Effects of ciguatoxin on current and voltage clamped frog myelinated nerve fibre. Off. J. Int. Soc. Toxinol. 1986, 24, 357–364. [Google Scholar] [CrossRef]
- Yamaoka, K.; Inoue, M.; Miyahara, H.; Miyazaki, K.; Hirama, M. A quantitative and comparative study of the effects of a synthetic ciguatoxin CTX3C on the kinetic properties of voltage-dependent sodium channels. Br. J. Pharmacol. 2004, 142, 879–889. [Google Scholar] [CrossRef]
- Helfrich, P.; Banner, A.H. Experimental Induction of Ciguatera Toxicity in Fish through Diet. Nature 1963, 197, 1025–1026. [Google Scholar] [CrossRef]
- Davin, W.T.; Kohler, C.C.; Tindall, D.R. Effects of Ciguatera Toxins on the Bluehead. Trans. Am. Fish. Soc. 1986, 115, 908–912. [Google Scholar] [CrossRef]
- Davin, W.T.; Kohler, C.C.; Tindall, D.R. Ciguatera Toxins Adversely Affect Piscivorous Fishes. Trans. Am. Fish. Soc. 1988, 117, 374–384. [Google Scholar] [CrossRef]
- Lewis, R.J. Ciguatoxins are potent ichthyotoxins. Off. J. Int. Soc. Toxinol. 1992, 30, 207–211. [Google Scholar] [CrossRef]
- Anadon, G.G.I. Morphometrical and enzymological disturbances on the liver of Serranus cabrilla (Teleostei, Serranidae) by ingestion of Gambierdiscus toxicus (Dinoflagellate). Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1993, 106, 757–760. [Google Scholar] [CrossRef]
- Ledreux, A.; Brand, H.; Chinain, M.; Bottein, M.-Y.D.; Ramsdell, J.S. Dynamics of ciguatoxins from Gambierdiscus polynesiensis in the benthic herbivore Mugil cephalus: Trophic transfer implications. Harmful Algae 2014, 39, 165–174. [Google Scholar] [CrossRef]
- Clausing, R.J.; Losen, B.; Oberhaensli, F.R.; Darius, H.T.; Sibat, M.; Hess, P.; Swarzenski, P.W.; Chinain, M.; Dechraoui Bottein, M.-Y. Experimental evidence of dietary ciguatoxin accumulation in an herbivorous coral reef fish. Aquat. Toxicol. 2018, 200, 257–265. [Google Scholar] [CrossRef]
- Yan, M.; Mak, M.Y.L.; Cheng, J.; Li, J.; Gu, J.R.; Leung, P.T.Y.; Lam, P.K.S. Effects of dietary exposure to ciguatoxin P-CTX-1 on the reproductive performance in marine medaka (Oryzias melastigma). Mar. Pollut. Bull. 2020, 152, 110837. [Google Scholar] [CrossRef]
- Li, J.; Mak, Y.L.; Chang, Y.-H.; Xiao, C.; Chen, Y.-M.; Shen, J.; Wang, Q.; Ruan, Y.; Lam, P.K.S. Uptake and Depuration Kinetics of Pacific Ciguatoxins in Orange-Spotted Grouper (Epinephelus coioides). Environ. Sci. Technol. 2020, 54, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Colman, J.R.; Dechraoui, M.Y.; Dickey, R.W.; Ramsdell, J.S. Characterization of the developmental toxicity of Caribbean ciguatoxins in finfish embryos. Off. J. Int. Soc. Toxinol. 2004, 44, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Leung, P.T.; Ip, J.C.; Cheng, J.P.; Wu, J.J.; Gu, J.R.; Lam, P.K. Developmental toxicity and molecular responses of marine medaka (Oryzias melastigma) embryos to ciguatoxin P-CTX-1 exposure. Aquat. Toxicol. 2017, 185, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Mak, Y.L.; Li, J.; Liu, C.N.; Cheng, S.H.; Lam, P.K.S.; Cheng, J.; Chan, L.L. Physiological and behavioural impacts of Pacific ciguatoxin-1 (P-CTX-1) on marine medaka (Oryzias melastigma). J. Hazard. Mater. 2017, 321, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.L.; Da Costa, J.M.; Teixeira, E.G.; Moreira, A.G.L.; De Moura, P.S.; Rocha, R.S.; Vieira, R.H.S.F. Performance of Carassius auratus with different food strategies in water recirculation system. Arch. Zootec. 2011, 60, 1203–1212. [Google Scholar] [CrossRef][Green Version]
- Wan, A.; Yu, S.; Hu, J.; Wan, Y.; Wei, Y.; Zhong, M.; Yang, H.; An, S.; Yan, Y. The Age, Growth, Fecundity of Carassius auratus in the Guohe River of Guoyang County. CLEAN Soil Air Water 2015, 43, 676–682. [Google Scholar] [CrossRef]
- Lorenzoni, M.; Corboli, M.; Ghetti, L.; Giovanni, P.; Carosi, A. Growth and Reproduction of the Goldfish Carassius Auratus: A Case Study from Italy; Springer: Berlin/Heidelberg, Germany, 2007; pp. 259–273. [Google Scholar]
- Estevez, P.; Castro, D.; Manuel Leao, J.; Yasumoto, T.; Dickey, R.; Gago-Martinez, A. Implementation of liquid chromatography tandem mass spectrometry for the analysis of ciguatera fish poisoning in contaminated fish samples from Atlantic coasts. Food Chem. 2019, 280, 8–14. [Google Scholar] [CrossRef]
- Iqbal, K. Influence of Feeding Frequency on Growth performance and Body Indices of Goldfish (Carrassius auratus). J. Aquac. Res. Dev. 2015, 6. [Google Scholar] [CrossRef]
- Lewis, R.J. Detection of toxins associated with ciguatera fish poisoning. In Manual on Harmful Marine Microalgae; UNESCO: Paris, France, 2003; pp. 267–277. [Google Scholar]
- Diogène, J. Marine Toxin Analysis for the Benefit of ‘One Health’ and for the Advancement of Science. In Comprehensive Analytical Chemistry; Diogène, J., Campàs, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–34. [Google Scholar]
- Lewis, R.; Sellin, M.; Poli, M.; Norton, R.; MacLeod, J.; Sheil, M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Off. J. Int. Soc. Toxinol. 1991, 29, 1115–1127. [Google Scholar] [CrossRef]
- Caillaud, A.; Eixarch, H.; de la Iglesia, P.; Rodriguez, M.; Dominguez, L.; Andree, K.B.; Diogene, J. Towards the standardisation of the neuroblastoma (neuro-2a) cell-based assay for ciguatoxin-like toxicity detection in fish: Application to fish caught in the Canary Islands. Food Addit. Contam. A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 1000–1010. [Google Scholar] [CrossRef]
- Soliño, L.; Widgy, S.; Pautonnier, A.; Turquet, J.; Loeffler, C.R.; Flores Quintana, H.A.; Diogène, J. Prevalence of ciguatoxins in lionfish (Pterois spp.) from Guadeloupe, Saint Martin, and Saint Barthélmy Islands (Caribbean). Off. J. Int. Soc. Toxinol 2015, 102, 62–68. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on marine biotoxins in shellfish—Emerging toxins: Ciguatoxin group. EFSA J. 2010, 8, 38. [Google Scholar] [CrossRef]
- Edmunds, J.S.G.; McCarthy, R.A.; Ramsdell, J.S. Ciguatoxin reduces larval survivability in finfish. Off. J. Int. Soc. Toxinol. 1999, 37, 1827–1832. [Google Scholar] [CrossRef]
- Catanese, F.; Fernández, P.; Villalba, J.J.; Distel, R.A. The physiological consequences of ingesting a toxic plant (Diplotaxis tenuifolia) influence subsequent foraging decisions by sheep (Ovis aries). Physiol. Behav. 2016, 167, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Manteifel, Y.B.; Karelina, M.A. Conditioned food aversion in the goldfish, Carassius Auratus. Comp. Biochem. Physiol. A Physiol. 1996, 115, 31–35. [Google Scholar] [CrossRef]
- Maier, K.J.; Tullis, R.E. The effects of diet and digestive cycle on the gastrointestinal tract pH values in the goldfish, Carassius auratus L., Mozambique tilapia, Oreochromis mossambicus (Peters), and channel catfish, Ictalurus punctatus (Rafinesque). J. Fish. Biol. 1984, 25, 151–165. [Google Scholar] [CrossRef]
- Dickey, R.W. Ciguatera Toxins: Chemistry, Toxicology, and Detection; CRC Press: Boca Raton, FL, USA, 2008; pp. 479–500. [Google Scholar]
- Castro, D.; Manger, R.; Vilariño, O.; Gago-Martínez, A. Evaluation of Matrix Issues in the Applicability of the Neuro-2a Cell Based Assay on the Detection of CTX in Fish Samples. Toxins 2020, 12, 308. [Google Scholar] [CrossRef]
- Chan, W.H.; Mak, Y.L.; Wu, J.J.; Jin, L.; Sit, W.H.; Lam, J.C.W.; Sadovy de Mitcheson, Y.; Chan, L.L.; Lam, P.K.S.; Murphy, M.B. Spatial distribution of ciguateric fish in the Republic of Kiribati. Chemosphere 2011, 84, 117–123. [Google Scholar] [CrossRef]
- Vernoux, J.P.; Lahlou, N.; Abbad el Andaloussi, S.; Riyeche, N.; Magras, L.P. A study of the distribution of ciguatoxin in individual Caribbean fish. Acta Trop. 1985, 42, 225–233. [Google Scholar]
| Experimental Fish (No) | Sampling Day a | Consecutive Dose Intakes b | Body Weight c | Muscle Weight d | Standard Length e | Observations |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 53.7 | 13.1 | 11.7 | Normal behavior |
| 2 | 1 | 1 | 46.9 | 11.4 | 11.5 | Normal behavior |
| 3 | 8 | 8 | 46.0 | 11.3 | 11.0 | Normal behavior |
| 4 | 8 | 8 | 43.7 | 11.6 | 11.0 | Normal behavior |
| 5 | 15 | 15 | 55.5 | 15.1 | 11.8 | Lethargy and loss of brightness |
| 6 | 15 | 15 | 59.4 | 17.8 | 12.5 | Lethargy and loss of brightness |
| 7 | 29 | 29 | 42.8 | 11.2 | 11.0 | Lethargy and loss of brightness |
| 8 | 29 | 29 | 63.3 | 15.8 | 12.5 | Lethargy and loss of brightness |
| 9 | 36 | 36 | 81.2 | 21.4 | 13.5 | Lethargy and loss of brightness |
| 10 | 36 | 36 | 41.2 | 12.0 | 10.9 | Lethargy and loss of brightness |
| 11 | 43 | 43 | 38.3 | 10.6 | 10.2 | Sideway swimming, drifting, jerking movements and difficulty on feeding |
| 12 | 60 | 57 | 38.3 | 10.0 | 10.0 | Starvation for 3 days and discarded after observing a tumor lesion in the cerebellum |
| 13 | 84 | 82 | 45.4 | 10.6 | 11.0 | It began to increase feeding time and then consciously refused toxic feeding on the last 2 days |
| 14 | 102 | 43 | 45.6 | 10.5 | 10.9 | Same symptomatology than fish no. 11 on day 43, then, it was separated and sampled after 60 days of commercial food feeding |
| Sampling Day | Experimental Fish Sampled (No.) | Mean Total Quantity of Toxin Ingested a (pg CTX) | Mean Muscle CTX-Like Toxicity Level b (ppb CTX) | Mean Muscle Toxin Burden c (pg CTX) | % Toxins Ingested Accumulated in Muscle d |
|---|---|---|---|---|---|
| 1 | 1, 2 | 681.82 | 0.000 ± 0.000 | 0.00 ± 0.00 | 0.0% |
| 8 | 3, 4 | 4864.55 | 0.020 ± 0.001 | 226.92 ± 10.90 | 4.7% |
| 15 | 5, 6 | 1168.13 | 0.025 ± 0.002 | 411.09 ± 13.81 | 3.5% |
| 29 | 7, 8 | 20,848.21 | 0.036 ± 0.010 | 504.36 ± 246.93 | 2.3% |
| 36 | 9, 10 | 29,877.93 | 0.031 ± 0.008 | 482.46 ± 63.09 | 1.8% |
| 43 | 11 | 22,334.22 * | 0.022 | 231.59 | 1.0% |
| 84 | 13 | 51,726.92 * | 0.035 | 368.17 | 0.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Henao, A.; García-Álvarez, N.; Padilla, D.; Ramos-Sosa, M.; Silva Sergent, F.; Fernández, A.; Estévez, P.; Gago-Martínez, A.; Diogène, J.; Real, F. Accumulation of C-CTX1 in Muscle Tissue of Goldfish (Carassius auratus) by Dietary Experience. Animals 2021, 11, 242. https://doi.org/10.3390/ani11010242
Sanchez-Henao A, García-Álvarez N, Padilla D, Ramos-Sosa M, Silva Sergent F, Fernández A, Estévez P, Gago-Martínez A, Diogène J, Real F. Accumulation of C-CTX1 in Muscle Tissue of Goldfish (Carassius auratus) by Dietary Experience. Animals. 2021; 11(1):242. https://doi.org/10.3390/ani11010242
Chicago/Turabian StyleSanchez-Henao, Andres, Natalia García-Álvarez, Daniel Padilla, María Ramos-Sosa, Freddy Silva Sergent, Antonio Fernández, Pablo Estévez, Ana Gago-Martínez, Jorge Diogène, and Fernando Real. 2021. "Accumulation of C-CTX1 in Muscle Tissue of Goldfish (Carassius auratus) by Dietary Experience" Animals 11, no. 1: 242. https://doi.org/10.3390/ani11010242
APA StyleSanchez-Henao, A., García-Álvarez, N., Padilla, D., Ramos-Sosa, M., Silva Sergent, F., Fernández, A., Estévez, P., Gago-Martínez, A., Diogène, J., & Real, F. (2021). Accumulation of C-CTX1 in Muscle Tissue of Goldfish (Carassius auratus) by Dietary Experience. Animals, 11(1), 242. https://doi.org/10.3390/ani11010242