Comparison of the Morphology and Developmental Potential of Oocytes Obtained from Prepubertal and Adult Domestic and Wild Cats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Ovaries and Oocyte Collection
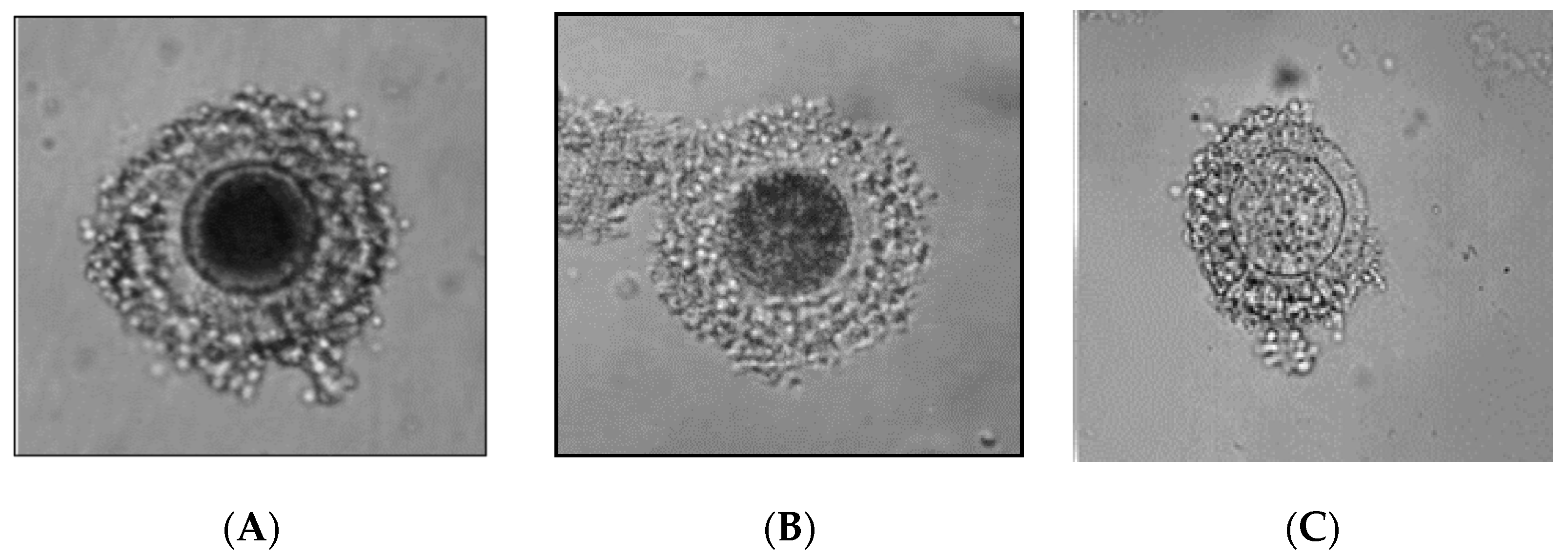
- oocytes tightly surrounded by at least three layers of cumulus cells,
- oocytes partially surrounded by cumulus cells,
- oocytes surrounded only by the corona radiata,
- degenerated oocytes with morphological defects, with damaged zona pellucida, distorted shape or fragmented cytoplasm.
- A-
- oocytes with very dark cytoplasm,
- B-
- oocytes with dark, mosaic cytoplasm,
- C-
- oocytes with pale cytoplasm,
2.2. In Vitro Maturation of Oocytes (IVM)
2.3. In Vitro Fertilization (IVF) and Embryo Culture
2.4. Differential Cell Staining and Counting of the Nuclei
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kochan, J.; Niżański, W.; Moreira, N.; Cubas, Z.; Nowak, A.; Prochowska, S.; Partyka, A.; Młodawska, W.; Skotnicki, J. ARTs in wild felid conservation programmes in Poland and in the world. J. Vet. Res. 2019, 63, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.C.; Pope, C.E.; Giraldo, A.; Lyons, A.; Harris, R.F.; King, A.L.; Cole, A.; Godke, R.A.; Dresser, B.L. Birth of African Wildcat cloned kittens born from domestic cats. Cloning Stem Cells 2004, 6, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Moro, L.N.; Sestelo, A.J.; Salamone, D.F. Evaluation of cheetah and leopard spermatozoa developmental capability after interspecific ICSI with domestic cat oocytes. Reprod. Domest. Anim. 2014, 49, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.E. Aspects of in vivo oocyte production, blastocyst development and embryo transfer in the cat. Theriogenology 2014, 81, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, A.M.; Johnston, L.A.; Seal, U.S.; Armstrong, D.L.; Tilson, R.L.; Wolf, P.; Petrini, L.G.; Simmons, T.; Groos, T.; Wildt, D.E. In vitro fertilization and embryo development in vitro and in vivo in the tiger (Panthera tigris). Biol. Reprod. 1990, 43, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Morato, R.G.; Conforti, V.A.; Azevedo, F.C.; Jacomo, A.T. Comparative analyses of semen and endocrine characteristics of free-living versus captive jaguars (Panthera onca). Reproduction 2001, 122, 745–751. [Google Scholar] [CrossRef]
- Swanson, W.F.; Brown, J.L. International training programs in reproductive sciences for conservation of Latin American felids. Anim. Reprod. Sci. 2004, 82, 21–34. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, L.; Hribal, R.; Stagegaard, J.; Zahmel, J.; Jewgenow, K. Production of lion (Panthera leo) blastocysts after in vitro maturation of oocytes and intracytoplasmic sperm injection. Theriogenology 2015, 83, 995–999. [Google Scholar] [CrossRef]
- Howard, J.G. Semen collection and analysis in carnivores. In Zoo Wildlife Medicine; Fowler, M.E., Ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1993; pp. 390–399. [Google Scholar]
- Swanson, W.F.; Maggs, D.J.; Clarke, H.E.; Newell, A.E.; Bond, J.B.; Bateman, H.L.; Kennedy-Stoskopf, S. Assessment of viral presence in semen and reproductive function of frozen-thawed spermatozoa from Pallas’ cats (Otocolobus manul) infected with feline herpesvirus. J. Zoo Wildl. Med. 2006, 37, 336–346. [Google Scholar] [CrossRef]
- Gañán, N.; González, R.; Garde, J.J.; Martínez, F.; Vargas, A.; Gomendio, M.; Roldan, E.R. Assessment of semen quality, sperm cryopreservation and heterologous IVF in the critically endangered Iberian lynx (Lynx pardinus). Reprod. Fertil. Dev. 2009, 21, 848–859. [Google Scholar] [CrossRef]
- Gañán, N.; González, R.; Sestelo, A.; Garde, J.J.; Sánchez, I.; Aguilar, J.M.; Gomendio, M.; Roldan, E.R. Male reproductive traits, semen cryopreservation, and heterologous in vitro fertilization in the bobcat (Lynx rufus). Theriogenology 2009, 72, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Paz, R.C.R. Wildlife Cats Reproductive Biotechnology. In Current Frontiers in Cryobiology; Katkov, I., Ed.; In Tech: Shanghai, China, 2012; pp. 369–388. [Google Scholar]
- Roth, T.L.; Swanson, W.F.; Collins, D.; Burton, M.; Garell, D.M. Snow leopard (Panthera uncia) spermatozoa are sensitive to alkaline pH, but motility in vitro is not influenced by protein or energy supplements. J. Androl. 1996, 17, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Goodrowe, K.L.; Wall, R.J.; O’Brien, S.J.; Schmidt, P.M.; Wildt, D.E. Developmental competence of domestic cat follicular oocytes after fertilization in vitro. Biol. Reprod. 1998, 39, 355–372. [Google Scholar] [CrossRef]
- Johnston, L.A.; O’Brien, S.J.; Wildt, D.E. In vitro maturation and fertilization of domestic cat follicular oocytes. Gamete Res. 1998, 24, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A.; Donoghue, A.M.; O’Brien, S.J.; Wildt, D.E. Rescue and maturation in Vitro of follicular oocytes collected from nondomestic felid species. Biol. Reprod. 1991, 45, 876–906. [Google Scholar] [CrossRef]
- Nowak, R.M.; Paradiso, J.L. Walker’s Mammals of the World, 4th ed.; The John Hopkins University Press: Baltimore, MD, USA, 1983; Volume 2, pp. 1061–1094. [Google Scholar]
- Armstrong, D.T.; Holm, P.; Irvin, B.; Petersen, B.A.; Stubbings, R.B.; McLean, D.; Stevens, G.; Seamark, R.F. Pregnancies and live birth from in vitro fertilization of calf oocytes collected by laparoscopic follicular aspiration. Theriogenology 1992, 38, 667–678. [Google Scholar] [CrossRef]
- Ptak, G.; Loi PDattena, M.; Tischner, M.; Cappai, P. Offspring from one-month-old lambs: Studies on the developmental capability of prepubertal oocytes. Biol. Reprod. 1999, 61, 1568–1574. [Google Scholar] [CrossRef]
- Morton, K.M. Developmental capabilities of embryos produced in vitro from prepubertal lamb oocytes. Reprod. Domest. Anim. 2008, 43, 137–143. [Google Scholar] [CrossRef]
- Leoni, G.G.; Palmerini, M.G.; Satta, V.; Succu, S.; Pasciu, V.; Zinellu, A.; Carru, C.; Macchiarelli, G.; Nottola, S.A.; Naitana, S.; et al. Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS ONE 2015, 20, e0124911. [Google Scholar] [CrossRef]
- Armstrong, D.T.; Kotaras, P.J.; Earl, C.R. Advances in production of embryos in vitro from juvenile and prepubertal oocytes from the calf and lamb. Reprod. Fertil. Dev. 1997, 9, 333–339. [Google Scholar] [CrossRef]
- Lasley, B.L.; Loskutoff, N.M.; Anderson, G.B. The limitation of conventional breeding programs and the need and promise of assisted reproduction in nondomestic species. Theriogenology 1994, 41, 119–132. [Google Scholar] [CrossRef]
- Niżański, W.; Dejneka, G.J.; Klimowicz, M.; Dubiel, A. Evaluating some properties of domestic cat epididymal spermatozoa and their cryopreservation. Med. Weter 2005, 61, 173–178. [Google Scholar]
- Pope, C.E.; Johnson, C.A.; McRae, M.A.; Keller, G.L.; Dresser, B.L. Development of embryos produced by intracytoplasmic sperm injection of cat oocytes. Anim. Reprod. Sci. 1998, 53, 221–236. [Google Scholar] [CrossRef]
- Comizzoli, P.; Wildt, D.E.; Pukazhenthi, B.S. Overcoming poor in vitro nuclear maturation and developmental competence of domestic cat oocytes during the non-breeding season. Reproduction 2003, 126, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P.; Wildt, D.E.; Pukazhenthi, B.S. Poor centrosomal function of cat testicular spermatozoa impairs embryo development in vitro after intracytoplasmic sperm injection. Biol. Reprod. 2006, 75, 252–260. [Google Scholar] [CrossRef]
- Merlo, B.; Iacono, E.; Regazzini, M.; Zambelli, D. Cat blastocysts produced in vitro from oocytes vitrified using the cryoloop technique and cryopreserved electroejaculated semen. Theriogenology 2008, 70, 126–130. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Vireque, A.A.; Adona, P.R.; Meirelles, F.V.; Ferriani, R.A.; Navarro, P.A. Cytoplasmic maturation of bovine oocytes: Structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef]
- Ledda, S.; Bogliolo, L.; Calvia, P.; Leoni, G.; Naitana, S. Meiotic progressionand developmental competence of oocytes collected from juvenile and adult ewes. J. Reprod. Fertil. 1997, 109, 73–78. [Google Scholar] [CrossRef]
- O’Brien, J.K.; Catt, S.L.; Ireland, K.A.; Maxwell, W.M.C.; Evans, G. In vitro and in vivo developmental capacity of oocytes from prepubertal and adult sheep. Theriogenology 1997, 47, 1433–1443. [Google Scholar] [CrossRef]


| Morphology of Oocytes | Adult | Prepubertal | ||||||
|---|---|---|---|---|---|---|---|---|
| Domestic Cat n = 20 | Pallas’s cat n = 2 | Lynx n = 2 | Serval n = 1 | Domestic Cat n = 15 | Pallas’s Cat n = 1 | Lynx n = 1 | Tiger n = 1 | |
| No. of oocytes/female (mean ± SD) | 23 ± 11 a | 28 ± 8 a | 5 ± 3 c | 30 a | 43± 29 b | 48 b | 41 b | 0 |
| Oocytes with darkcytoplasm (grade A,B) (%) | 77% a | 74% a | 80% a | 73% a | 48% b | 54% b | 53% b | 0 |
| Oocytes with light cytoplasm (grade C) (%) | 23% a | 26% a | 20% a | 27% a | 52% c | 46% c | 47% c | 0 |
| Diameter (µm) of oocytes with dark cytoplasm (mean ± SD) | 161 ± 5 a | 162 ± 3 a | 162 ± 2 a | 160 ± 5 a | 161 ± 7 a | 160 ± 4 a | 162 ± 5 a | - |
| Diameter (µm of oocytes with light cytoplasm (mean ± SD) | 152 ± 11 a | 154 ± 9 a | 152 ± 5 a | 153 ± 13 a | 153 ± 15 a | 151 ± 8 a | 152 ± 10 a | - |
| In Vitro Maturation of Oocytes | Adult | Prepubertal | ||||
|---|---|---|---|---|---|---|
| Domestic Cat | Pallas’s Cat | Lynx | Domestic Cat | Pallas’s Cat | Lynx | |
| Total number of oocytes | 120 | 56 | 10 | 108 | 48 | 37 |
| Oocytes selected for IVM | 72 (60%) | 23 (64%) | 6 (60%) | 49 (45%) | 25 (52%) | 19 (51%) |
| Oocytes in met II after IVM | 38 (52%) | 11 (52%) | 3 (50%) | 24 (49%) | 13 (52%) | 9 (47%) |
| Group of Females | No. of Fertilized Oocytes n | No of Cleaved Embryos n (%) | No of Blastocyst n (%) | No of Hatching Blastocyst n (%) |
|---|---|---|---|---|
| Adult | 203 | 104 (51) b | 41(39) b | 8 (19) d |
| Prepubertal | 218 | 92 (42) a | 26 (28) a | 2 (8) c |
| Group of Females | TCN: Total Cell Number | TE: Trophoblast Cells Number | ICM: Inner Cell Mass Number | ICM% |
|---|---|---|---|---|
| Adult | 192 ± 27 b | 101 ± 21 b | 91 ± 17 d | 48 |
| Prepubertal | 144 ± 44 a | 86± 9 a | 58 ± 11 c | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochan, J.; Nowak, A.; Młodawska, W.; Prochowska, S.; Partyka, A.; Skotnicki, J.; Niżański, W. Comparison of the Morphology and Developmental Potential of Oocytes Obtained from Prepubertal and Adult Domestic and Wild Cats. Animals 2021, 11, 20. https://doi.org/10.3390/ani11010020
Kochan J, Nowak A, Młodawska W, Prochowska S, Partyka A, Skotnicki J, Niżański W. Comparison of the Morphology and Developmental Potential of Oocytes Obtained from Prepubertal and Adult Domestic and Wild Cats. Animals. 2021; 11(1):20. https://doi.org/10.3390/ani11010020
Chicago/Turabian StyleKochan, Joanna, Agnieszka Nowak, Wiesława Młodawska, Sylwia Prochowska, Agnieszka Partyka, Józef Skotnicki, and Wojciech Niżański. 2021. "Comparison of the Morphology and Developmental Potential of Oocytes Obtained from Prepubertal and Adult Domestic and Wild Cats" Animals 11, no. 1: 20. https://doi.org/10.3390/ani11010020
APA StyleKochan, J., Nowak, A., Młodawska, W., Prochowska, S., Partyka, A., Skotnicki, J., & Niżański, W. (2021). Comparison of the Morphology and Developmental Potential of Oocytes Obtained from Prepubertal and Adult Domestic and Wild Cats. Animals, 11(1), 20. https://doi.org/10.3390/ani11010020







