The Modulating Effect of Dietary Beta-Glucan Supplementation on Expression of Immune Response Genes of Broilers during a Coccidiosis Challenge
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diets, and Eimeria Challenge
2.2. Tissue Sampling for Gene Expression Analysis
2.3. Total RNA Extraction and Reverse Transcription
2.4. Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
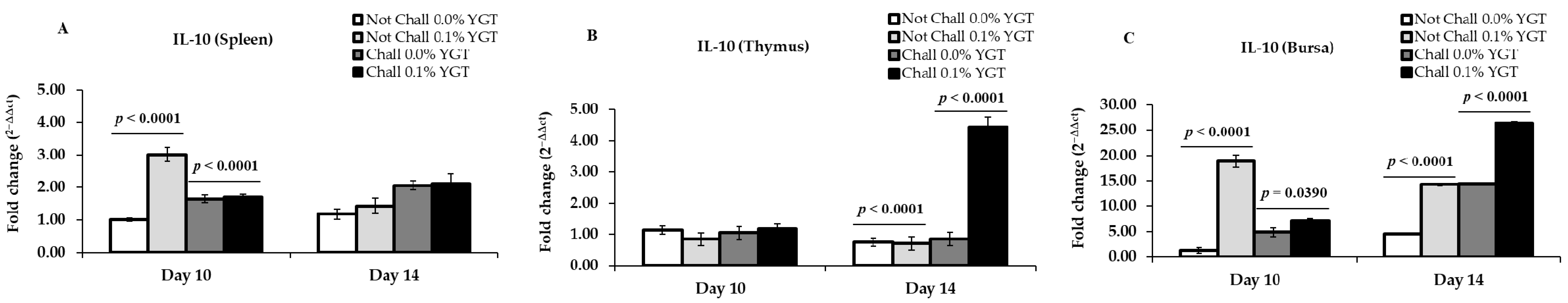
3.1. IL-10 mRNA Expression Levels (Table 2; Figure 1)
| Gene Target 1 | Organ | p-Value 3 at d 10 | p-Value 3 at d 14 | ||||
|---|---|---|---|---|---|---|---|
| Diet 2 | Challenge | Diet × Challenge | Diet 2 | Challenge | Diet × Challenge | ||
| IL-10 | Spleen | <0.0001 | 0.0345 | <0.0001 | 0.5437 | 0.0020 | 0.6702 |
| Thymus | 0.6790 | 0.4993 | 0.2482 | <0.0001 | <0.0001 | <0.0001 | |
| Bursa | <0.0001 | 0.0005 | <0.0001 | 0.0014 | 0.0012 | 0.7347 | |
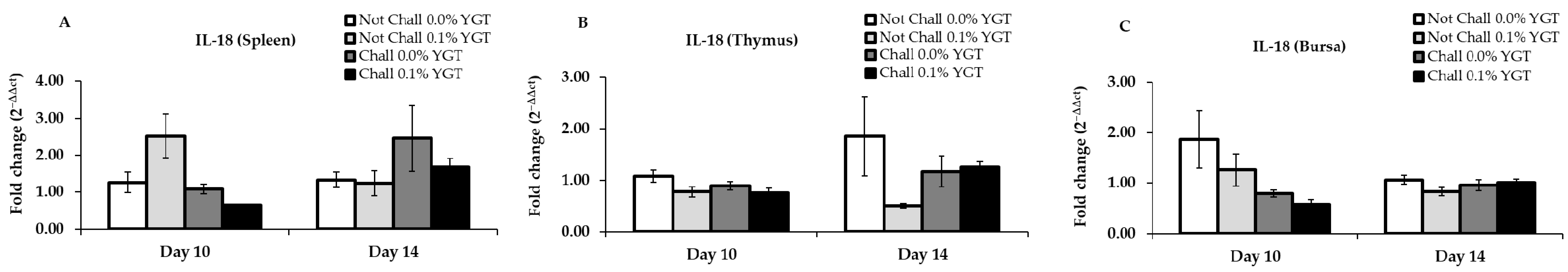
| IL-18 | Spleen | 0.2546 | 0.0055 | 0.0207 | 0.3002 | 0.0707 | 0.4103 |
| Thymus | 0.0585 | 0.3737 | 0.4735 | 0.1183 | 0.9320 | 0.0763 | |
| Bursa | 0.2281 | 0.0114 | 0.5750 | 0.3330 | 0.7018 | 0.1474 | |
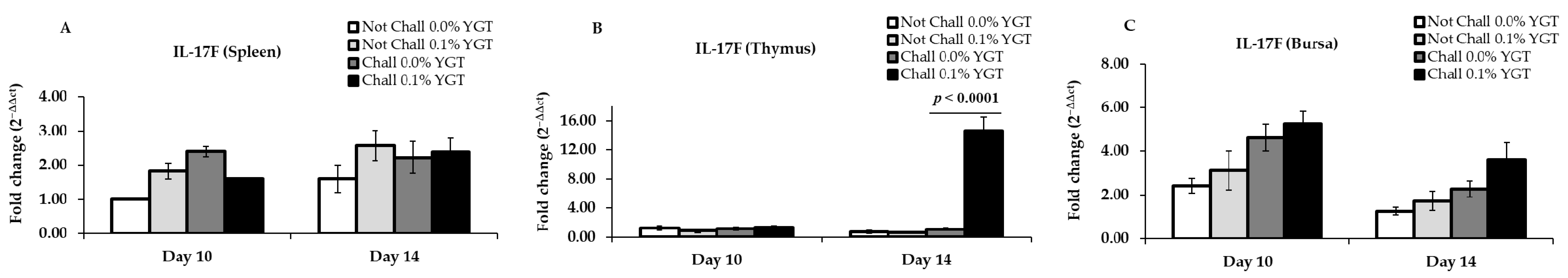
| IL-17F | Spleen | 0.9937 | 0.0013 | <0.0001 | 0.2069 | 0.6390 | 0.3660 |
| Thymus | 0.6774 | 0.4761 | 0.3153 | <0.0001 | <0.0001 | <0.0001 | |
| Bursa | 0.2800 | 0.0008 | 0.9596 | 0.0735 | 0.0048 | 0.3903 | |
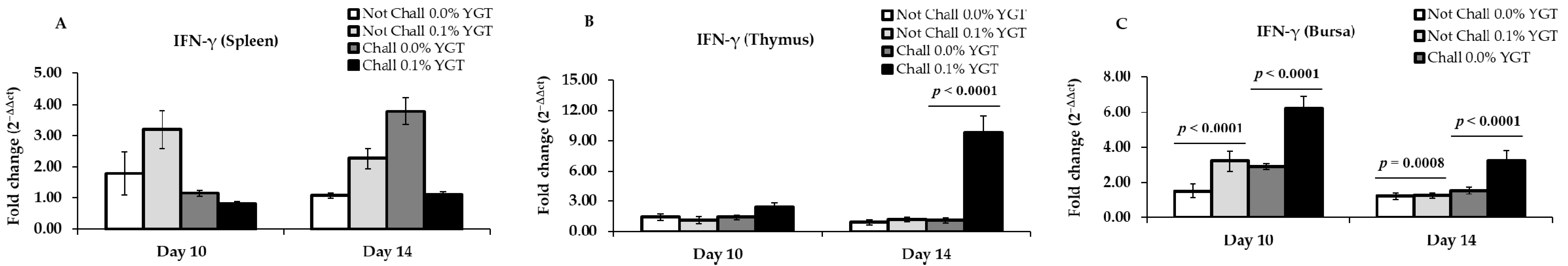
| IFN-γ | Spleen | 0.2719 | 0.0033 | 0.0792 | 0.1884 | 0.1676 | 0.0011 |
| Thymus | 0.0774 | 0.0785 | 0.3234 | <0.0001 | <0.0001 | <0.0001 | |
| Bursa | <0.0001 | <0.0001 | 0.1031 | 0.0192 | 0.0016 | 0.0152 | |
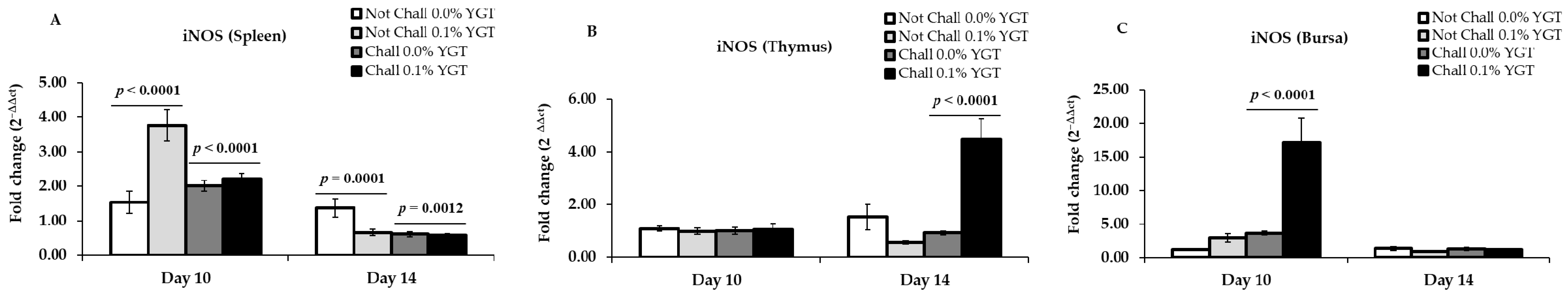
| iNOS | Spleen | 0.0007 | 0.1142 | 0.0040 | 0.0466 | 0.0266 | 0.0635 |
| Thymus | 0.8977 | 0.9987 | 0.6537 | 0.0037 | 0.0003 | <0.0001 | |
| Bursa | 0.0009 | 0.0003 | 0.0095 | 0.2475 | 0.8261 | 0.4419 | |
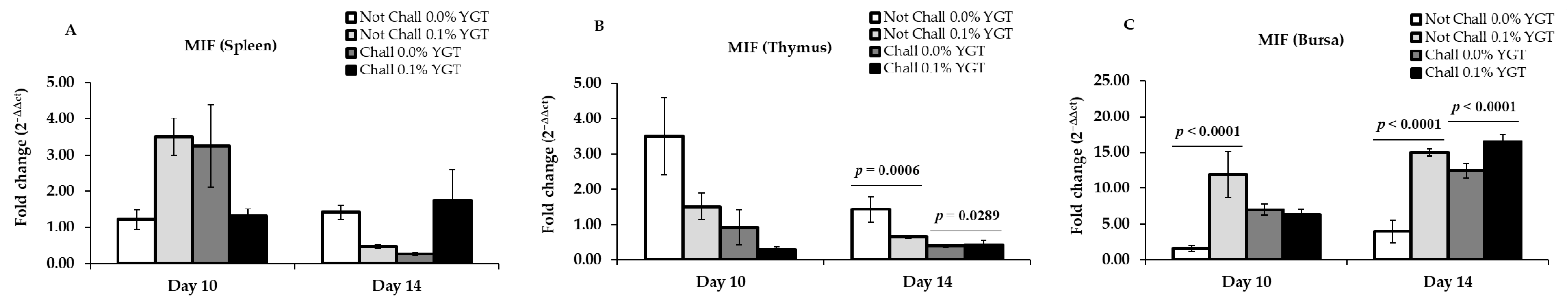
| MIF | Spleen | 0.8068 | 0.9164 | 0.0058 | 0.5617 | 0.9049 | 0.0100 |
| Thymus | 0.0855 | 0.0139 | 0.3614 | 0.0408 | 0.0011 | 0.0274 | |
| Bursa | 0.0007 | 0.9470 | 0.0001 | <0.0001 | <0.0001 | 0.0022 | |
3.2. IL-18 mRNA Expression Levels (Table 2; Figure 2)
3.3. IL-17F mRNA Expression Levels (Table 2; Figure 3)
3.4. IFN-γ mRNA Expression Levels (Table 2; Figure 4)
3.5. iNOS mRNA Expression Levels (Table 2; Figure 5)
3.6. MIF mRNA Expression Levels (Table 2; Figure 6)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalloul, R.A.; Lillehoj, H.S. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, R.A.; Lillehoj, H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccines 2006, 5, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.P.; Omara, I.I.; Persia, M.; Dalloul, R.A. The impact of β-glucans on performance and response of broiler chickens during a coccidiosis challenge. Poult. Sci. 2018, 97, 2713–2721. [Google Scholar] [PubMed]
- Cox, C.M.; Sumners, L.H.; Kim, S.; McElroy, A.P.; Bedford, M.R.; Dalloul, R.A. Immune responses to dietary β-glucan in broiler chicks during an Eimeria challenge. Poult. Sci. 2010, 89, 2597–2607. [Google Scholar] [CrossRef]
- Leung, H.; Yitbarek, A.; Snyder, R.; Patterson, R.; Barta, J.R.; Karrow, N.; Kiarie, E. Responses of broiler chickens to Eimeria challenge when fed a nucleotide-rich yeast extract. Poult. Sci. 2019, 98, 1622–1633. [Google Scholar]
- Pender, C.M.; Kim, S.; Potter, T.D.; Ritzi, M.M.; Young, M.; Dalloul, R.A. Effects of in ovo supplementation of probiotics on performance and immunocompetence of broiler chicks to an Eimeria challenge. Benef. Microb. 2016, 7, 699–705. [Google Scholar] [CrossRef]
- Wang, X.; Peebles, E.D.; Kiess, A.S.; Wamsley, K.G.S.; Zhai, W. Effects of coccidial vaccination and dietary antimicrobial alternatives on the growth performance, internal organ development, and intestinal morphology of Eimeria-challenged male broilers. Poult. Sci. 2019, 98, 2054–2065. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by beta-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar]
- Harada, T.; Ohno, N. Contribution of dectin-1 and granulocyte macrophage-colony stimulating factor (GMCSF) to immunomodulating actions of beta-glucan. Int. Immunopharmacol. 2008, 8, 556–566. [Google Scholar] [CrossRef]
- Soltanian, S.; Stuyven, E.; Cox, E.; Sorgeloos, P.; Bossier, P. β-Glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 2009, 35, 109–138. [Google Scholar]
- Markazi, A.D.; Perez, V.; Sifri, M.; Shanmugasundaram, R.; Selvaraj, R.K. Effect of whole yeast cell product supplementation (CitriStim®) on immune responses and cecal microflora species in pullet and layer chickens during an experimental coccidial challenge. Poult. Sci. 2017, 96, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.; Horst, G.; Tonda, R.; Lumpkins, B.; Mathis, G. Evaluation of the effects of feeding dried algae containing beta-1,3-glucan on broilers challenged with Eimeria. Poult. Sci. 2018, 97, 3494–3500. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ali, R.A.; Qureshi, M.A. The influence of beta-glucan on immune responses in broiler chicks. Immunopharmacol. Immunotoxicol. 2003, 25, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Weng, B.C.; Chang, M.T.; Liao, Y.H.; Chen, T.T.; Chu, C. Direct enhancement of the phagocytic and bactericidal capability of abdominal macrophage of chicks by beta-1,3–1,6-glucan. Poult. Sci. 2008, 87, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Chuammitri, P.; Redmond, S.B.; Kimura, K.; Andreasen, C.B.; Lamont, S.J.; Palić, D. Heterophil functional responses to dietary immunomodulators vary in genetically distinct chicken lines. Vet. Immunol. Immunopathol. 2011, 142, 219–227. [Google Scholar] [CrossRef]
- Lowry, V.K.; Farnell, M.B.; Ferro, P.J.; Swaggerty, C.L.; Bahl, A.; Kogut, M.H. Purified beta-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 2005, 98, 309–318. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Wang, Z. The modulating effect of β-1,3/1,6-glucan supplementation in the diet on performance and immunological responses of broiler chickens. Asian-Aust. J. Anim. Sci. 2008, 21, 237–244. [Google Scholar] [CrossRef]
- Cox, C.M.; Stuard, L.H.; Kim, S.; McElroy, A.P.; Bedford, M.R.; Dalloul, R.A. Performance and immune responses to dietary β-glucan in broiler chicks. Poult. Sci. 2010, 89, 1924–1933. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, Y.; Wang, Z. Beta-1,3/1,6-glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 2013, 92, 1764–1773. [Google Scholar] [CrossRef]
- Cox, C.M.; Dalloul, R.A. Beta-glucans as immunomodulators in poultry: Use and potential applications. Avian Biol. Rev. 2010, 3, 171–178. [Google Scholar] [CrossRef]
- Huff, G.R.; Huff, W.E.; Farnell, M.B.; Rath, N.C.; de Los Santos, F.S.; Donoghue, A.M. Bacterial clearance heterophil function, and hematological parameters of transport-stressed turkey poults supplemented with dietary yeast extract. Poult. Sci. 2010, 89, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Horst, G.; Levine, R.; Chick, R.; Hofacre, C. Effects of beta-1,3-glucan (Aleta TM) on vaccination response in broiler chickens. Poult. Sci. 2019, 98, 1643–1647. [Google Scholar] [CrossRef]
- Tian, X.; Shao, Y.; Wang, Z.; Guoa, Y. Effects of dietary yeast-glucans supplementation on growth performance, gut morphology, intestinal Clostridium perfringens population and immune response of broiler chickens challenged with necrotic enteritis. Anim. Feed Sci. Technol. 2016, 215, 144–155. [Google Scholar] [CrossRef]
- Anwar, M.I.; Muhammad, F.; Awais, M.M.; Akhtar, M. A review of β-glucans as a growth promoter and antibiotic alternative against enteric pathogens in poultry. World Poult. Sci. J. 2017, 73, 651–657. [Google Scholar] [CrossRef]
- Alizadeh, M.; Rogiewicz, A.; McMillan, E.; Rodriguez-LeCompte, J.C.; Patterson, R.; Slominski, B.A. Effect of yeast-derived products and distillers dried grains with solubles (DDGS) on growth performance and local innate immune response of broiler chickens challenged with Clostridium perfringens. Avian Pathol. 2016, 45, 334–345. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Kim, S.; Miska, K.B.; Jenkins, M.C.; Fetterer, R.H.; Cox, C.M.; Stuard, L.H.; Dalloul, R.A. Molecular cloning and functional characterization of the avian macrophage migration inhibitory factor (MIF). Dev. Comp. Immunol. 2010, 34, 1021–1032. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hoelscher, C. The power of combinatorial immunology: IL-12 and IL-12-related dimeric cytokines in infectious diseases. Med. Microbiol. Immunol. 2004, 193, 1–17. [Google Scholar] [CrossRef]
- Arendt, M.K.; Sand, J.M.; Marcone, T.M.; Cook, M.E. Interleukin-10 neutralizing antibody for detection of intestinal luminal levels and as a dietary additive in Eimeria challenged broiler chicks. Poult. Sci. 2016, 95, 430–438. [Google Scholar] [CrossRef]
- Haritova, A.M.; Stanilova, S.A. Enhanced expression of IL-10 in contrast to IL-12B mRNA in poultry with experimental coccidiosis. Exp. Parasitol. 2012, 132, 378–382. [Google Scholar] [CrossRef]
- Sand, J.M.; Arendt, M.K.; Repasy, A.; Deniz, G.; Cook, M.E. Oral antibody to interleukin-10 reduces growth rate depression due to Eimeria spp. infection in broiler chickens. Poult. Sci. 2016, 95, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bremner, A. Innate Responses and Biomarkers of Resistance to Eimeria Infection in the Chicken. Ph.D. Thesis, The University of Edinburgh, Edinburgh, UK, 30 June 2018. [Google Scholar]
- Boulton, K.; Nolan, M.J.; Wu, Z.; Psifidi, A.; Riggio, V.; Harman, K.; Bishop, S.C.; Kaiser, P.; Abrahamsen, M.S.; Hawken, R.; et al. Phenotypic and genetic variation in the response of chickens to Eimeria tenella induced coccidiosis. Genet. Sel. Evol. 2018, 50, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wigley, P.; Kaiser, P. Avian cytokines in health and disease. Rev. Bras. Cienc. Avic. 2003, 5, 1–14. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Lee, Y.; Lillehoj, H.S. Beneficial effects of dietary supplementation of Bacillus strains on growth performance and gut health in chickens with mixed coccidiosis infection. Vet. Parasitol. 2020, 277, 1–11. [Google Scholar] [CrossRef]
- Göbel, T.W.; Schneider, K.; Schaerer, B.; Mejri, I.; Puehler, F.; Weigend, S.; Staeheli, P.; Kaspers, B. IL-18 stimulates the proliferation and IFN-gamma release of CD4+ T cells in the chicken: Conservation of a Th1-like system in a non-mammalian species. J. Immunol. 2003, 171, 1809–1815. [Google Scholar] [CrossRef]
- Li, J.; Xing, J.; Li, D.; Wang, X.; Zhao, L.; Lv, S.; Huang, D. Effects of β-glucan extracted from Saccharomyces cerevisiae on humoral and cellular immunity in weaned piglets. Arch. Anim. Nutr. 2005, 59, 303–312. [Google Scholar] [CrossRef]
- Bedirli, A.; Kerem, M.; Pasaoglu, H.; Akyurek, N.; Tezcaner, T.; Elbeg, S.; Memis, L.; Sakrak, O. β-Glucan attenuates inflammatory cytokine release and prevents acute lung injury in an experimental model of sepsis. Shock 2007, 27, 397–401. [Google Scholar] [CrossRef]
- Kim, W.H.; Jeong, J.; Park, A.R.; Yim, D.; Kim, Y.H.; Kim, K.D.; Chang, H.H.; Lillehoj, H.; Lee, S.B.; Min, W. Chicken IL-17F: Identification and comparative expression analysis in Eimeria-infected chickens. Dev. Comp. Immunol. 2012, 38, 401–409. [Google Scholar] [CrossRef]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Onuchic, L.F.; Li, X.D.; Essayan, D.M.; Schroeder, J.; Xiao, H.Q.; Liu, M.C.; Krishnaswamy, G.; Germino, G.; Huang, S.K. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J. Immunol. 2001, 167, 4430–4435. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kokubu, F.; Matsukura, S.; Ieki, K.; Odaka, M.; Watanabe, S.; Suzuki, S.; Adachi, M.; Huang, S.K. Induction of C-X-C chemokines, growth-related oncogene α expression, and epithelial cell-derived neutrophil-activating protein-78 by ML-1 (interleukin 17F) involves activation of Raf1-mitogenactivated protein kinase kinase-extracellular signal-regulated kinase ½ pathway. J. Pharmacol. Exp. Ther. 2003, 307, 1213–1220. [Google Scholar] [PubMed]
- Tizard, I.R. Veterinary Immunology. An Introduction, 8th ed.; Saunders Elsevier: St. Louis, MO, USA, 2009. [Google Scholar]
- Broom, L.J.; Kogut, M.H. Deciphering desirable immune responses from disease models with resistant and susceptible chickens. Poult. Sci. 2019, 98, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.I.; Miura, N.N.; Adachi, Y.; Ogura, N.; Tamura, H.; Tanaka, S.; Ohno, N. Relationship between the physical properties of Candida albicans cell well β-glucan and activation of leukocytes in vitro. Int. Immunopharmacol. 2002, 2, 1109–1122. [Google Scholar] [PubMed]
- Ljungman, A.G.; Leanderson, P.; Tagesson, C. (1–3)-b-D-glucan stimulates nitric oxide generation and cytokine mRNA expression in macrophages. Environ. Toxicol. Pharmacol. 1998, 5, 273–281. [Google Scholar] [CrossRef]
- Mucksová, J.; Babicek, K.; Pospisil, M. Particulate 1,3-beta-D-glucan, carboxymethylglucan and sulfoethylglucan–Influence of their oral or intraperitoneal administration on immunological respondence of mice. Folia Microbiol. 2001, 46, 559–563. [Google Scholar]
- de Dios Rosado, J.; Rodriguez-Sosa, M. Macrophage migration inhibitory factor (MIF): A key player in protozoan infections. Int. J. Biol. Sci. 2011, 7, 1239–1256. [Google Scholar] [CrossRef]
- Bucala, R. A most interesting factor. Nature 2000, 408, 146–147. [Google Scholar] [CrossRef]
- Kleemann, R.; Hausser, A.; Geiger, G.; Mischke, R.; Burger-Kentischer, A.; Flieger, O.; Johannes, F.; Roger, J.T.; Calandra, T.; Kapurniotuk, A.; et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature 2000, 408, 211–216. [Google Scholar]
- Miska, K.B.; Fetterer, R.H.; Lillehoj, H.S.; Jenkins, M.C.; Allen, P.C.; Harper, S.B. Characterization of macrophage migration inhibitory factor from Eimeria species infectious to chickens. Mol. Biochem. Parasitol. 2007, 151, 173–183. [Google Scholar] [CrossRef]
- Hong, Y.H.; Lillehoj, H.S.; Lee, S.H.; Dalloul, R.A.; Lillehoj, E.P. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 2006, 114, 209–223. [Google Scholar] [CrossRef] [PubMed]
| Target | Accession No. | Nucleotide Sequence (5′ → 3′) |
|---|---|---|
| GAPDH_F | NM_204305 | CCTAGGATACACAGAGGACCAGGTT |
| GAPDH_R | GGTGGAGGAATGGCTGTCA | |
| IL-10_F | NM_001004414 | CGCTGTCACCGCTTCTTCA |
| IL-10_R | CGTCTCCTTGATCTGCTTGATG | |
| IL-18_F | NM_204608 | AGGTGAAATCTGGCAGTGGAAT |
| IL-18_R | TGAAGGCGCGGTGGTTT | |
| IL-17F_F | XM_426223 | CGCTTCCCCCAGGTGATT |
| IL-17F_R | CGCTTCCCCCAGGTGATT | |
| IFN-γ_F | NM_205149 | GCTCCCGATGAACGACTTGA |
| IFN-γ_R | TGTAAGATGCTGAAGAGTTCATTCG | |
| iNOS_F | D85422 | CCTGTACTGAAGGTGGCTATTGG |
| iNOS_R | AGGCCTGTGAGAGTGTGCAA | |
| MIF_F | M95776 | GCCCGCGCAGTACATAGC |
| MIF_R | CCCCCGAAGGACATCATCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omara, I.I.; Pender, C.M.; White, M.B.; Dalloul, R.A. The Modulating Effect of Dietary Beta-Glucan Supplementation on Expression of Immune Response Genes of Broilers during a Coccidiosis Challenge. Animals 2021, 11, 159. https://doi.org/10.3390/ani11010159
Omara II, Pender CM, White MB, Dalloul RA. The Modulating Effect of Dietary Beta-Glucan Supplementation on Expression of Immune Response Genes of Broilers during a Coccidiosis Challenge. Animals. 2021; 11(1):159. https://doi.org/10.3390/ani11010159
Chicago/Turabian StyleOmara, Islam I., Chasity M. Pender, Mallory B. White, and Rami A. Dalloul. 2021. "The Modulating Effect of Dietary Beta-Glucan Supplementation on Expression of Immune Response Genes of Broilers during a Coccidiosis Challenge" Animals 11, no. 1: 159. https://doi.org/10.3390/ani11010159
APA StyleOmara, I. I., Pender, C. M., White, M. B., & Dalloul, R. A. (2021). The Modulating Effect of Dietary Beta-Glucan Supplementation on Expression of Immune Response Genes of Broilers during a Coccidiosis Challenge. Animals, 11(1), 159. https://doi.org/10.3390/ani11010159





