Physiological Response of Pelophylax nigromaculatus Adults to Salinity Exposure
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design and Treatment
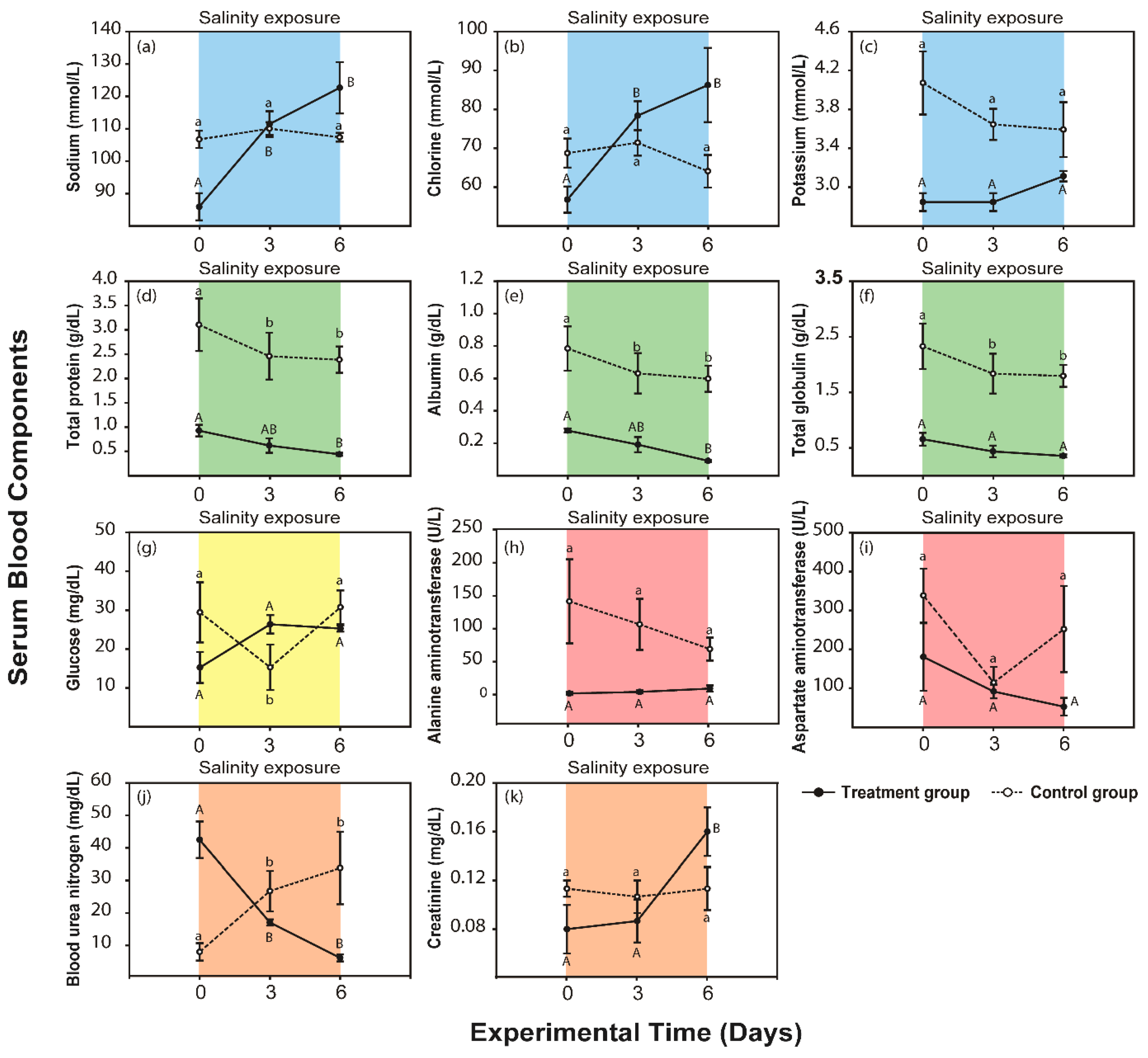
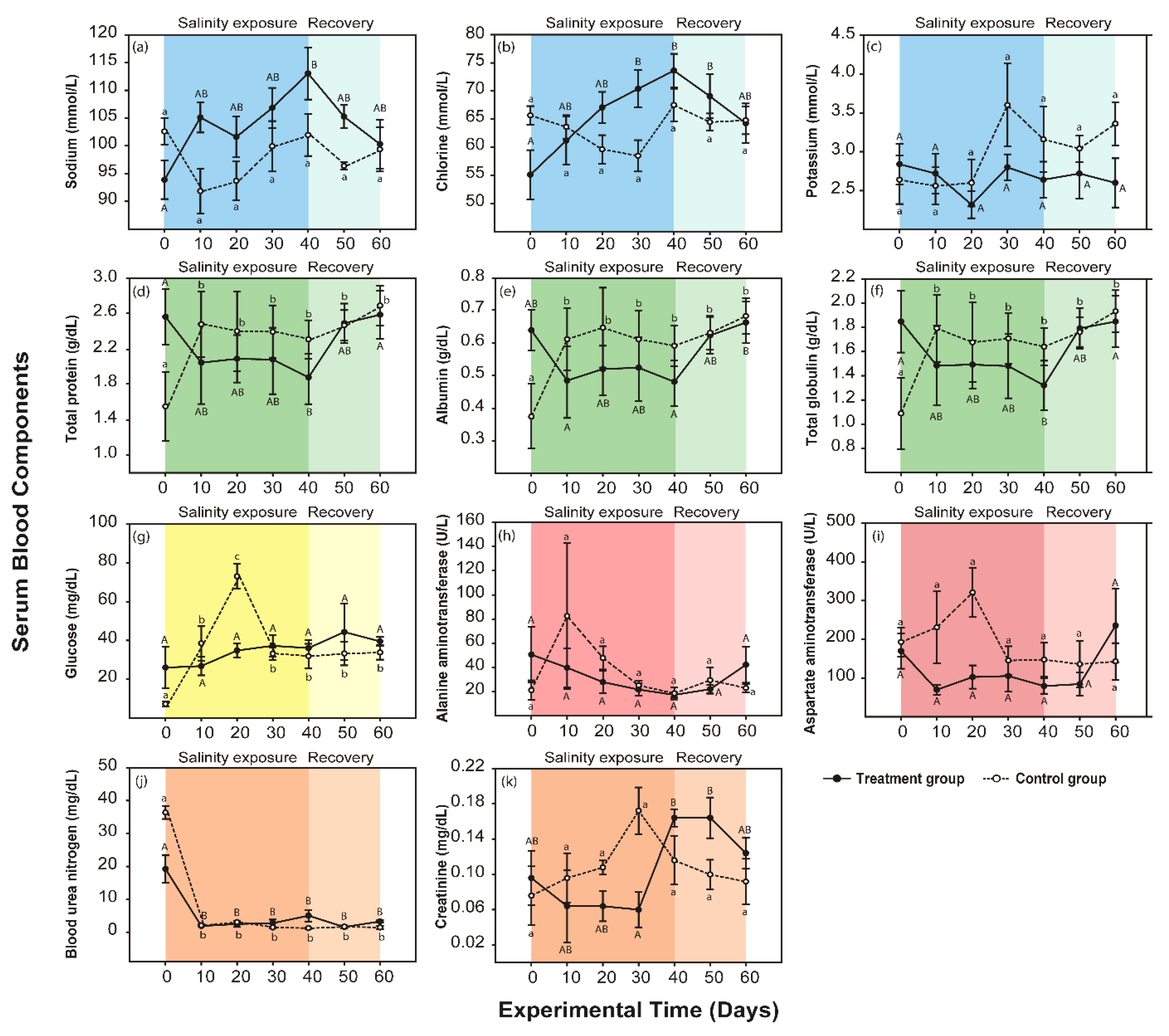
2.3. Blood Samples and Serum Chemistry Analysis
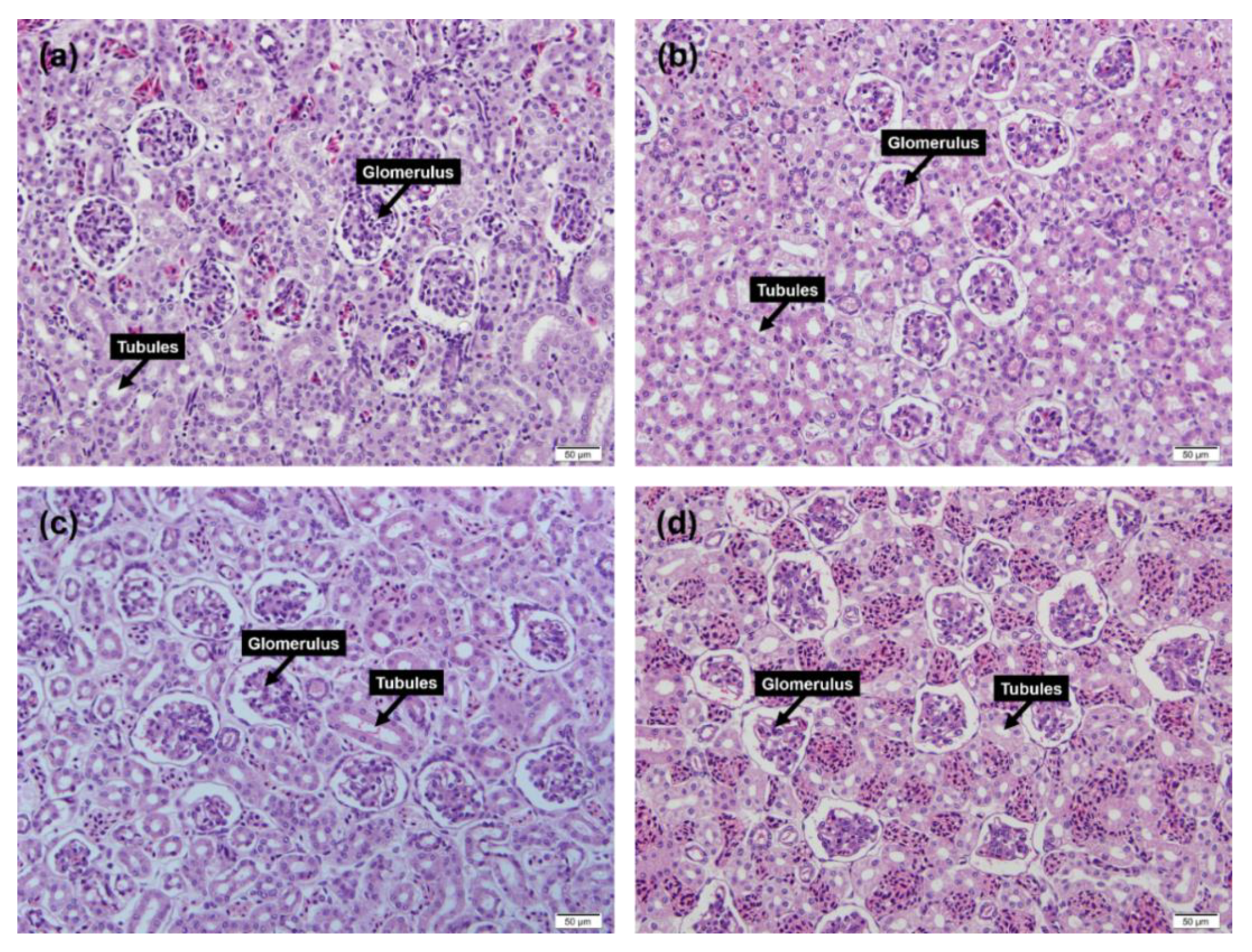
2.4. Histology
2.5. Statistical Analysis
3. Results
3.1. Response to the Severe Condition
3.2. Response to the Moderate Condition
3.3. Renal Tissue Inspection
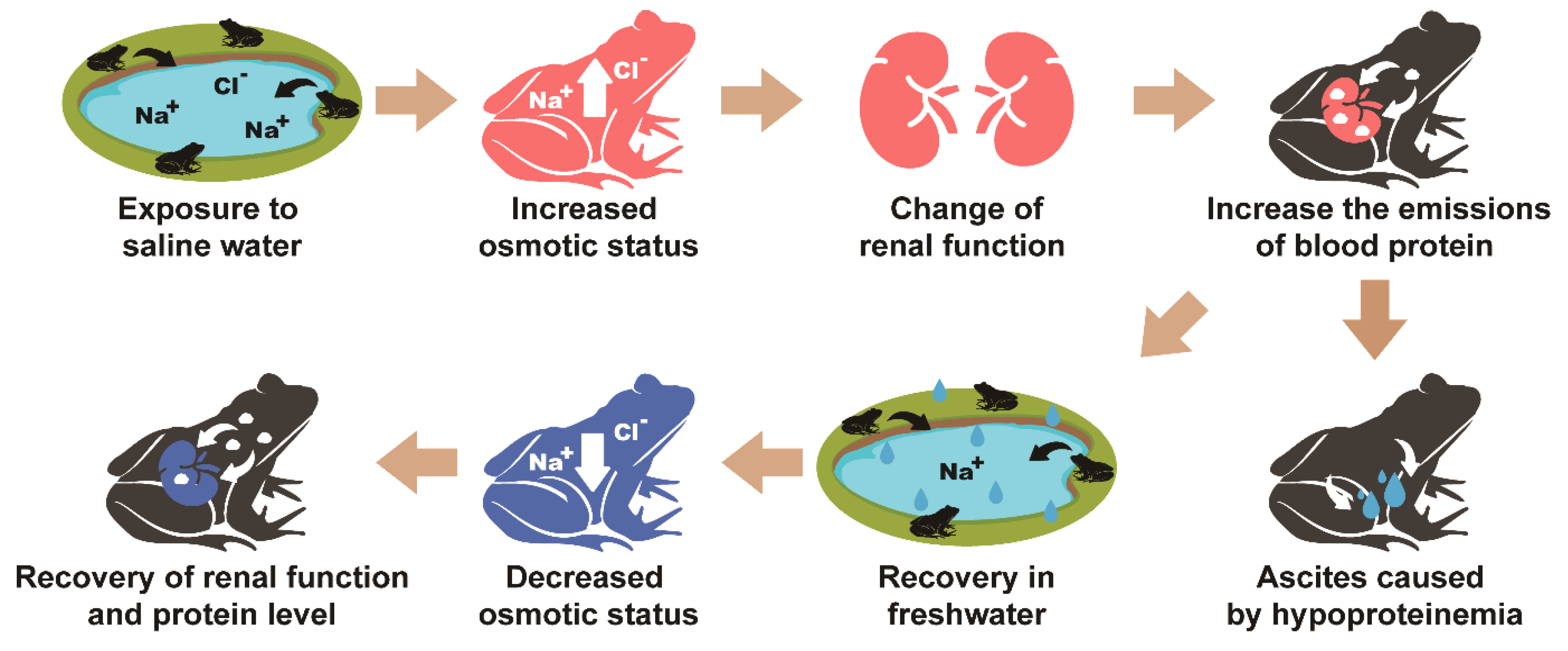
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Parameters | Field | Severe Condition | Moderate Condition | |||
| EXP | CON | EXP-T | EXP-R | CON | ||
| Temperature (°C) | 17.9 ± 1.8 | 21.5 ± 0.1 | 22.5 ± 0.1 | 23.3 ± 1.3 | 22.8 ± 1.8 | 22.5 ± 1.4 |
| SPC (μS/cm) | 292.1 ± 194.5 | 9385 ± 77 | 351 ± 5 | 8991 ± 549 | 507 ± 149 | 585 ± 176 |
| C (μS/cm) | 250.4 ± 167.6 | 8736 ± 62 | 335 ± 4 | 8696 ± 505 | 453 ± 152 | 564 ± 178 |
| Salinity (‰) | 0.14 ± 0.1 | 5.25 ± 0.04 | 0.17 ± 0.01 | 5.23 ± 0.9 | 0.24 ± 0.07 | 0.28 ± 0.08 |
| pH | 7.6 ± 0.1 | 8.4 ± 0.2 | 8.02 ± 0.16 | 8.1 ± 0.2 | 8.1 ± 0.1 | 8.2 ± 0.3 |
References
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Utz, R.M.; Haq, S.; Gorman, J.; Grese, M. Freshwater salinization syndrome on a continental. Proc. Natl. Acad. Sci. USA 2018, 115, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.M.; Gell, P. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 2015, 6, 1–43. [Google Scholar] [CrossRef]
- Venâncio, C.; Castro, B.B.; Ribeiro, R.; Antunes, S.C.; Lopes, I. Sensitivity to salinization and acclimation potential of amphibian (Pelophylax perezi) and fish (Lepomis gibbosus) models. Ecotoxicol. Environ. Saf. 2019, 172, 348–355. [Google Scholar] [CrossRef]
- Hopkins, G.R.; Brodie, E.D., Jr. Occurrence of amphibians in saline habitats: A review and evolutionary perspective. Herpetol. Monogr. 2015, 29, 1–27. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Hall, E.M.; Brady, S.P.; Mattheus, N.M.; Earley, R.L.; Diamond, M.; Crespi, E.J. Physiological consequences of exposure to salinized roadside ponds on wood frog larvae and adults. Biol. Conserv. 2017, 209, 98–106. [Google Scholar] [CrossRef]
- Albecker, M.A.; Michael, W.M. Adaptive responses to salinity stress across multiple life stages in anuran amphibians. Front. Zool. 2017, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Karraker, N.E.; Arrigoni, J.; Dudgeon, D. Effects of increased salinity and an introduced predator on lowland amphibians in Southern China: Species identity matters. Biol. Conserv. 2010, 143, 1079–1086. [Google Scholar] [CrossRef]
- Jena, S.; Palita, S.; Mahapatra, M. Anurans of Bhitarkanika mangroves, Odisha, east coast of India. Check List 2013, 9, 400–404. [Google Scholar] [CrossRef]
- Wu, C.-S.; Gomez-Mestre, I.; Kam, Y.-C. Irreversibility of a bad start: Early exposure to osmotic stress limits growth and adaptive developmental plasticity. Oecologia 2012, 169, 15–22. [Google Scholar] [CrossRef]
- Collins, S.J.; Russell, R.W. Toxicity of road salt to Nova Scotia amphibians. Environ. Pollut. 2009, 157, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Gunzburger, M.S.; Hughes, W.B.; Barichivich, W.J.; Staiger, J.S. Hurricane storm surge and amphibian communities in coastal wetlands of northwestern Florida. Wetl. Ecol. Manag. 2010, 18, 651–663. [Google Scholar] [CrossRef]
- Moreira, L.F.B.; Knauth, D.S.; Maltchik, L. Intermittently closed estuaries and tadpole communities: Influence of artificial breaching. Estuaries Coasts 2015, 38, 979–987. [Google Scholar] [CrossRef]
- de Olivera, I.S.; Roedder, D.; Toledo, L.F. Potential worldwide impacts of sea level rise on coastal-lowland anurans. North West. J. Zool. 2016, 12, 91–101. [Google Scholar]
- Haramura, T. Experimental test of spawning site selection by Buergeria japonica (Anura: Rhacophoridae) in response to salinity level. Copeia 2008, 2008, 64–67. [Google Scholar] [CrossRef]
- Wu, C.S.; Yang, W.K.; Lee, T.H.; Gomez-Mestre, I.; Kam, Y.C. Salinity acclimation enhances salinity tolerance in tadpoles living in brackish water through increased Na+, K+-ATPase expression. J. Exp. Zool. A Ecol. Genet. Physiol. 2014, 321, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.T.; Wu, C.S.; Hatch, K.; Chang, Y.M.; Kam, Y.C. Full compensation of growth in salt-tolerant tadpoles after release from salinity stress. J. Zool. 2018, 304, 141–149. [Google Scholar] [CrossRef]
- Squires, Z.E.; Bailey, P.C.; Reina, R.D.; Wong, B.B. Compensatory growth in tadpoles after transient salinity stress. Mar. Freshw. Res. 2010, 61, 219–222. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Tejedo, M. Local adaptation of an anuran amphibian to osmotically stressful environments. Evolution 2003, 57, 1889–1899. [Google Scholar] [CrossRef]
- Weinbach, A.; Cayuela, H.; Grolet, O.; Besnard, A.; Joly, P. Resilience to climate variation in a spatially structured amphibian population. Sci. Rep. 2018, 8, 14607. [Google Scholar] [CrossRef]
- Hodgson, D.; McDonald, J.L.; Hosken, D.J. What do you mean, ‘resilient’? Trends Ecol. Evol. 2015, 30, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T. A developmentally informed perspective on the relation between stress and psychopathology: When the problem with stress is that there is not enough. J. Abnorm. Psychol. 2015, 124, 80–92. [Google Scholar] [CrossRef]
- Hopkins, G.R.; Brodie, E.D., Jr.; Neuman-Lee, L.A.; Mohammadi, S.; Brusch, G.A., IV; Hopkins, Z.M.; French, S.S. Physiological responses to salinity vary with proximity to the ocean in a coastal amphibian. Physiol. Biochem. Zool. 2016, 89, 322–330. [Google Scholar] [CrossRef]
- Albecker, M.A.; Brantley, W.B.; McCoy, M.W. Diet and Nematode Infection Differ Between Coastal and Inland Populations of Green Treefrogs (Hyla cinerea). Southeast Nat. 2018, 17, 155–166. [Google Scholar] [CrossRef]
- Stockwell, M.; Clulow, J.; Mahony, M. Evidence of a salt refuge: Chytrid infection loads are suppressed in hosts exposed to salt. Oecologia 2015, 177, 901–910. [Google Scholar] [CrossRef]
- Klop-Toker, K.L.; Valdez, J.W.; Stockwell, M.P.; Edgar, M.E.; Fardell, L.; Clulow, S.; Clulow, J.; Mahony, M.J. Assessing host response to disease treatment: How chytrid-susceptible frogs react to increased water salinity. Wildl. Res. 2018, 44, 648–659. [Google Scholar] [CrossRef]
- Katz, U. Strategies of adaptation to osmotic stress in anuran amphibia under salt and burrowing conditions. Comp. Biochem. Physiol. A Physiol. 1989, 93, 499–503. [Google Scholar] [CrossRef]
- Balinsky, J.B. Adaptation of nitrogen metabolism to hyperosmotic environment in Amphibia. J. Exp. Zool. 1981, 215, 335–350. [Google Scholar] [CrossRef]
- Wake, D.B.; Koo, M.S. Amphibians. Curr. Biol. 2018, 28, R1237–R1241. [Google Scholar] [CrossRef] [PubMed]
- Amphibiaweb. Available online: https://amphibiaweb.org/lists/index.shtml (accessed on 15 April 2020).
- Divers, S.J.; Cooper, J.E. Reptile hepatic lipidosis. Semin. Avian. Exot. Pet. Med. 2000, 9, 153–164. [Google Scholar] [CrossRef]
- Salazar, J.H. Overview of urea and creatinine. Lab. Med. 2014, 45, 19–20. [Google Scholar] [CrossRef]
- Medina, M.F.; Gonzalez, M.E.; Klyver, S.M.R.; Odstrcil, I.M.A. Histopathological and biochemical changes in the liver, kidney, and bloodof amphibians intoxicated with cadmium. Turk. J. Biol. 2016, 40, 229–238. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Tejedo, M.; Ramayo, E.; Estepa, J. Developmental alterations and osmoregulatory physiology of a larval anuran under osmotic stress. Physiol. Biochem. Zool. 2004, 77, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Forzán, M.J.; Heatley, J.; Russell, K.E.; Horney, B. Clinical pathology of amphibians: A review. Vet. Clin. Pathol. 2017, 46, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, W.M.; Connor, W.E.; Abboud, F.; Rastogi, S.; Anderson, T.A.; Fry, M. The effect of dietary sodium chloride on blood pressure, body fluids, electrolytes, renal function, and serum lipids of normotensive man. J. Lab. Clin. Med. 1976, 87, 418–434. [Google Scholar]
- Buranakarl, C.; Mathur, S.; Brown, S.A. Effects of dietary sodium chloride intake on renal function and blood pressure in cats with normal and reduced renal function. Am. J. Vet. Res. 2004, 65, 620–627. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Casey, A.; Greenhaff, P.L. Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance? Am. J. Clin. Nutr. 2000, 72, 607–617. [Google Scholar] [CrossRef]
- Kaneko, J.J. Carbohydrate metabolism and its diseases. In Clinical Biochemistry of Domestic Animals, 6th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 45–81. [Google Scholar]
- Hwang, P.P.; Lee, T.H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 479–497. [Google Scholar] [CrossRef]
- Lai, J.-C.; Kam, Y.-C.; Lin, H.-C.; Wu, C.-S. Enhanced salt tolerance of euryhaline tadpoles depends on increased Na+, K+-ATPase expression after salinity acclimation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 227, 84–91. [Google Scholar] [CrossRef]
- Hillman, S.S.; Withers, P.C.; Drewes, R.C.; Hillyard, S.D. Ecological and Environmental Physiology of Amphibians; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Hillman, S.S. Anuran amphibians as comparative models for understanding extreme dehydration tolerance: A unique negative feedback lymphatic mechanism for blood volume regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R790–R798. [Google Scholar] [CrossRef] [PubMed]
- Degani, G. Salinity tolerance and osmoregulation in Salamandra salamandra (L.) from different populations. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1981, 145, 133–137. [Google Scholar] [CrossRef]
- Hoffman, J. Physiological response of Bufo viridis (Laurenti, 1768) populations across an aridity gradient. Alytes 2014, 30, 33–41. [Google Scholar]
- Degani, G.; Silanikove, N.; Shkolnik, A. Adaptation of green toad (Bufo viridis) to terrestrial life by urea accumulation. Comp. Biochem. Physiol. A Physiol. 1984, 77, 585–587. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Tejedo, M. Adaptation or exaptation? An experimental test of hypotheses on the origin of salinity tolerance in Bufo calamita. J. Evol. Biol. 2005, 18, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Bernabò, I.; Bonacci, A.; Coscarelli, F.; Tripepi, M.; Brunelli, E. Effects of salinity stress on Bufo balearicus and Bufo bufo tadpoles: Tolerance, morphological gill alterations and Na+/K+-ATPase localization. Aquat. Toxicol. 2013, 132, 119–133. [Google Scholar] [CrossRef]
- Brady, S.P. Microgeographic maladaptive performance and deme depression in response to roads and runoff. PeerJ 2013, 1, e163. [Google Scholar] [CrossRef]


| Components | Mean ± SD | 95% CI | Median | 25% to 75% |
|---|---|---|---|---|
| Sodium | 105.6 ± 12.3 | 101.6–109.6 | 105.6 | 98.5–111.7 |
| Chlorine | 70.7 ± 3.2 | 67.5–73.9 | 69.4 | 63.9–76.2 |
| Potassium | 3.3 ± 0.8 | 3.00–3.5 | 3.1 | 2.6–3.6 |
| Total protein | 2.2 ± 1.1 | 1.8–2.6 | 2.07 | 1.5–2.7 |
| Albumin | 0.6 ± 0.3 | 0.47–0.66 | 0.53 | 0.37–0.66 |
| Total globulin | 1.7 ± 0.8 | 1.3–1.9 | 1.57 | 1.1–2.0 |
| Glucose | 25.1 ± 31.6 | 14.7–35.4 | 14.32 | 10.1–30.2 |
| ALT | 66.2 ± 115.1 | 28.6–103.8 | 28.63 | 17.5–60.1 |
| AST | 264 ± 257 | 180–348 | 200.41 | 127.3–306.7 |
| BUN | 24.1 ± 15.3 | 19.1–29.2 | 25.4 | 9.9–36.2 |
| Creatinine | 0.1 ± 0.06 | 0.08–0.12 | 0.1 | 0.06–0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-K.; Do, Y. Physiological Response of Pelophylax nigromaculatus Adults to Salinity Exposure. Animals 2020, 10, 1698. https://doi.org/10.3390/ani10091698
Park J-K, Do Y. Physiological Response of Pelophylax nigromaculatus Adults to Salinity Exposure. Animals. 2020; 10(9):1698. https://doi.org/10.3390/ani10091698
Chicago/Turabian StylePark, Jun-Kyu, and Yuno Do. 2020. "Physiological Response of Pelophylax nigromaculatus Adults to Salinity Exposure" Animals 10, no. 9: 1698. https://doi.org/10.3390/ani10091698
APA StylePark, J.-K., & Do, Y. (2020). Physiological Response of Pelophylax nigromaculatus Adults to Salinity Exposure. Animals, 10(9), 1698. https://doi.org/10.3390/ani10091698




