Trefoil Factor Family Member 2 (TFF2) as an Inflammatory-Induced and Anti-Inflammatory Tissue Repair Factor
Abstract
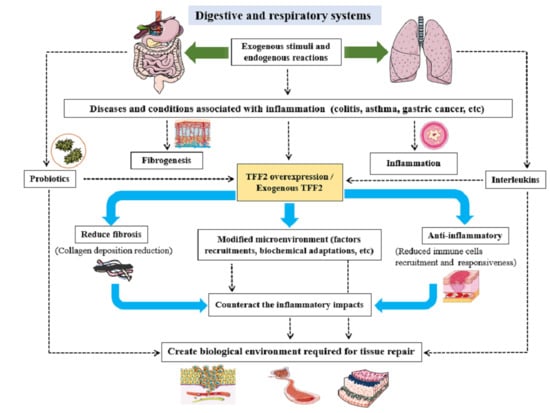
Dear Editor,
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jørgensen, K.H.; Thim, L.; Jacobsen, H.E. Pancreatic spasmolytic polypeptide (PSP): I. Preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul. Pept. 1982, 3, 207–219. [Google Scholar] [CrossRef]
- Royce, S.G.; Lim, C.; Muljadi, R.C.; Samuel, C.S.; Ververis, K.; Karagiannis, T.C.; Giraud, A.S.; Tang, M.L. Trefoil factor-2 reverses airway remodeling changes in allergic airways disease. Am. J. Respir. Cell Mol. Biol. 2013, 48, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Taupin, D.; Koh, T.J.; Chen, D.; Zhao, C.-M.; Podolsky, D.K.; Wang, T.C. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J. Clin. Investig. 2002, 109, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Aamann, L.; Vestergaard, E.M.; Grønbæk, H. Trefoil factors in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Marchbank, T.; Chinery, R.; Evison, R.; Pignatelli, M.; Boulton, R.A.; Thim, L.; Hanby, A.M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology 1995, 108, 108–116. [Google Scholar] [CrossRef]
- McKenzie, C.; Marchbank, T.; Playford, R.J.; Otto, W.; Thim, L.; Parsons, M.E. Pancreatic spasmolytic polypeptide protects the gastric mucosa but does not inhibit acid secretion or motility. Am. J. Physiol. 1997, 273 Pt 1, G112–G117. [Google Scholar] [CrossRef]
- Aihara, E.; Engevik, K.A.; Montrose, M.H. Trefoil Factor Peptides and Gastrointestinal Function. Annu. Rev. Physiol. 2017, 79, 357–380. [Google Scholar] [CrossRef]
- Greeley, M.A.; Van Winkle, L.S.; Edwards, P.C.; Plopper, C.G. Airway trefoil factor expression during naphthalene injury and repair. Toxicol. Sci. 2010, 113, 453–467. [Google Scholar] [CrossRef]
- Heuer, F.; Stürmer, R.; Heuer, J.; Kalinski, T.; Lemke, A.; Meyer, F.; Hoffmann, W. Different Forms of TFF2, A Lectin of the Human Gastric Mucus Barrier: In Vitro Binding Studies. Int. J. Mol. Sci. 2019, 20, 5871. [Google Scholar] [CrossRef]
- Royce, S.G.; Lim, C.; Muljadi, R.C.; Tang, M.L. Trefoil factor 2 regulates airway remodeling in animal models of asthma. J. Asthma 2011, 48, 653–659. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Mucunguzi, O.; Yoshioka, M.; St-Amand, J. Energy and metabolic pathways in trefoil factor family member 2 (Tff2) KO mice beyond the protection from high-fat diet-induced obesity. Life Sci. 2018, 215, 190–197. [Google Scholar] [CrossRef]
- Katoh, M. Trefoil factors and human gastric cancer (review). Int. J. Mol. Med. 2003, 12, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Pierzchalski, P.; Kwiecien, S.; Pajdo, R.; Hahn, E.G.; Konturek, S.J. Activation of genes for spasmolytic peptide, transforming growth factor alpha and for cyclooxygenase (COX)-1 and COX-2 during gastric adaptation to aspirin damage in rats. Aliment. Pharm. 1998, 12, 767–777. [Google Scholar] [CrossRef]
- Hanisch, F.G.; Bonar, D.; Schloerer, N.; Schroten, H. Human trefoil factor 2 is a lectin that binds α-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori. J. Biol. Chem. 2014, 289, 27363–27375. [Google Scholar] [CrossRef] [PubMed]
- Sébert, M.; Sola-Tapias, N.; Mas, E.; Barreau, F.; Ferrand, A. Protease-Activated Receptors in the Intestine: Focus on Inflammation and Cancer. Front. Endocrinol. (Lausanne) 2019, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Lu, N.; Zou, F.; Meng, F.Z. Sirtuins as novel targets in the pathogenesis of airway inflammation in bronchial asthma. Eur. J. Pharm. 2019, 865, 172670. [Google Scholar] [CrossRef]
- Schuliga, M. NF-kappaB Signaling in Chronic Inflammatory Airway Disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more (Review). Int. J. Oncol. 2015, 47, 806–816. [Google Scholar] [CrossRef]
- Ortiz-Masiá, D.; Hernández, C.; Quintana, E.; Velázquez, M.; Cebrián, S.; Riaño, A.; Calatayud, S.; Esplugues, J.V.; Barrachina, M.D. iNOS-derived nitric oxide mediates the increase in TFF2 expression associated with gastric damage: Role of HIF-1. FASEB J. 2010, 24, 136–145. [Google Scholar] [CrossRef]
- Nikolaidis, N.M.; Zimmermann, N.; King, N.E.; Mishra, A.; Pope, S.M.; Finkelman, F.D.; Rothenberg, M.E. Trefoil factor-2 is an allergen-induced gene regulated by Th2 cytokines and STAT6 in the lung. Am. J. Respir. Cell Mol. Biol. 2003, 29, 458–464. [Google Scholar] [CrossRef]
- Knipper, J.A.; Willenborg, S.; Brinckmann, J.; Bloch, W.; Maaß, T.; Wagener, R.; Krieg, T.; Sutherland, T.; Munitz, A.; Rothenberg, M.E.; et al. Interleukin-4 Receptor α Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity 2015, 43, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Wang, X.; King, A.; Le, L.D.; Bhattacharya, S.S.; Moles, C.M.; Butte, M.J.; de Jesus Perez, V.A.; Liechty, K.W.; Wight, T.N.; et al. Interleukin-10-mediated regenerative postnatal tissue repair is dependent on regulation of hyaluronan metabolism via fibroblast-specific STAT3 signaling. FASEB J. 2017, 31, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Hadian, Y.; Bagood, M.D.; Dahle, S.E.; Sood, A.; Isseroff, R.R. Interleukin-17: Potential Target for Chronic Wounds. Mediat. Inflamm. 2019, 2019, 1297675. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Santamatilde, E.; McCreath, K.J.; Cervera, A.M.; Díez, I.; Ortiz-Masiá, D.; Martínez, N.; Calatayud, S.; Esplugues, J.V.; Barrachina, M.D. Induction of trefoil factor (TFF)1, TFF2 and TFF3 by hypoxia is mediated by hypoxia inducible factor-1: Implications for gastric mucosal healing. Br. J. Pharmacol. 2009, 156, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Azarschab, P.; Al-Azzeh, E.; Kornberger, W.; Gött, P. Aspirin promotes TFF2 gene activation in human gastric cancer cell lines. FEBS Lett. 2001, 488, 206–210. [Google Scholar] [CrossRef]
- Hu, G.Y.; Yu, B.P.; Dong, W.G.; Li, M.Q.; Yu, J.P.; Luo, H.S.; Rang, Z.X. Expression of TFF2 and Helicobacter pylori infection in carcinogenesis of gastric mucosa. World J. Gastroenterol. 2003, 9, 910–914. [Google Scholar] [CrossRef]
- Tran, C.P.; Cook, G.A.; Yeomans, N.D.; Thim, L.; Giraud, A.S. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut 1999, 44, 636–642. [Google Scholar] [CrossRef]
- Judd, L.M.; Chalinor, H.V.; Walduck, A.; Pavlic, D.I.; Däbritz, J.; Dubeykovskaya, Z.; Wang, T.C.; Menheniott, T.R.; Giraud, A.S. TFF2 deficiency exacerbates weight loss and alters immune cell and cytokine profiles in DSS colitis, and this cannot be rescued by wild-type bone marrow. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G12–G24. [Google Scholar] [CrossRef]
- Ellermann, M.; Gharaibeh, R.Z.; Fulbright, L.; Dogan, B.; Moore, L.N.; Broberg, C.A.; Lopez, L.R.; Rothemich, A.M.; Herzog, J.W.; Rogala, A.; et al. Yersiniabactin-Producing Adherent/Invasive Escherichia coli Promotes Inflammation-Associated Fibrosis in Gnotobiotic Il10(-/-) Mice. Infect. Immun. 2019, 87, e00587-19. [Google Scholar] [CrossRef]
- Gordon, I.O.; Agrawal, N.; Willis, E.; Goldblum, J.R.; Lopez, R.; Allende, D.; Liu, X.; Patil, D.Y.; Yerian, L.; El-Khider, F.; et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment. Pharmacol. Ther. 2018, 47, 922–939. [Google Scholar] [CrossRef]
- Soriano-Izquierdo, A.; Gironella, M.; Massaguer, A.; May, F.E.; Salas, A.; Sans, M.; Poulsom, R.; Thim, L.; Piqué, J.M.; Panés, J. Trefoil peptide TFF2 treatment reduces VCAM-1 expression and leukocyte recruitment in experimental intestinal inflammation. J. Leukoc. Biol. 2004, 75, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Giraud, A.S.; Pereira, P.M.; Thim, L.; Parker, L.M.; Judd, L.M. TFF-2 inhibits iNOS/NO in monocytes, and nitrated protein in healing colon after colitis. Peptides 2004, 25, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.K.; Wang, T.C.; Maruyama, R.; Udagawa, J.; Kubota, H.; Fuji, T.; Tachibana, M.; Ono, T.; Otani, H.; Nagasue, N. Expression of Cytoplasmic TFF2 Is a Marker of Tumor Metastasis and Negative Prognostic Factor in Gastric Cancer. Lab. Investig. 2003, 83, 1343–1352. [Google Scholar] [CrossRef]
- Schmidt, P.H.; Lee, J.R.; Joshi, V.; Playford, R.J.; Poulsom, R.; Wright, N.A.; Goldenring, J.R. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab. Investig. 1999, 79, 639–646. [Google Scholar]
- Cai, Y.; Yi, M.; Chen, D.; Liu, J.; Guleng, B.; Ren, J.; Shi, H. Trefoil factor family 2 expression inhibits gastric cancer cell growth and invasion in vitro via interactions with the transcription factor Sp3. Int. J. Mol. Med. 2016, 38, 1474–1480. [Google Scholar] [CrossRef]
- Longman, R.J.; Douthwaite, J.; Sylvester, P.A.; Poulsom, R.; Corfield, A.P.; Thomas, M.G.; Wright, N.A. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut 2000, 47, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Khoder, G.; Al-Yassir, F.; Al Menhali, A.; Saseedharan, P.; Sugathan, S.; Tomasetto, C.; Karam, S.M. Probiotics Upregulate Trefoil Factors and Downregulate Pepsinogen in the Mouse Stomach. Int. J. Mol. Sci. 2019, 20, 3901. [Google Scholar] [CrossRef] [PubMed]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-Tov, H.; Davis, S.C.; Tomic-Canic, M.; Pastar, I. Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017, 25, 912–922. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef]
- Liu, Y.; Alookaran, J.J.; Rhoads, J.M. Probiotics in Autoimmune and Inflammatory Disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef]
- Yoshioka, M.; Bolduc, C.; Raymond, V.; St-Amand, J. High-fat meal-induced changes in the duodenum mucosa transcriptome. Obesity (Silver Spring) 2008, 16, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, M.R.; Yoshioka, M.; Riedl, I.; Moreault, O.; Cherizol, R.G.; Shah, A.A.; Blin, N.; Richard, D.; St-Amand, J. Trefoil factor family member 2 (Tff2) KO mice are protected from high-fat diet-induced obesity. Obesity (Silver Spring) 2013, 21, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; St-Amand, J. Interleukin-6 as a “metabolic hormone”. Cytokine 2018, 112, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.T.; Son, D.J.; Lee, C.K.; Yoon, D.Y.; Lee, D.H.; Park, M.H. Interleukin 32, inflammation and cancer. Pharmacol. Ther. 2017, 174, 127–137. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Wagner, E.F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016, 30, 489–501. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Judd, L.M.; Alderman, B.M.; Howlett, M.; Shulkes, A.; Dow, C.; Moverley, J.; Grail, D.; Jenkins, B.J.; Ernst, M.; Giraud, A.S. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology 2004, 126, 196–207. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 2019, 11, 2175. [Google Scholar] [CrossRef]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Broken Energy Homeostasis and Obesity Pathogenesis: The Surrounding Concepts. J. Clin. Med. 2018, 7, 453. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise and High-Fat Diet in Obesity: Functional Genomics Perspectives of Two Energy Homeostasis Pillars. Genes (Basel) 2020, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Akhter, N.; Kochumon, S.; Thomas, R.; Wilson, A.; Shenouda, S.; Tuomilehto, J.; Ahmad, R. Increased Expression of the Innate Immune Receptor TLR10 in Obesity and Type-2 Diabetes: Association with ROS-Mediated Oxidative Stress. Cell. Physiol. Biochem. 2018, 45, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; St-Amand, J. Redefining obesity toward classifying as a disease. Eur. J. Intern. Med. 2018, 55, 20–22. [Google Scholar] [CrossRef]
- Taupin, D.; Wu, D.-C.; Jeon, W.-K.; Devaney, K.; Wang, T.C.; Podolsky, D.K. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor– and MAP kinase–dependent interregulation. J. Clin. Investig. 1999, 103, R31–R38. [Google Scholar] [CrossRef]
- Lefebvre, O.; Chenard, M.-P.; Masson, R.; Linares, J.; Dierich, A.; LeMeur, M.; Wendling, C.; Tomasetto, C.; Chambon, P.; Rio, M.-C. Gastric Mucosa Abnormalities and Tumorigenesis in Mice Lacking the pS2 Trefoil Protein. Science 1996, 274, 259–262. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef]
- Kirikoshi, H.; Katoh, M. Expression of TFF1, TFF2 and TFF3 in gastric cancer. Int. J. Oncol. 2002, 21, 655–659. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and inflammation: Another homeostatic property? Cytokine 2020, 133, 155179. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and cancer: A homeostatic hormone? Cytokine 2020, 127, 154996. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine: Metabolic and Homeostatic Properties beyond the Extracellular Matrix Structure. Appl. Sci. 2020, 10, 2388. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and bioenergetics: Extracellular matrix, adipocytes remodeling and skeletal muscle metabolism. Int. J. Biochem. Cell Biol. 2019, 117, 105627. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanemi, A.; Yoshioka, M.; St-Amand, J. Trefoil Factor Family Member 2 (TFF2) as an Inflammatory-Induced and Anti-Inflammatory Tissue Repair Factor. Animals 2020, 10, 1646. https://doi.org/10.3390/ani10091646
Ghanemi A, Yoshioka M, St-Amand J. Trefoil Factor Family Member 2 (TFF2) as an Inflammatory-Induced and Anti-Inflammatory Tissue Repair Factor. Animals. 2020; 10(9):1646. https://doi.org/10.3390/ani10091646
Chicago/Turabian StyleGhanemi, Abdelaziz, Mayumi Yoshioka, and Jonny St-Amand. 2020. "Trefoil Factor Family Member 2 (TFF2) as an Inflammatory-Induced and Anti-Inflammatory Tissue Repair Factor" Animals 10, no. 9: 1646. https://doi.org/10.3390/ani10091646
APA StyleGhanemi, A., Yoshioka, M., & St-Amand, J. (2020). Trefoil Factor Family Member 2 (TFF2) as an Inflammatory-Induced and Anti-Inflammatory Tissue Repair Factor. Animals, 10(9), 1646. https://doi.org/10.3390/ani10091646