Hyperhomocysteinemia Induced by Methionine Excess is Effectively Suppressed by Betaine in Geese
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design, Diets, and Management
2.3. Sample Collection and Analyses
2.4. Clinical Blood Parameters
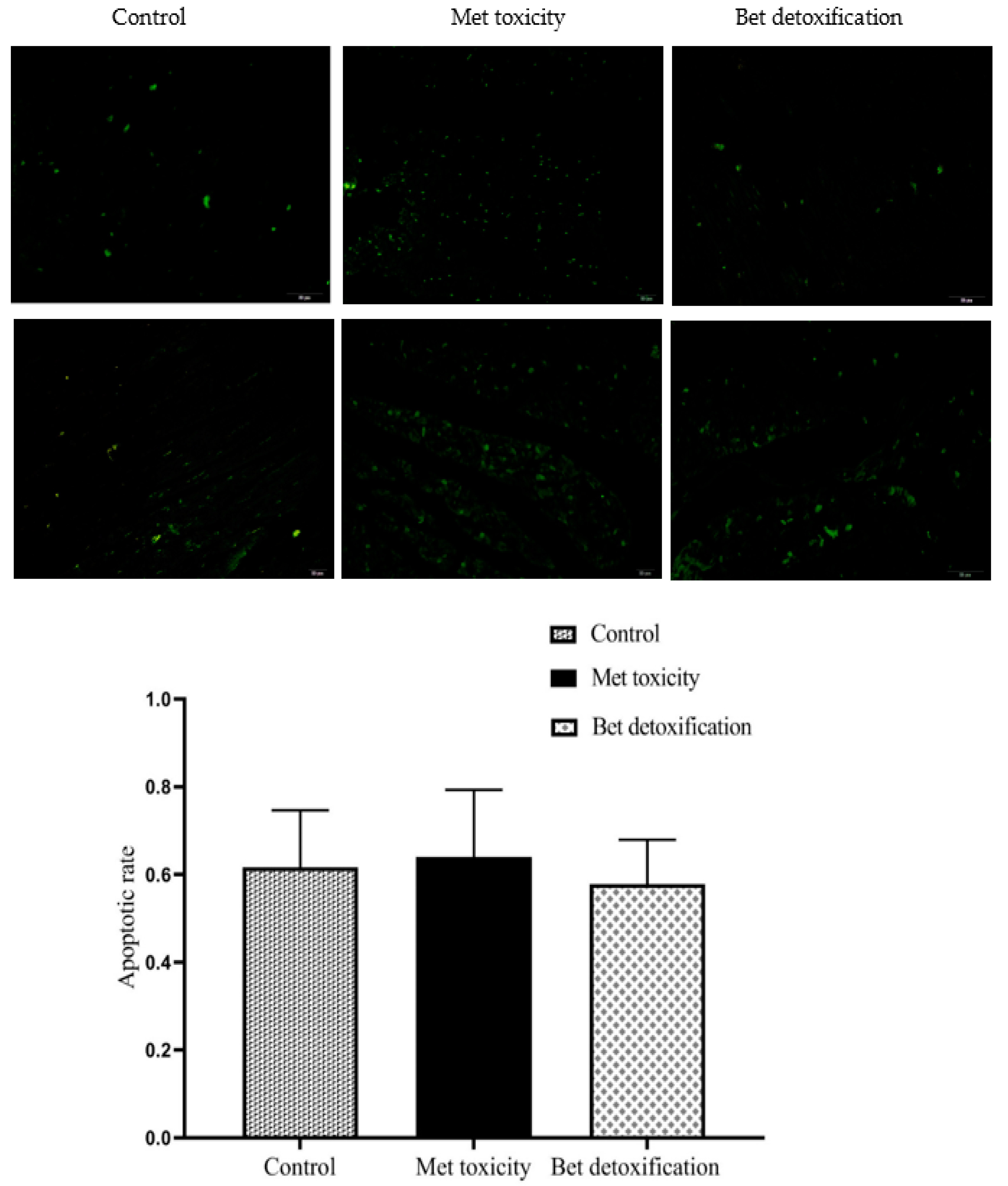
2.5. Apoptosis Assay
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Clinical Blood Parameters
3.3. Cardiomyocyte Apoptosis
3.4. Expression of Apoptosis-Related Proteins
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Moghadam, M.H.B.; Shehab, A.; Cherian, G. Methionine supplementation augments tissue n-3 fatty acid and tocopherol content in broiler birds fed flaxseed. Anim. Feed Sci. Technol. 2017, 228, 149–158. [Google Scholar] [CrossRef]
- Hasek, B.E.; Boudreau, A.; Shin, J.; Feng, D.; Hulver, M.; Van, N.T.; Laque, A.; Stewart, L.K.; Stone, K.P.; Wanders, D.; et al. Remodeling the integration of lipid metabolism between liver and adipose tissue by dietary methionine restriction in rats. Diabetes 2013, 62, 3362–3372. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Shi, S.R.; Zhou, Q.Y.; Yang, H.M.; Zou, J.M.; Zhang, K.N.; Han, H.M. Response of growing goslings to dietary methionine from 28 to 70 days of age. Br. Poult. Sci. 2010, 51, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Z.Y.; Yang, H.M.; Zhao, F.Z.; Kong, L.L. Response of growing goslings to dietary supplementation with methionine and betaine. Br. Poult. Sci. 2016, 57, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Abouelezz, K.F.M.; Cheng, Z.; Gad-Elkareem, A.E.G.; Fan, Q.; Ding, F.; Gao, J.; Jiang, S.; Jiang, Z. Modelling Methionine Requirements of Fast- and Slow-Growing Chinese Yellow-Feathered Chickens during the Starter Phase. Animals 2020, 10, 443. [Google Scholar]
- Xie, M.; Hou, S.S.; Huang, W.; Fan, H.P. Effect of excess methionine and methionine hydroxy analogue on growth performance and plasma homocysteine of growing pekin ducks. Poult. Sci. 2007, 86, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Carew, L.B.; Evarts, K.G.; Alster, F.A. Growth, feed intake, and plasma thyroid hormone levels in chicks fed dietary excesses of essential amino acids. Poult. Sci. 1998, 77, 295–298. [Google Scholar] [CrossRef]
- Fukada, S.I.; Morita, T.; Sugiyama, K. Effects of various amino acids on methionine-induced hyperhomocysteinemia in rats. Biosci. Biotechnol. Biochem. 2008, 72, 1940–1943. [Google Scholar] [CrossRef][Green Version]
- Zhu, B.T. On the mechanism of homocysteine pathophysiology and pathogenesis: A unifying hypothesis. Histol. Histopathol. 2002, 17, 1283–1291. [Google Scholar]
- McCully, K.S. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am. J. Pathol. 1969, 56, 111–128. [Google Scholar]
- Costa, M.Z.; da Silva, T.M.; Flores, N.P.; Schmitz, F.; Scherer, E.B.D.S.; Viau, C.M.; Saffi, J.; Barschak, A.G.; Wyse, A.T.d.S.; Spanevello, R.M.; et al. Methionine and methionine sulfoxide alter parameters of oxidative stress in the liver of young rats: In vitro and in vivo studies. Mol. Cell. Biochem. 2013, 384, 21. [Google Scholar] [CrossRef] [PubMed]
- Kockx, M.M.; Meyer, G.R.D.; Bult, J.; Bultinck, J.; Herman, A.G. Distribution of cell replication and apoptosis in atherosclerotic plaques of cholesterol-fed rabbits. Atherosclerosis 1996, 120, 115–124. [Google Scholar] [CrossRef]
- Hegyi, L.; Skepper, J.N.; Cary, N.R.B.; Mitchinson, M.J. Foam cell apoptosis and the development of the lipid core of human atherosclerosis. J. Pathol. 1996, 180, 423–429. [Google Scholar] [CrossRef]
- Clarke, M.C.H.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Littlewood, T.D.; Bennett, M.R. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 2006, 12, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Austin, R.C.; Lentz, S.R.; Werstuck, G.H. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004, 11, 56–64. [Google Scholar] [CrossRef]
- Setoue, M.; Ohuchi, S.; Morita, T.; Sugiyama, K. Hyperhomocysteinemia induced by guanidinoacetic acid is effectively suppressed by choline and betaine in rats. Biosci. Biotechol. Biochem. 2008, 72, 1696–1703. [Google Scholar] [CrossRef]
- Zhou, J.; MøLler, J.; Ritskes-Hoitinga, M.; Larsen, M.L.; Austin, R.C.; Falk, E. Effects of vitamin supplementation and hyperhomocysteinemia on atherosclerosis in apoE-deficient mice. Atherosclerosis 2003, 168, 255–262. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, 152–158. [Google Scholar] [CrossRef]
- Yamada, H.; Akahoshi, N.; Kamata, S.; Hagiya, Y.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Takana, N.; Mori, M.; Ishizaki, Y.; et al. Methionine excess in diet induces acute lethal hepatitis in mice lacking cystathionine γ-lyase, an animal model of cystathioninuria. Free Radic. Biol. Med. 2012, 52, 1716–1726. [Google Scholar] [CrossRef]
- Dibner, J.J.; Kitchell, M.L.; Robey, W.W.; Yersin, A.G.; Dunn, P.A.; Wideman, R.F. Liver damage and supplemental methionine sources in the diets of mature laying hens. J. Appl. Poult. Res. 1994, 3, 367–372. [Google Scholar] [CrossRef]
- Feussner, A.; Rolinski, B.; Weiss, N.; Deufel, T.; Wolfram, G.; Roscher, A.A. Determination of total homocysteine in human plasma by isocratic high-performance liquid chromatography. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 687–691. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steenge, G.R.; Verhoef, P.; Katan, M.B. Betaine supplementation lowers plasma homocysteine in healthy men and women. J. Nutr. 2003, 133, 1291–1295. [Google Scholar] [CrossRef]
- Brouwer, I.A. Betaine supplementation and plasma homocysteine in healthy volunteers. Arch. Intern. Med. 2000, 160, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.J.; Xie, M.; Tang, J.; Huang, W.; Zhang, Q.; Hou, S.S. Effects of excess DL- and L-methionine on growth performance of starter pekin ducks. Poult. Sci. 2017, 97, 946–950. [Google Scholar] [CrossRef]
- Dilger, R.N.; Kobler, C.; Weckbecker, C.; Hoehler, D.; Baker, D.H. 2-keto-4-(methylthio) butyric acid (keto analog of methionine) is a safe and efficacious precursor of L-methionine in chicks. J. Nutr. 2007, 137, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Jia, Z.F.; Han, T.; Inakuma, T.; Miyashita, T.; Sugiyama, K.; Sun, L.C.; Xiang, X.S.; Huang, Z.W. Suppression effects of betaine-enriched spinach on hyperhomocysteinemia induced by guanidinoacetic acid and choline deficiency in rats. Sci. World J. 2014, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nasir, K.; Tsai, M.; Rosen, B.D.; Fernandes, V.; Bluemke, D.A.; Folsom, A.R.; Liam, J.A.C. Elevated homocysteine is associated with reduced regional left ventricular function: The multi-ethnic study of atherosclerosis. Circulation 2007, 115, 180–187. [Google Scholar] [CrossRef]
- Kim, J.M.; Stewart, R.; Kim, S.W.; Yang, S.J.; Shin, I.S.; Yoon, J.S. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br. J. Psychiatry 2008, 192, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, D.E.; Wilcken, B. The pathogenesis of coronary disease. A possible role for methionine metabolism. J. Clin. Investig. 1976, 57, 1079–1082. [Google Scholar] [CrossRef]
- Ansari, R.; Manta, A.; Mallack, E.; Luo, J.J. Hyperhomocysteinemia and neurologic disorders: A review. J. Clin. Neurol. 2014, 10, 281. [Google Scholar] [CrossRef]
- Folstein, M.; Liu, T.; Peter, I.; Buel, J.; Arsenault, L.; Scott, T.; Qiu, W.W. The homocysteine hypothesis of depression. Am. J. Psychiatry 2007, 164, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Alirezaei, M.; Jelodar, G.; Niknam, P.; Ghayemi, Z.; Nazifi, S. Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat cerebellum. J. Physiol. Biochem. 2011, 67, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Schwahn, B.C.; Wendel, U.; Lussier-Cacan, S.; Mar, M.; Zeisel, S.H.; Leclerc, D.; Castro, C.; Garrow, T.A.; Rozen, R. Effects of betaine in a murine model of mild cystathionine-β-synthase deficiency. Metabolism 2004, 53, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Barak, A.J.; Beckenhauer, H.C.; Tuma, D.J. Betaine effects on hepatic methionine metabolism elicited by short-term ethanol feeding. Alcohol 1996, 13, 483–486. [Google Scholar] [CrossRef]
- Saarinen, M.T.; Kettunen, H.; Pulliainen, K.; Peuranen, S.; Tihonen, K.; Remus, J. A novel method to analyze betaine in chicken liver: Effect of dietary betaine and choline supplementation on the hepatic betaine concentration in broiler chicks. J. Agric. Food Chem. 2001, 49, 559–563. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J.; Harris, B.J.; Kyle, W.E. Regulation of hepatic betaine-homocysteine methyltransferase by dietary betaine. J. Nutr. 1983, 113, 519–521. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J.; Harris, B.J.; Kyle, W.E. Regulation of the betaine content of rat liver. Arch. Biochem. Biophys. 1982, 218, 169–173. [Google Scholar] [CrossRef]
- Carson, M.D.; Ribeiro, J.M. Apoptosis and disease. Lancet 1993, 14, 367–374. [Google Scholar] [CrossRef]
- McCully, K.S. Homocysteine and vascular disease. Nat. Med. 1996, 2, 386–389. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Ghasemi, M.; Hoseini, T. Association between plasma homocysteine concentrations and extracranial carotid stenosis. Ann. Saudi Med. 2006, 26, 120–122. [Google Scholar] [CrossRef]
- Buemi, M.; Marino, D.; Di Pasquale, G.; Floccari, F.; Ruello, A.; Aloisi, C.; Corica, F.; Senatore, M.; Romeo, A.; Frisina, N. Effects of homocysteine on proliferation, necrosis, and apoptosis of vascular smooth muscle cells in culture and influence of folic acid. Thromb. Res. 2001, 104, 207–213. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Rogers, D.D.; Mailliard, M.E.; Siford, G.L.; Barak, A.J.; Beckenhauer, H.C.; Sorrell, M.F.; Tuma, D.J. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: Protection by betaine. Biochem. Pharmacol. 2005, 70, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Barak, A.J.; Beckenhauer, H.C.; Mailliard, M.E.; Kharbanda, K.K.; Tuma, D.J. Betaine lowers elevated S-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J. Nutr. 2003, 133, 2845–2848. [Google Scholar] [CrossRef]
- Zavad’áková, P.; Fowler, B.; Zeman, J.; Suormala, T.; Pšistoupilová, K.; Kožich, V. Cble type of homocystinuria due to methionine synthase reductase deficiency: Clinical and molecular studies and prenatal diagnosis in two families. J. Inherit. Metab. Dis. 2002, 25, 461–476. [Google Scholar] [CrossRef]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | 14–28 d | 29–70 d |
|---|---|---|
| Maize | 61.4 | 64.4 |
| Soybean meal | 25.5 | 23.0 |
| Wheat bran | 6.0 | 2.0 |
| Rice husk | 3.5 | 7.0 |
| Limestone | 1.0 | 1.2 |
| Calcium hydrogen phosphate | 1.2 | 1.0 |
| DL-methionine | 0.1 | 0.1 |
| Salt | 0.3 | 0.3 |
| Premix | 1.0 1 | 1.0 2 |
| Total | 100.0 | 100.0 |
| Analyzed nutrient concentrations | ||
| Metabolizable energy 3 (MJ/kg) | 11.26 | 11.29 |
| Crude protein (%) | 17.00 | 15.63 |
| Crude fiber (%) | 4.30 | 5.33 |
| Calcium (%) | 0.82 | 0.82 |
| Available phosphorus (%) | 0.41 | 0.37 |
| Methionine (%) | 0.35 | 0.36 |
| Lysine (%) | 0.86 | 0.83 |
| Arginine (%) | 1.14 | 1.13 |
| Histidine (%) | 0.44 | 0.40 |
| Isoleucine (%) | 0.73 | 0.60 |
| Leucine (%) | 1.49 | 1.25 |
| Phenylalanine (%) | 0.83 | 0.74 |
| Threonine (%) | 0.68 | 0.54 |
| Cysteine (%) | 0.16 | 0.25 |
| Variable | Control | Met Toxicity | Bet Detoxification | p-Value | |
|---|---|---|---|---|---|
| 14 d BW (g) | 473 ± 0.31 | 473 ± 0.42 | 473 ± 0.56 | 0.826 | |
| 28 d BW (g) | 1459 ± 70 a | 1355 ± 40 b | 1224 ± 77 c | <0.001 | |
| 49 d BW (g) | 3298 ± 135 a | 2910 ± 189 b | 2539 ± 107 c | <0.001 | |
| 70 d BW (g) | 4311 ± 131 a | 3973 ± 122 b | 3820 ± 261 b | 0.003 | |
| Mortality at 70 d (%) | 5.72 ± 7.83 | 17.14 ± 18.63 | 8.57 ± 12.78 | 0.422 | |
| 14–28 d | ADFI (g) | 154 ± 7.02 a | 136 ± 16 b | 117 ± 9.85c | 0.001 |
| ADG (g) | 104 ± 4.98 a | 97 ± 2.86 b | 88 ± 5.50 c | <0.001 | |
| F/G | 2.20 ± 0.16 | 2.16 ± 0.24 | 2.20 ± 0.16 | 0.951 | |
| 29–70 d | ADFI (g) | 227 ± 13 a | 193 ± 22 b | 176 ± 15 | 0.002 |
| ADG (g) | 68 ± 3.59 | 62 ± 3.24 | 62 ± 5.37 | 0.074 | |
| F/G | 3.35 ± 0.32 | 3.10 ± 0.31 | 2.87 ± 0.42 | 0.140 | |
| Variable | Control | Met Toxicity | Bet Detoxification | p-Value |
|---|---|---|---|---|
| 28 d serum Hcy (µmol/L) | 21.58 ± 6.30 b | 38.90 ± 11.27 a | 33.84 ± 12.61 a | 0.003 |
| 49 d serum Hcy (µmol/L) | 14.82 ± 2.20 b | 44.54 ± 5.86 a | 42.92 ± 9.54 a | <0.001 |
| 70 d serum Hcy (µmol/L) | 16.16 ± 2.33 b | 30.51 ± 11.08 a | 19.75 ± 3.82 b | 0.017 |
| Groups | Bax | Bcl-2 | Bcl-2/Bax |
|---|---|---|---|
| Control | 0.863 ± 0.480 | 1.128 ± 0.305 a | 1.454 ± 0.417 |
| Met toxicity | 0.318 ± 0.106 | 0.696 ± 0.147 b | 2.477 ± 1.152 |
| Bet detoxification | 0.359 ± 0.217 | 0.602 ± 0.246 b | 1.961 ± 0.670 |
| p-Value | 0.062 | 0.029 | 0.252 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Yang, Y.; Yang, J.; Wan, X.; Yang, H.; Wang, Z. Hyperhomocysteinemia Induced by Methionine Excess is Effectively Suppressed by Betaine in Geese. Animals 2020, 10, 1642. https://doi.org/10.3390/ani10091642
Yang Z, Yang Y, Yang J, Wan X, Yang H, Wang Z. Hyperhomocysteinemia Induced by Methionine Excess is Effectively Suppressed by Betaine in Geese. Animals. 2020; 10(9):1642. https://doi.org/10.3390/ani10091642
Chicago/Turabian StyleYang, Zhi, Yu Yang, Jinjin Yang, Xiaoli Wan, Haiming Yang, and Zhiyue Wang. 2020. "Hyperhomocysteinemia Induced by Methionine Excess is Effectively Suppressed by Betaine in Geese" Animals 10, no. 9: 1642. https://doi.org/10.3390/ani10091642
APA StyleYang, Z., Yang, Y., Yang, J., Wan, X., Yang, H., & Wang, Z. (2020). Hyperhomocysteinemia Induced by Methionine Excess is Effectively Suppressed by Betaine in Geese. Animals, 10(9), 1642. https://doi.org/10.3390/ani10091642






