Pearsonema spp. (Family Capillariidae, Order Enoplida) Infection in Domestic Carnivores in Central–Northern Italy and in a Red Fox Population from Central Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Domestic Carnivores
2.2. Red Foxes (Vulpes Vulpes)
2.3. Statistical Analysis
3. Results
3.1. Cats
3.2. Dogs
3.3. Red Foxes (Vulpes Vulpes)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gibbons, L.M. Keys to the Nematode Parasites of Vertebrates; CAB International: Wallingford, UK, 2010; p. 416. [Google Scholar]
- Moravec, F. Proposal of a new systematic arrangement of nematodes of the family Capillariidae. Folia Parasitol. (Praha) 1982, 29, 119–132. [Google Scholar]
- Bowman, D.D.; Hendrix, C.M.; Lindsay, D.S.; Barr, S.C. Feline Clinical Parasitology; Iowa State University Press: Ames, IA, USA, 2002; pp. 342–345. [Google Scholar]
- Aleksić, J.; Stepanović, P.; Dimitrijević, S.; Gajić, B.; Bogunović, D.; Davidov, I.; Aleksić-Agelidis, A.; Ilić, T. Capillaria plica in Red Foxes (Vulpes vulpes) from Serbia: Epidemiology and Diagnostic Approaches to Urinary Capillariosis in Domestic Carnivores. Acta Parasitol. 2020. [Google Scholar] [CrossRef]
- Alić, A.; Hodxić Adrić, M.; BesiroKavić, H.; Prasović, S. Pearsonema plica (Capillaria plica) infection and associated urinary bladder pathology in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasitol. Res. 2015, 114, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Magi, M.; Guardone, L.; Prati, M.C.; Mignone, W.; Macchioni, F. Extraintestinal nematodes of the red fox (Vulpes vulpes) in north-west Italy. J. Helminthol. 2015, 11, 1–6. [Google Scholar]
- Mariacher, A.; Eleni, C.; Fico, R.; Ciarrocca, E.; Perrucci, S. Pearsonema plica and Eucoleus böhmi infections and associated lesions in wolves (Canis lupus) from Italy. Helminthologia 2015, 52, 364–369. [Google Scholar] [CrossRef]
- Mariacher, A.; Millanta, F.; Guidi, G.; Perrucci, S. Urinary capillariosis in six dogs from Italy. Open Vet. J. 2016, 6, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed.; CABI Publishing: Wallingford, UK, 2000; p. 672. [Google Scholar]
- Basso, W.; Spänhauer, Z.; Arnold, S.; Deplazes, P. Capillaria plica (syn. Pearsonema plica) infection in a dog with chronic pollakiuria: Challenges in the diagnosis and treatment. Parasitol. Int. 2003, 63, 140–142. [Google Scholar] [CrossRef]
- Rossi, M.; Messina, N.; Ariti, G.; Riggio, F.; Perrucci, S. Symptomatic Capillaria plica infection in a young European cat. J. Feline Med. Surg. 2011, 13, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.D. Nematode Parasites of Domestic Animals and of Man; Burgess Publishing Company: Minneapolis, MN, USA, 1968; p. 600. [Google Scholar]
- Mariacher, A.; Eleni, C.; Fico, R.; Perrucci, S. Urinary capillariosis in a free-ranging Marsican brown bear (Ursus arctos marsicanus). Int. J. Parasitol. Parasites Wildl. 2018, 7, 429–431. [Google Scholar] [CrossRef]
- Ribas, A.; Milazzo, C.; Foronda, P.; Casanova, J.C. New data on helminths of stone marten, Martes foina (Carnivora, Mustelidae), in Italy. Helminthology 2004, 1, 59–61. [Google Scholar]
- Petersen, H.H.; Nielsen, S.T.; Larsen, G.; Holm, E.; Chriél, M. Prevalence of Capillaria plica in Danish wild carnivores. Int. J. Parasitol. Parasites Wildl. 2018, 7, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Iori, A.; Costantini, R.; Cancrini, G. Parassiti in volpi provenienti da alcune regioni italiane. Parassitologia 1990, 32, 153–154. (In Italian) [Google Scholar]
- Rossi, L.; Iori, A.; Cancrini, G. Osservazioni sulla fauna parassitaria della popolazione di volpi presente nel parco regionale “La Mandria”. Parassitologia 1983, 25, 340–343. (In Italian) [Google Scholar]
- Bork-Mimm, S.; Rinder, H. High prevalence of Capillaria plica infections in red foxes (Vulpes vulpes) in Southern Germany. Parasitol. Res. 2011, 108, 1063–1067. [Google Scholar] [CrossRef]
- Senior, D.F.; Solomon, G.B.; Goldschmidt, M.H.; Joyce, T.; Bovee, K.C. Capillaria plica infection in dogs. J. Am. Vet. Med. Assoc. 1980, 176, 901–905. [Google Scholar]
- Knaus, M.; Schukullari, E.; Rosentel, J.; Rehbein, S. Efficacy of a novel topical combination of fipronil, (S)-methoprene, eprinomectin and praziquantel against feline urinary bladder worm (Capillaria plica) infection. Vet. Parasitol. 2014, 202, 45–48. [Google Scholar] [CrossRef]
- Krone, O.; Guminsky, O.; Meinig, H.; Herrmann, M.; Trinzen, M.; Wibbelt, G. Endoparasite spectrum of wild cats (Felis silvestris Schreber, 1777) and domestic cats (Felis catus L.) from the Eifel, Pfalz region and Saarland, Germany. Eur. J. Wildl. Res. 2008, 54, 95–100. [Google Scholar] [CrossRef]
- Schuster, R.; Kaufmann, A.; Hering, S. Investigations on the endoparasitic fauna of domestic cats in eastern Brandenburg. Berl. Munch. Tierarztl. Wochenschr. 1997, 110, 48–50. (In German) [Google Scholar]
- de Souza Ramos, D.G.; De Cruz Scheremeta, R.G.A.; De Oliveira, A.C.S.; Sinkoc, A.L.; De Campos Pacheco, R. Survey of helminth parasites of cats from the metropolitan area of Cuiabà, Mato Grosso, Brazil. Rev. Bras. Parasitol. Vet. 2013, 22, 201–206. [Google Scholar] [CrossRef]
- Wilson-Hanson, S.; Prescott, C.W. Capillaria in the bladder of the domestic cat. Aust. Vet. J. 1982, 59, 190–191. [Google Scholar] [CrossRef]
- Bedard, C.; Desnoyers, M.; Lavallée, M.C.; Poirier, D. Capillaria in the bladder of an adult cat. Can. Vet. J. 2002, 43, 973–974. [Google Scholar] [PubMed]
- Cazelles, C.; Bourdeau, P.; Vidal, J. Capillariose vesicale chez un chien: A propos d’un cas. Point Vetérinaire 1989, 21, 41–44. (In French) [Google Scholar]
- Guimarães, A.; Aguilera, V.C.O.; Gomes, D.P.P.; Zanesco, E.V.; Oliveira, Á.F.X.; Stocco, N.V.; Andrade, G.F.P.; Souza, N.C.; Souza, H.J.M.; Baldani, C.D. Urinary capillariosis in a cat from Rio de Janeiro, Brazil-Clinical, morphological and phylogenetic characterization. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100409. [Google Scholar]
- Komorova, P.; Kasicovà, Z.; Zbojanova, K.; Kocisovà, A. First documented cases of Pearsonema plica (syn. Capillaria plica) infections in dogs from Western Slovakia. Helmintologia 2020, 57, 158–162. [Google Scholar] [CrossRef]
- Van der Linden, B. Persistent cystitis in a male cat. Tijdschr Diergeneeskd 1986, 111, 638–639. [Google Scholar] [PubMed]
- Van Veen, L. Bladder infection with Capillaria plica in a male dog. Tijdschr Diergeneesk 2002, 127, 393–394. (In Dutch) [Google Scholar]
- Whitehead, M. Urinary capillariosis in a cat in the UK. Vet. Rec. 2009, 165, 757. [Google Scholar]
- Callegari, D.; Kramer, L.; Cantoni, A.M.; Di Lecce, R.; Dodi, P.L.; Grandi, G. Canine bladderworm (Capillaria plica) infection associated with glomerular amyloidosis. Vet. Parasitol. 2002, 168, 338–341. [Google Scholar] [CrossRef]
- Maurelli, M.P.; Rinaldi, L.; Rubino, G.; Lia, R.; Musella, V.; Cringoli, G. FLOTAC and Mini-FLOTAC for uro-microscopic diagnosis of Capillaria plica (syn. Pearsonema plica) in dogs. BMC Res. Notes 2018, 7, 591. [Google Scholar]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Parassitologia e Malattie Parassitarie Degli Animali, 1st ed.; Emsi: Roma, Italy, 2010; p. 989. [Google Scholar]
- Fernández-Aguilar, X.; Mattsson, R.; Meijer, T.; Osterman-Lind, E.; Gavier-Widén, D. Pearsonema (syn. Capillaria plica) associated cystitis in a Fennoscandian arctic fox (Vulpes lagopus): A case report. Acta Vet. Scand. 2010, 52, 39. [Google Scholar]
- Studzińska, M.B.; Obara-Gałek, J.; Demkowska-Kutrzepa, M.; Tomczuk, K. Diagnosis and therapy of Capillaria plica infection: Report and literature review. Acta Parasitol. 2015, 60, 563–566. [Google Scholar] [CrossRef]
- Loftin, C.M.; Donnett, U.B.; Schneider, L.G.; Varela-Stokes, A.S. Prevalence of endoparasites in northern Mississippi shelter cats. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100322. [Google Scholar] [CrossRef] [PubMed]
- Del-Angel-Caraza, J.; Quijano-Hernández, I.A.; Soriano-Vargas, E.; Barbosa-Mireles, M.A.; Martínez-Castañeda, J.S. Urinary bladder worm (Pearsonema sp.) infection in domestic dogs and cats in Mexico at a high altitude. Parasitol. Res. 2018, 117, 1979–1983. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; van Knapen, F.; Schweiger, A.; Overgaauw, P.A. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet. Parasitol. 2011, 182, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Cantacessi, C.; Dantas-Torres, F.; Brianti, E.; Pfeffer, M.; Genchi, C.; Guberti, V.; Capelli, G.; Deplazes, P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Vet. Parasitol. 2015, 213, 24–37. [Google Scholar] [CrossRef]
- Inforzato, G.R.; Santos, W.R.M.; Neves, M.F. Capilariose em gatos. Rev. Científica Eletrônica Med. Veterinária 2009, 12, 1–5. [Google Scholar]
- Pagnoncelli, M.; França, R.T.; Martins, D.B.; Howes, F.; dos Anjos Lopes, S.T.; Mazzanti, C.M. Capillaria sp. in a cat. Acta Sci. Vet. 2011, 39, 1–3. [Google Scholar]
- Graham, J.A.; Sato, M.; Moore, A.R.; McGrew, A.K.; Ballweber, L.R.; Byas, A.D.; Dowers, K.L. Disseminated Strongyloides stercoralis infection in a dog following long-term treatment with budesonide. J. Am. Vet. Med. Assoc. 2019, 254, 974–978. [Google Scholar] [CrossRef]
- Ita, O.I.; Akpayak, I.C.; Onyedibe, K.I.; Otu, A.A. Strongyloides stercoralis larvae in the urine of a patient with transitional cell carcinoma of the bladder: A case report. J. Parasit. Dis. 2019, 43, 154–157. [Google Scholar] [CrossRef]
- Wulcan, J.M.; Dennis, M.M.; Ketzis, J.K.; Bevelock, T.J.; Verocai, G.G. Strongyloides spp. in cats: A review of the literature and the first report of zoonotic Strongyloides stercoralis in colonic epithelial nodular hyperplasia in cats. Parasites Vectors 2019, 12, 349. [Google Scholar] [CrossRef]
- Spagnesi, M.; De Marinis, A.M. Mammiferi d’Italia. Quad. Cons. Nat. 2002, 14. Available online: https://www.minambiente.it/sites/default/files/archivio/biblioteca/qcn_14.pdf (accessed on 1 August 2020). (In Italian).
- Davidson, R.; Gjerde, B.; Vikoren, T.; Lillehaug, A.; Handeland, K. Prevalence of Trichinella larvae and extra intestinal nematodes in Norwegian red foxes (Vulpes vulpes). Vet. Parasitol. 2006, 136, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Eira, C.; Viganda, J.; Torres, J.; Miquel, J. The helminth community of the red fox, Vulpes vulpes, in Dunas de Mira (Portugal) and its effect on host condition. Wildl. Biol. Pract. 2006, 2, 26–36. [Google Scholar] [CrossRef]
- Segovia, J.M.; Torres, J.; Miquel, J. Helminth parasites of the red fox (Vulpes vulpes L., 1758) in the Iberian Peninsula: An ecological study. Acta Parasitol. 2001, 49, 67–79. [Google Scholar]
- Saeed, I.; Maddox-Hyttel, C.; Monrad, J.; Kapel, C.M.O. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006, 139, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Sréter, T.; Szell, Z.; Marucci, G.; Pozio, E.; Vargac, I. Extraintestinal nematode infections of red foxes (Vulpes vulpes) in Hungary. Vet. Parasitol. 2003, 115, 329–334. [Google Scholar] [CrossRef]
- Cignini, B.; Riga, F. Red Fox Sightings in Rome. Hjstrix 1997, 9, 71–74. [Google Scholar]
- Pandolfi, M.; Forconi, P.; Montecchiari, L. Spatial behaviour of the red fox (Vulpes vulpes) in a rural area of central Italy. Ital. J. Zool. 1997, 64, 351–358. [Google Scholar] [CrossRef]
- Guardone, L.; Deplazes, P.; Macchioni, F.; Magi, M.; Mathis, A. Ribosomal and mitochondrial DNA analysis of Trichuridae nematodes of carnivores and small mammals. Vet. Parasitol. 2013, 197, 364–369. [Google Scholar] [CrossRef]
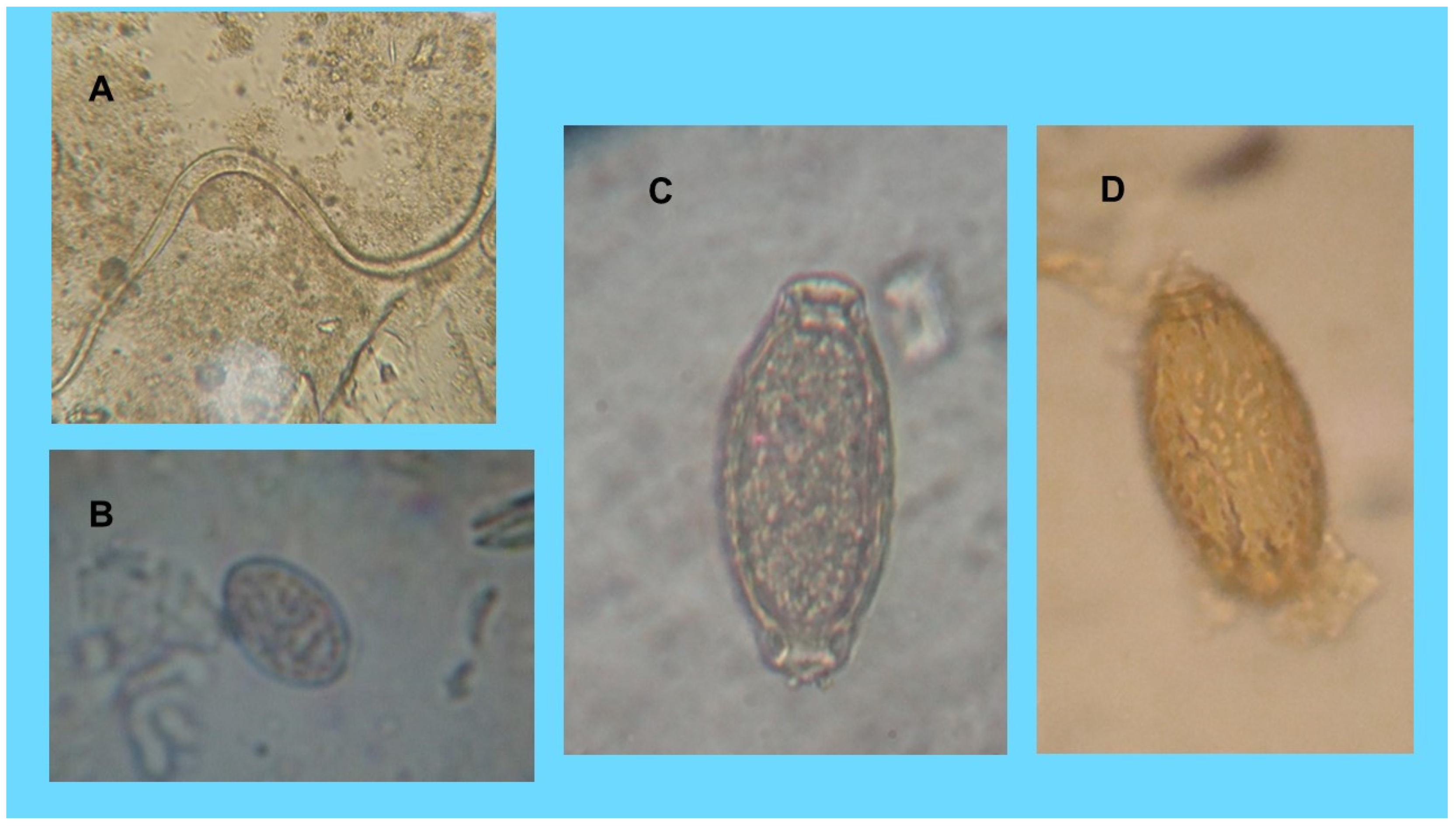
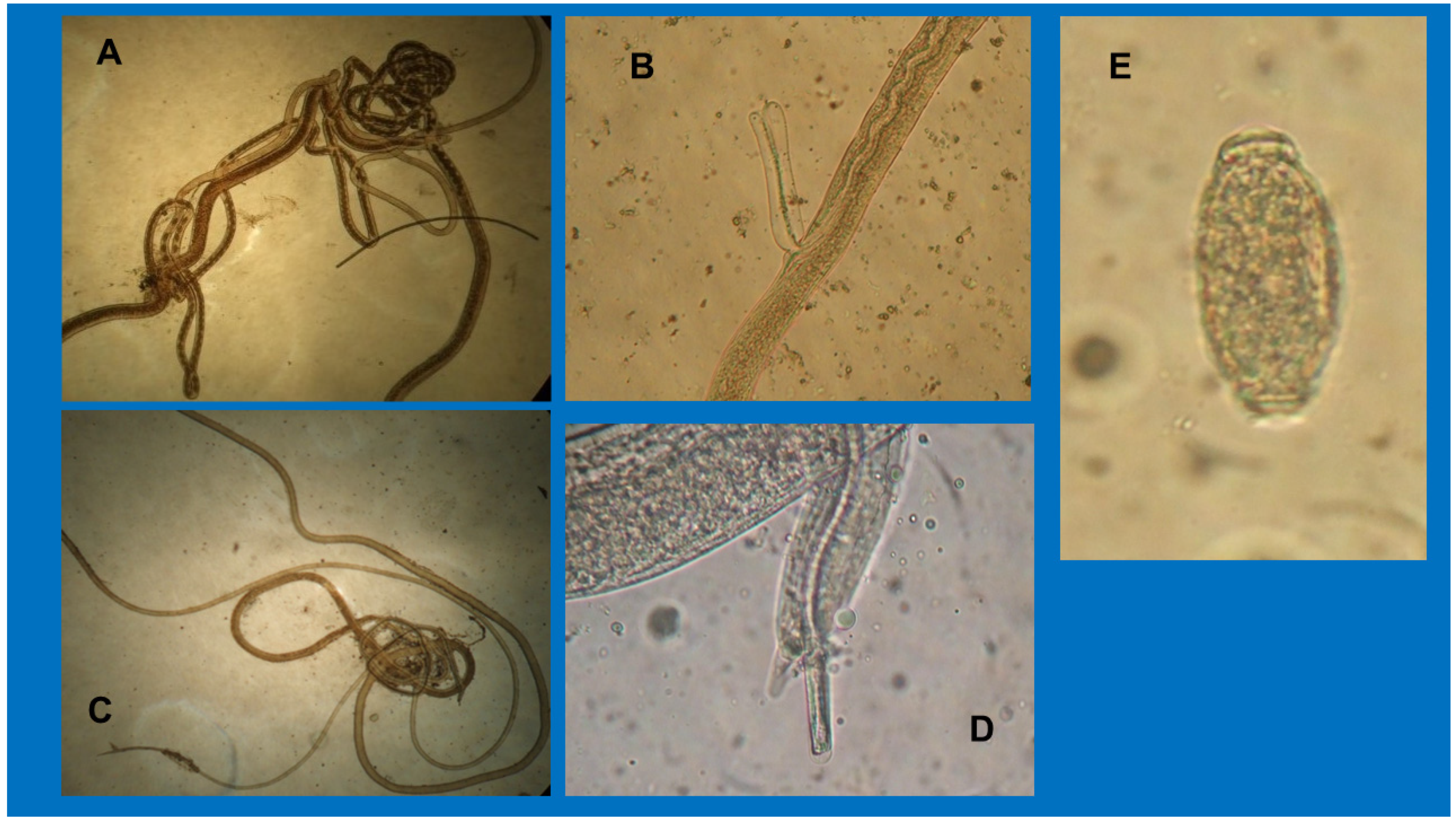
| Patients Description | Cats | Dog |
|---|---|---|
| Age range | 1.5–14 years old | 1–16 years old |
| Males | 14 | 43 |
| Females | 12 | 40 |
| Mixed breed | 19 a | 28 c |
| Pure breed | 7 b | 55 d |
| Mainly indoor lifestyle | 21 | 72 |
| Mainly outdoor lifestyle | 5 | 11 |
| Symptomatic | 20 | 62 |
| Asymptomatic | 6 | 21 |
| Piedmont | 4 (3 m, 1 f) | 10 (6 m, 4 f) |
| Lombardy | 11 (7 m, 4 f) | 26 (11 m, 5 f) |
| Tuscany | 11 (4 m, 7 f) | 47 (26 m, 21 f) |
| Pearsonema spp. positive | 2 (2/26, 7.7%, 95% CI 0–17.9%) | 1 (1/83, 1.2%, 95% CI 0–3.6%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelligra, S.; Guardone, L.; Riggio, F.; Parisi, F.; Maestrini, M.; Mariacher, A.; Perrucci, S. Pearsonema spp. (Family Capillariidae, Order Enoplida) Infection in Domestic Carnivores in Central–Northern Italy and in a Red Fox Population from Central Italy. Animals 2020, 10, 1607. https://doi.org/10.3390/ani10091607
Pelligra S, Guardone L, Riggio F, Parisi F, Maestrini M, Mariacher A, Perrucci S. Pearsonema spp. (Family Capillariidae, Order Enoplida) Infection in Domestic Carnivores in Central–Northern Italy and in a Red Fox Population from Central Italy. Animals. 2020; 10(9):1607. https://doi.org/10.3390/ani10091607
Chicago/Turabian StylePelligra, Salvatore, Lisa Guardone, Francesca Riggio, Francesca Parisi, Michela Maestrini, Alessia Mariacher, and Stefania Perrucci. 2020. "Pearsonema spp. (Family Capillariidae, Order Enoplida) Infection in Domestic Carnivores in Central–Northern Italy and in a Red Fox Population from Central Italy" Animals 10, no. 9: 1607. https://doi.org/10.3390/ani10091607
APA StylePelligra, S., Guardone, L., Riggio, F., Parisi, F., Maestrini, M., Mariacher, A., & Perrucci, S. (2020). Pearsonema spp. (Family Capillariidae, Order Enoplida) Infection in Domestic Carnivores in Central–Northern Italy and in a Red Fox Population from Central Italy. Animals, 10(9), 1607. https://doi.org/10.3390/ani10091607







