Development and Long-Term Follow-Up of an Experimental Model of Myocardial Infarction in Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
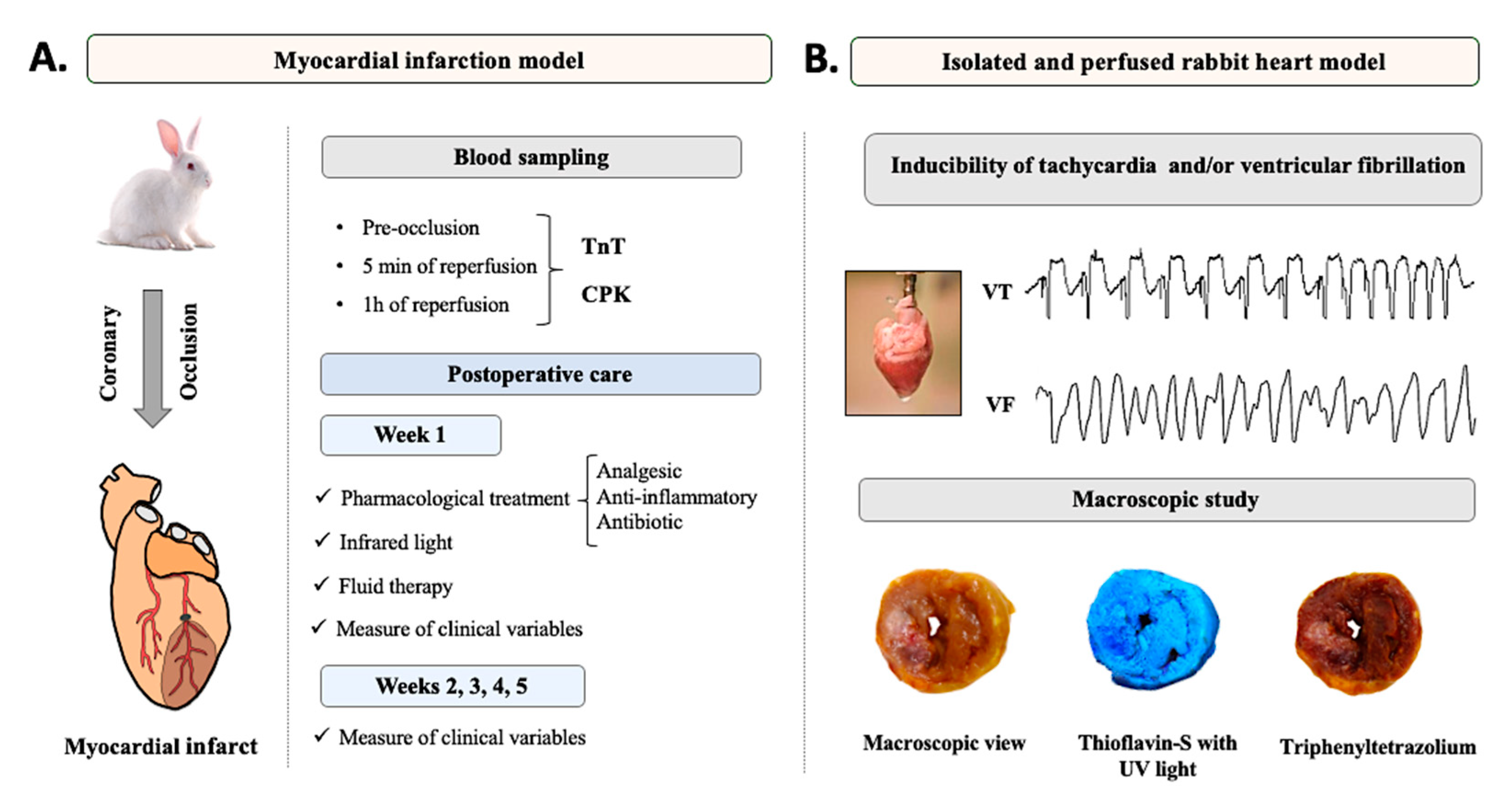
2.2. Experimental Design
2.3. Presurgical Phase
2.4. Anesthesia, Endotracheal Intubation, Measurement of Clinical Parameters, and Surgical Field Preparation
2.5. Surgical Procedure
2.6. Blood Sampling
2.7. Recovery Protocol
2.8. Postoperative Care
2.8.1. Week 1
2.8.2. Weeks 2, 3, 4, and 5
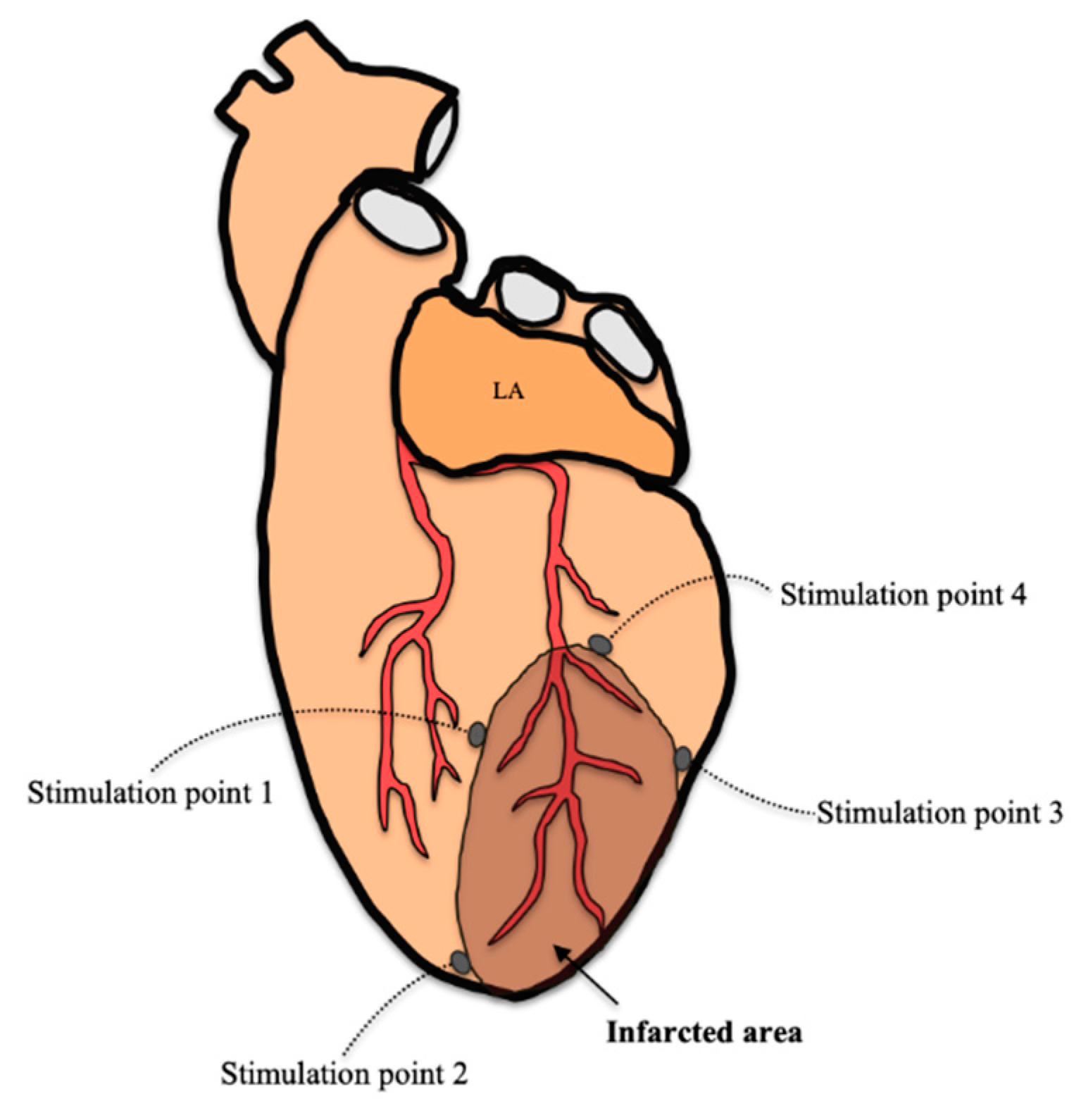
2.9. Arrhythmia Inducibility
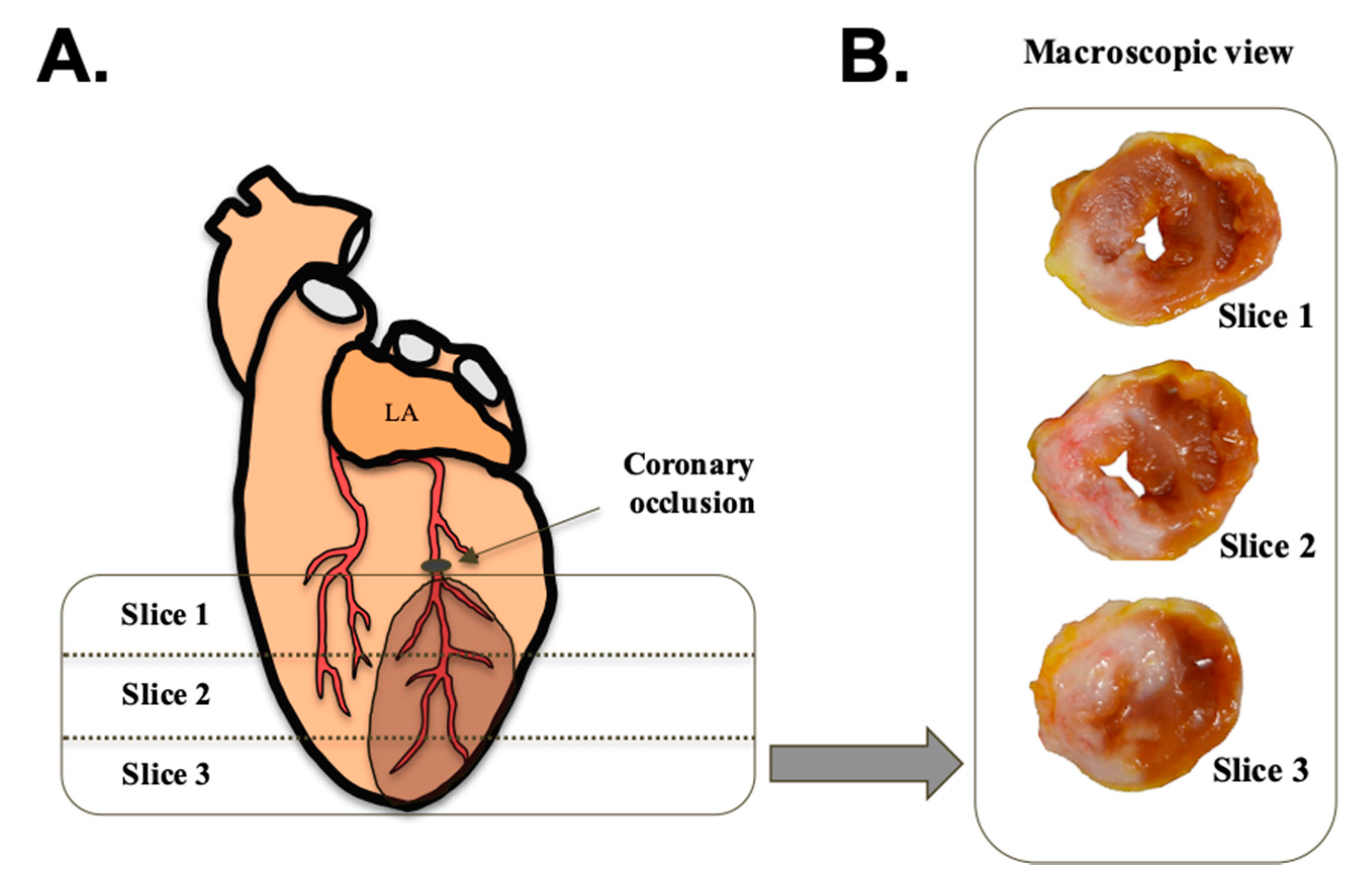
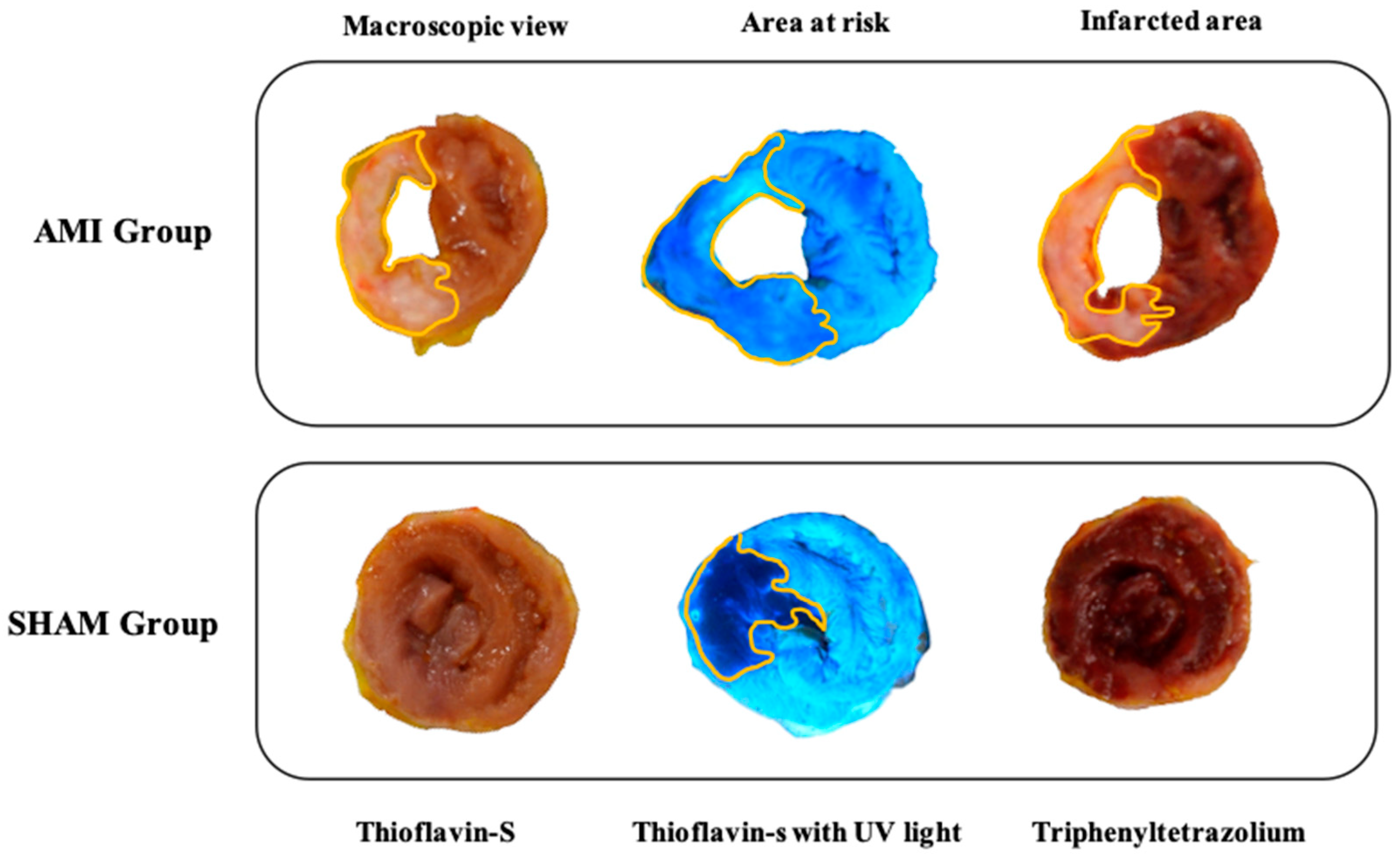
2.10. Macroscopic Study
2.11. Statistics
3. Results
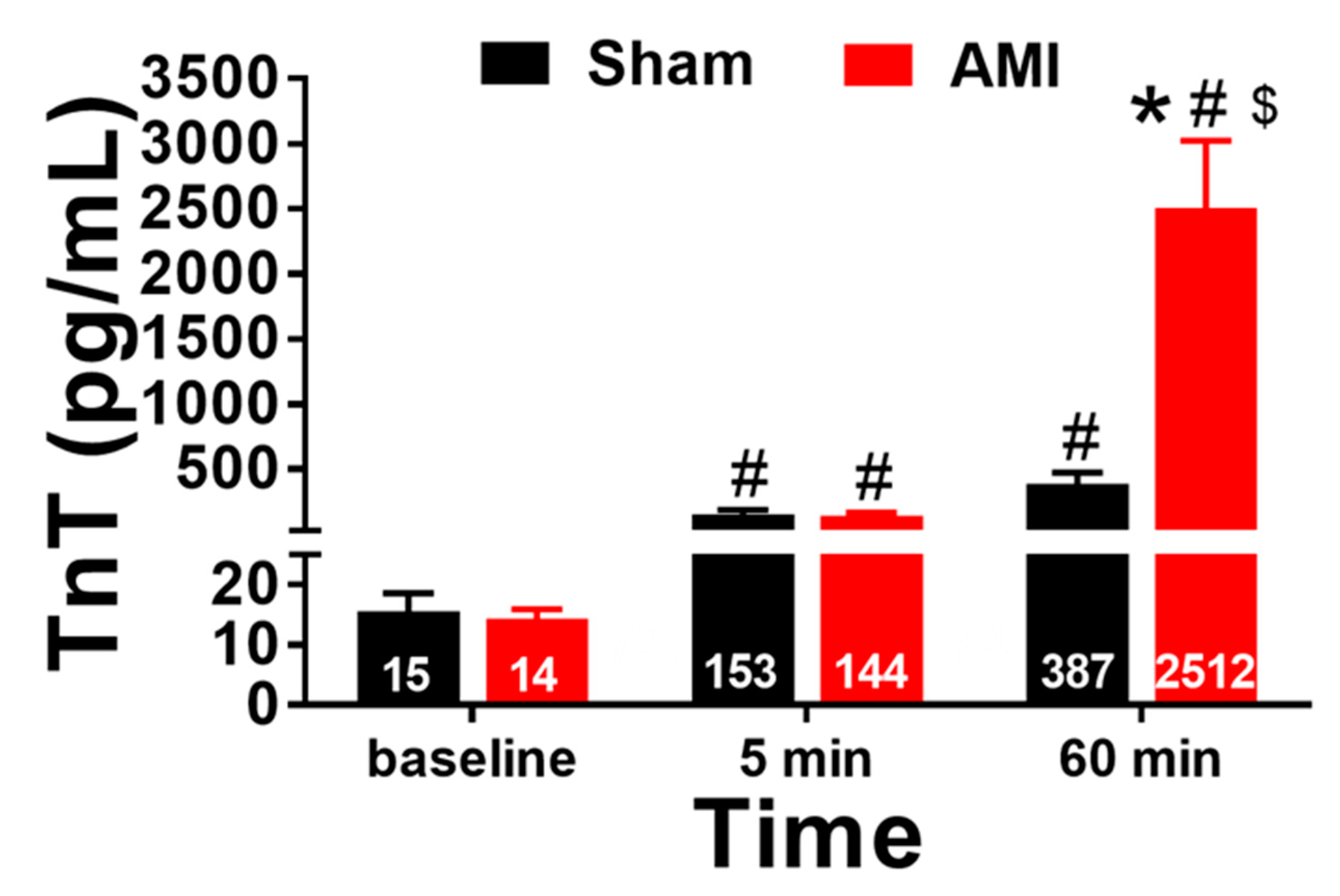
3.1. Blood Sampling: Biomarkers of Myocardial Necrosis
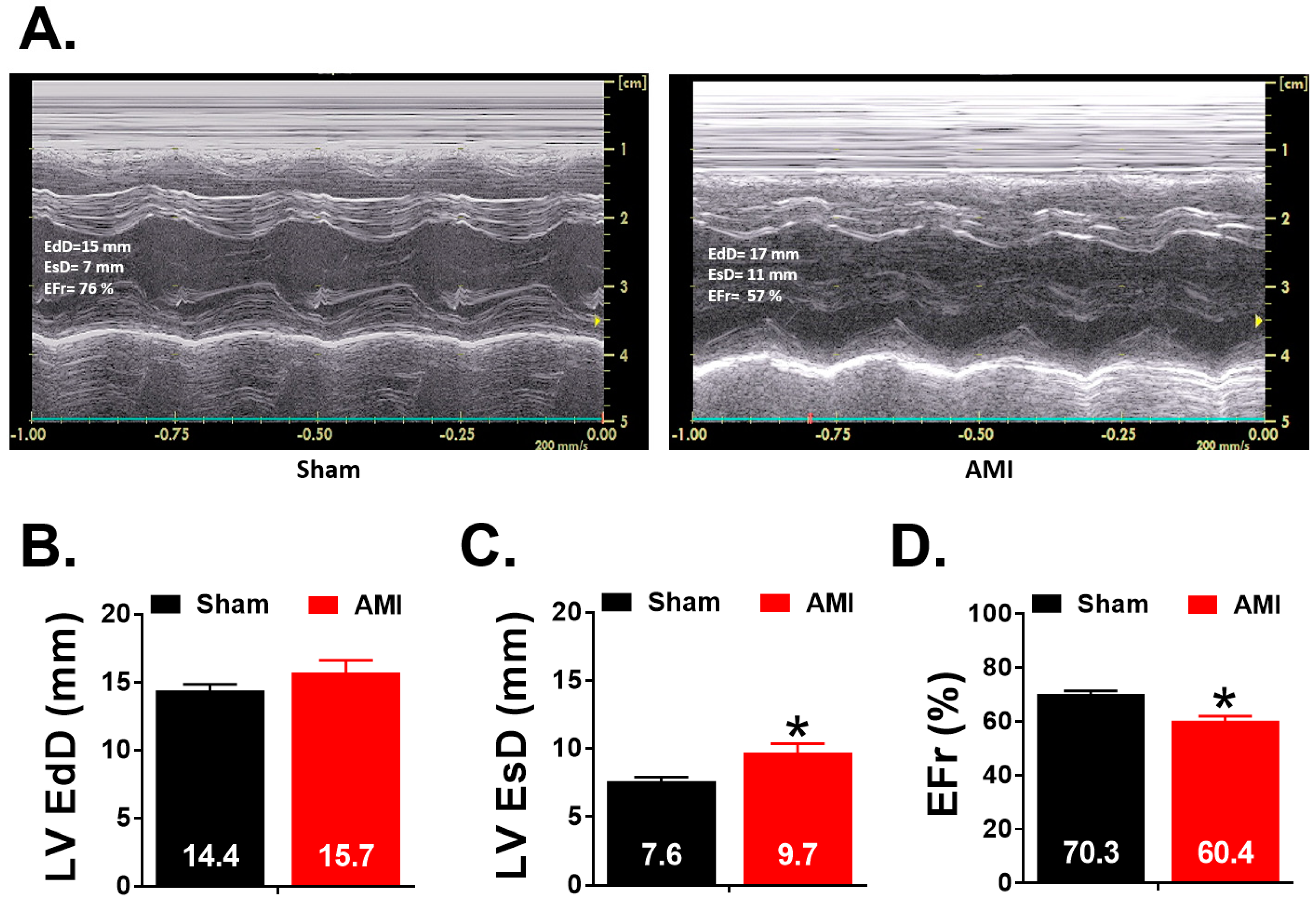
3.2. Echocardiographic Study
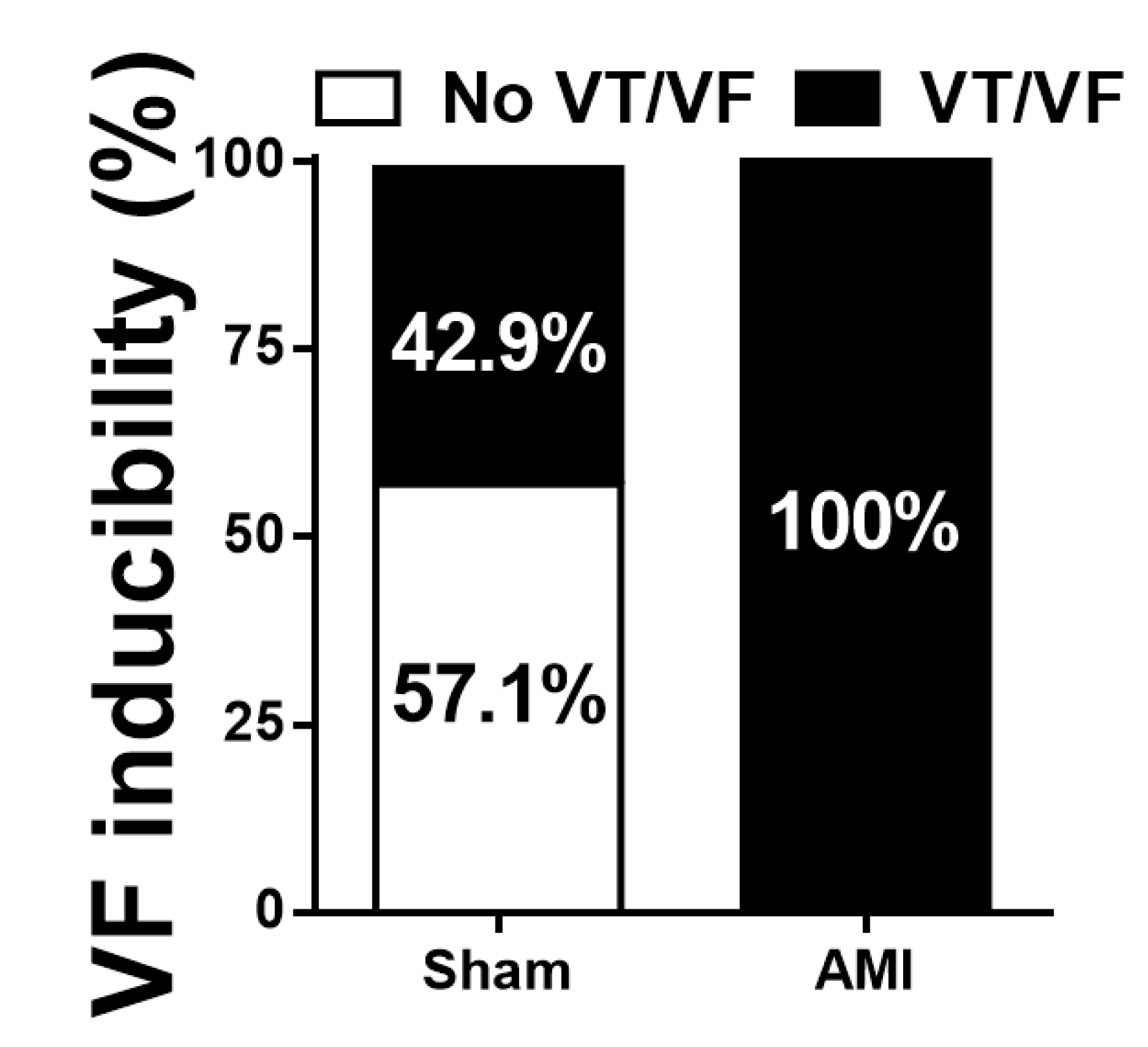
3.3. Arrhythmia Inducibility
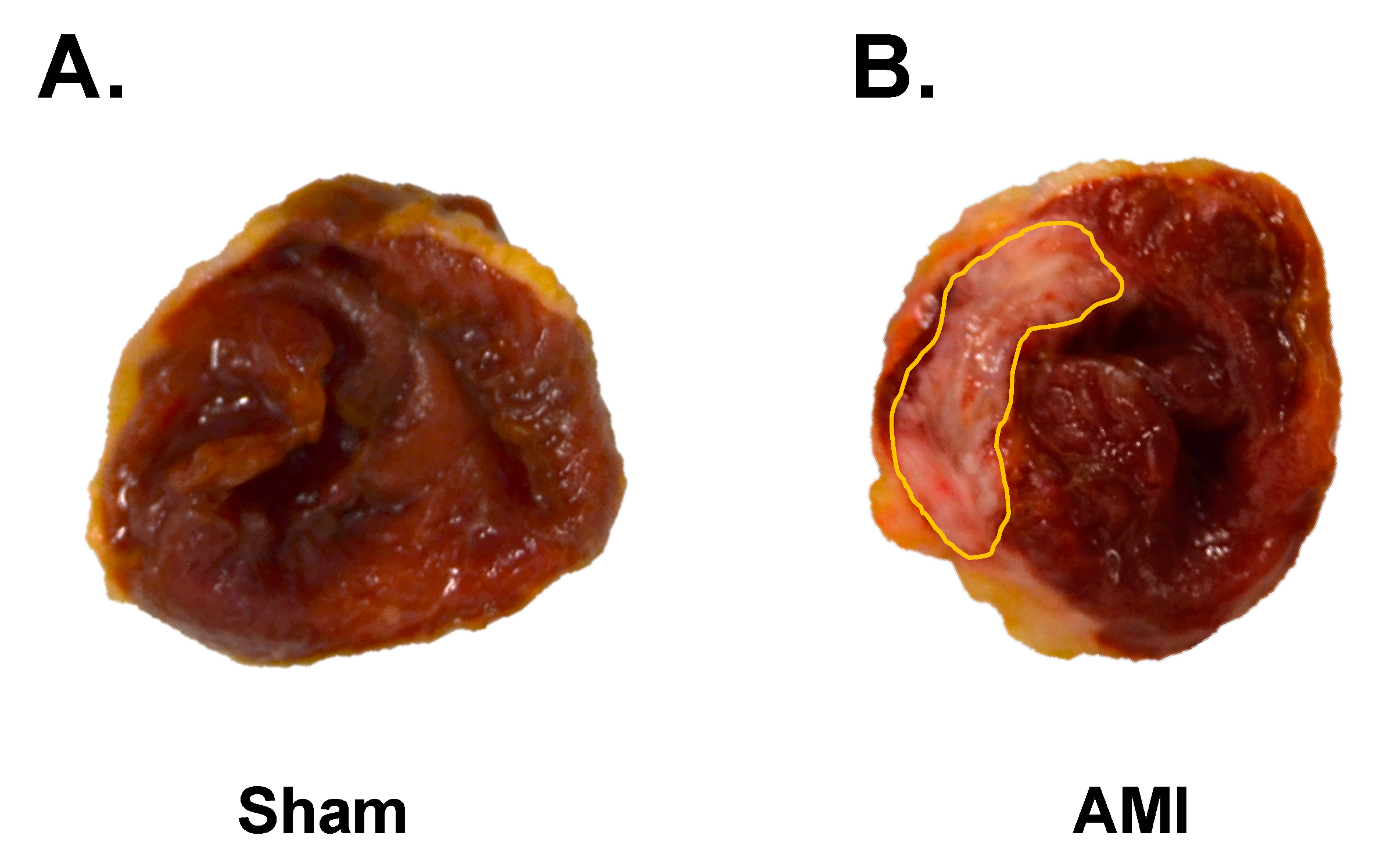
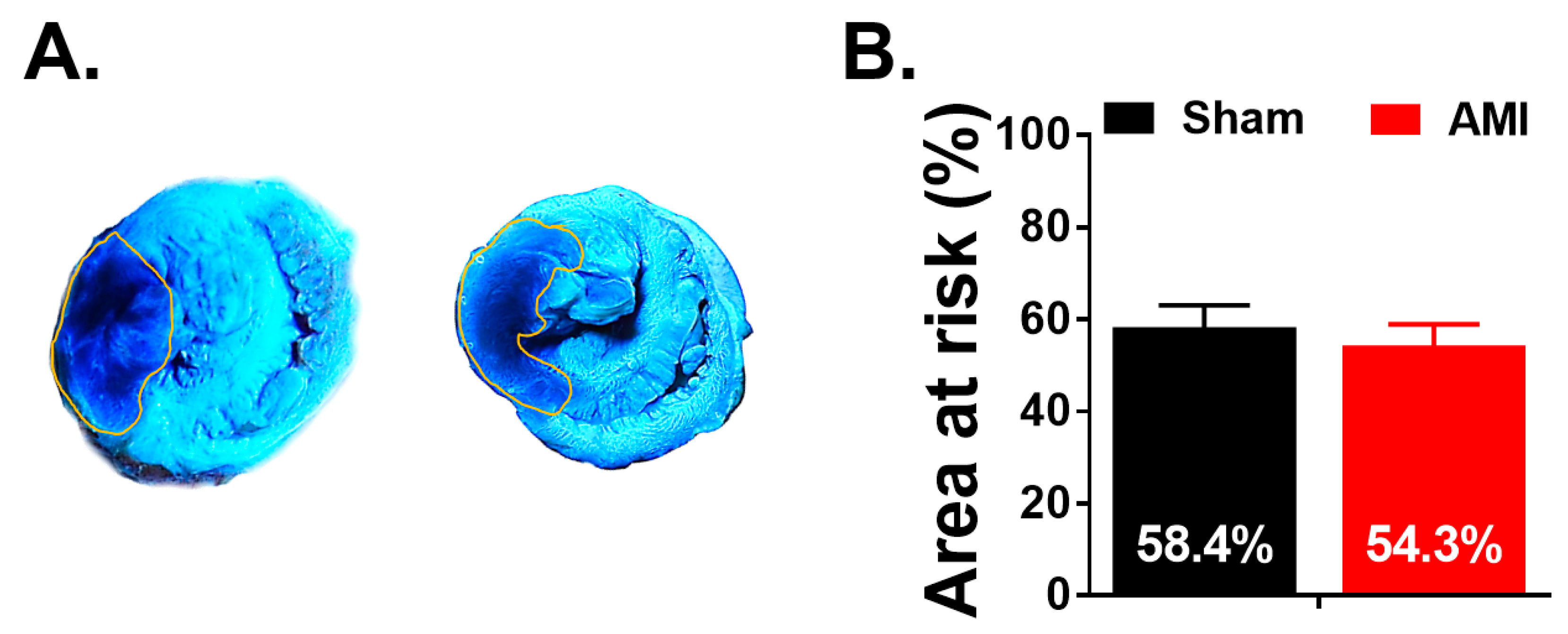
3.4. Macroscopic Study
4. Discussion
4.1. Animal Care
4.2. Viability of the Model
4.3. Induction of Ischemia/Reperfusion
4.4. Extent of Myocardial Damage and Characteristics of the Injured Area
4.5. Arrhythmia Inducibility
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santos, J.V.; Souza, J.; Valente, J.; Alonso, V.; Ramalho, A.; Viana, J.; Ricciardi, W.; Freitas, A. The state of health in the European Union (EU-28) in 2017: An analysis of the burden of diseases and injuries. Eur. J. Public Health 2019, 30, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.D.; Adair, T. Is the long-term decline in cardiovascular-disease mortality in high-income countries over? Evidence from national vital statistics. Int. J. Epidemiol. 2019, 48, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- On Behalf of the Atlas Writing Group; ESC Atlas of Cardiology is a compendium of cardiovascular statistics compiled by the European Heart Agency, a department of the European Society of Cardiology; Developed in collaboration with the national societies of the European Society of Cardiology member countries; Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019 (Executive Summary). Eur. Hear. J. Qual. Care Clin. Outcomes 2020, 6, 7–9. [Google Scholar] [CrossRef]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-Cause and cause-Specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Rossello, X.; Lobo-Gonzalez, M.; Ibanez, B. Editor´s choice—Pathophysiology and therapy of myocardial ischaemia/reperfusion syndrome. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 443–456. [Google Scholar] [CrossRef]
- Sattler, S.M.; Skibsbye, L.; Linz, D.; Lubberding, A.F.; Tfelt-Hansen, J.; Jespersen, T. Ventricular Arrhythmias in First Acute Myocardial Infarction: Epidemiology, Mechanisms, and Interventions in Large Animal Models. Front. Cardiovasc. Med. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Chorro, F.J.; Such-Belenguer, L.; López-Merino, V. Animal Models of Cardiovascular Disease. Rev. Esp. Cardiol. 2009, 62, 69–84. [Google Scholar] [CrossRef]
- Heusch, G. Critical Issues for the Translation of Cardioprotection. Circ. Res. 2017, 120, 1477–1486. [Google Scholar] [CrossRef]
- Bøtker, H.E.; Hausenloy, D.; Andreadou, I.; Antonucci, S.; Boengler, K.; Davidson, S.M.; Deshwal, S.; Devaux, Y.; Di Lisa, F.; Di Sante, M.; et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res. Cardiol. 2018, 113, 39. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Mendieta, G.; Ben-Aicha, S.; Vilahur, G. Post-Genomic Methodologies and Preclinical Animal Models: Chances for the Translation of Cardioprotection to the Clinic. Int. J. Mol. Sci. 2019, 20, 514. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Bogaert, J.; Oyen, R.; Ni, Y. An overview on development and application of an experimental platform for quantitative cardiac imaging research in rabbit models of myocardial infarction. Quant. Imaging Med. Surg. 2014, 4, 358–375. [Google Scholar] [PubMed]
- Lindsey, M.L.; Bolli, R.; Canty, J.M.; Du, X.-J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef]
- Allahwala, U.K.; Weaver, J.; Bhindi, R. Animal chronic total occlusion models: A review of the current literature and future goals. Thromb. Res. 2019, 177, 83–90. [Google Scholar] [CrossRef]
- Andreadou, I.; Bell, R.M.; Bøtker, H.E.; Zuurbier, C.J. SGLT2 inhibitors reduce infarct size in reperfused ischemic heart and improve cardiac function during ischemic episodes in preclinical models. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165770. [Google Scholar] [CrossRef]
- Solanes, N.; Bobi, J.; Dagleish, M.; Jiménez, F.; Gray, G.; Sabaté, M.; Tura-Ceide, O.; Rigol, M. Targeting the Main Anatomopathological Features in Animal Models of Myocardial Infarction. J. Comp. Pathol. 2020, 176, 33–38. [Google Scholar] [CrossRef]
- Hundahl, L.A.; Tfelt-Hansen, J.; Jespersen, T. Rat Models of Ventricular Fibrillation Following Acute Myocardial Infarction. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 514–528. [Google Scholar] [CrossRef]
- Khodayari, S.; Khodayari, H.; Amiri, A.Z.; Eslami, M.; Farhud, D.; Hescheler, J.; Nayernia, K. Inflammatory Microenvironment of Acute Myocardial Infarction Prevents Regeneration of Heart with Stem Cells Therapy. Cell. Physiol. Biochem. 2019, 53, 887–909. [Google Scholar] [CrossRef]
- Houang, E.M.; Bartos, J.A.; Hackel, B.J.; Lodge, T.P.; Yannopoulos, D.; Bates, F.S.; Metzger, J.M. Cardiac Muscle Membrane Stabilization in Myocardial Reperfusion Injury. JACC: Basic Transl. Sci. 2019, 4, 275–287. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Hervas, A.; Ruiz, A.-S.; Gavara, J.; Monmeneu, J.V.; De Dios, E.; Rios, C.-N.; Perez-Sole, N.; Perez, I.; Monleon, D.; Morales, J.M.; et al. A Multidisciplinary Assessment of Remote Myocardial Fibrosis After Reperfused Myocardial Infarction in Swine and Patients. J. Cardiovasc. Transl. Res. 2016, 9, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Bodí, V.; Sanchis, J.; Mainar, L.; Chorro, F.J.; Núñez, J.; Monmeneu, J.V.; Chaustre, F.; Forteza, M.J.; Ruiz-Sauri, A.; López-Lereu, M.P.; et al. Right ventricular involvement in anterior myocardial infarction: A translational approach. Cardiovasc. Res. 2010, 87, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Navarro, C.; Hueso, L.; Díaz, A.; Marcos-Garcés, V.; Bonanad, C.; Ruiz-Sauri, A.; Vila, J.M.; Sanz, M.J.; Chorro, F.J.; Piqueras, L.; et al. Role of antiangiogenic VEGF-A165b in angiogenesis and systolic function after reperfused myocardial infarction. Rev. Esp. Cardiol. 2020, 30161–30164. [Google Scholar] [CrossRef]
- Jazayeri, M.-A.; Emert, M.P. Sudden Cardiac Death: Who Is at Risk? Med. Clin. N. Am. 2019, 103, 913–930. [Google Scholar] [CrossRef]
- Ibanez, B.; Aletras, A.H.; Arai, A.E.; Arheden, H.; Bax, J.; Berry, C.; Bucciarelli-Ducci, C.; Croisille, P.; Dall’Armellina, E.; Dharmakumar, R.; et al. Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials. J. Am. Coll. Cardiol. 2019, 74, 238–256. [Google Scholar] [CrossRef]
- Jones, S.P.; Tang, X.L.; Guo, Y.; Steenbergen, C.; Lefer, D.J.; Kukreja, R.C.; Kong, M.; Li, Q.; Bhushan, S.; Zhu, X.; et al. The NHLBI-sponsored consortium for preclinical assesment of cardioprotective therapies (CAESAR): A new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ. Res. 2015, 116, 572–586. [Google Scholar] [CrossRef]
- Calvo, C.J.; Lozano, W.M.; Arias, Ó.J.-M.; Such-Miquel, L.; Such, L.; Genovés, P.; Guill, A.; Millet, J.; Chorro, F.J.; Alberola, A.; et al. Modifications of short-term intrinsic pacemaker variability in diet-induced metabolic syndrome: A study on isolated rabbit heart. J. Physiol. Biochem. 2019, 75, 173–183. [Google Scholar] [CrossRef]
- Such-Miquel, L.; Chorro, F.J.; Guerrero, J.; Trapero, I.; Brines, L.; Zarzoso, M.; Parra, G.; Soler, C.; Del Canto, I.; Alberola, A.; et al. Evaluation of the Complexity of Myocardial Activation During Ventricular Fibrillation. An Experimental Study. Rev. Esp. Cardiol. 2013, 66, 177–184. [Google Scholar] [CrossRef]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Hacker, T.A. Animal Models and Cardiac Extracellular Matrix Research. In Cardiac Extracellular Matrix. Advances in Experimental Medicine and Biology; Schmuck, E., Hematti, P., Raval, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 1098, pp. 45–58. [Google Scholar]
- Andreadou, I.; Bibli, S.-I.; Mastromanolis, E.; Zoga, A.; Efentakis, P.; Papaioannou, N.; Farmakis, D.; Kremastinos, D.T.; Iliodromitis, E.K. Transient carotid ischemia as a remote conditioning stimulus for myocardial protection in anesthetized rabbits: Insights into intracellular signaling. Int. J. Cardiol. 2015, 184, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, M.P.; Hearse, D.J.; Yellon, D.M. Species variation in the coronary collateral circulation during regional myocardial ischemia: A critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc. Res. 1987, 21, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-P.; Liu, Y.; Fan, Y.-J.; Zhao, Y.-Y.; Feng, J.-Q.; Liu, Y. To develop a novel animal model of myocardial infarction: A research imperative. Anim. Model. Exp. Med. 2018, 1, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, E.; Magyari, B.; Töth, L.; Petrasi, Z.; Repa, I.; Koller, A.; Horváth, I.G. Overview of large animal myocardial infarction models (review). Acta Physiol. Hung. 2012, 99, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Isorni, M.-A.; Casanova, A.; Piquet, J.; Bellamy, V.; Pignon, C.; Puymirat, E.; Menasché, P. Comparative Analysis of Methods to Induce Myocardial Infarction in a Closed-Chest Rabbit Model. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Arias-Mutis, O.J.; Marrachelli, V.G.; Ruiz-Sauri, A.; Alberola, A.; Morales, J.M.; Such-Miquel, L.; Monleón, D.; Chorro, F.J.; Such, L.; Zarzoso, M. Development and characterization of an experimental model of diet-induced metabolic syndrome in rabbit. PLoS ONE 2017, 12, e0178315. [Google Scholar] [CrossRef]
- Nerbonne, J.M.; Kass, R.S. Molecular Physiology of Cardiac Repolarization. Physiol. Rev. 2005, 85, 1205–1253. [Google Scholar] [CrossRef]
- Edwards, A.G.; Louch, W.E. Species-Dependent Mechanisms of Cardiac Arrhythmia: A Cellular Focus. Clin. Med. Insights Cardiol. 2017, 11, 1–14. [Google Scholar] [CrossRef]
- Chorro, F.J.; Trapero, I.; Such, L.-M.; Pelechano, F.; Mainar, L.; Cánoves, J.; Tormos, Á.; Alberola, A.; Hove-Madsen, L.; Cinca, J.; et al. Pharmacological modifications of the stretch-induced effects on ventricular fibrillation in perfused rabbit hearts. Am. J. Physiol. Circ. Physiol. 2009, 297, H1860–H1869. [Google Scholar] [CrossRef]
- Feng, Y.; Hemmeryckx, B.; Frederix, L.; Lox, M.; Wu, J.; Heggermont, W.; Lu, H.R.; Gallacher, D.; Oyen, R.; Lijnen, H.R.; et al. Monitoring reperfused myocardial infarction with delayed left ventricular systolic dysfunction in rabbits by longitudinal imaging. Quant. Imaging Med. Surg. 2018, 8, 754–769. [Google Scholar] [CrossRef]
- Hu, N.; Straub, C.M.; Garzarelli, A.A.; Sabey, K.H.; Yockman, J.W.; Bull, D.A. Ligation of the Left Circumflex Coronary Artery with Subsequent MRI and Histopathology in Rabbits. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 838–844. [Google Scholar] [PubMed]
- Maroko, P.R.; Kjekshus, J.K.; Sobel, B.E.; Watanabe, T.; Covell, J.W.; Ross, J., Jr.; Braunwald, E. Factors Influencing Infarct Size Following Experimental Coronary Artery Occlusions. Circulation 1971, 43, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.; Lindahl, B.; Hammarsten, O.; Müller, C.; Giannitsis, E.; Huber, K.; Möckel, M.; Plebani, M.; Thygesen, K.; Jaffe, A.S.; et al. How is cardiac troponin released from injured myocardium? Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Archan, S.; Fleisher, L.A. From Creatine Kinase-MB to Troponin. Anesthesiology 2010, 112, 1005–1012. [Google Scholar] [CrossRef]
- Santaló, M.B.; Guindo Soldevila, J.; Ordóñez Llanos, J. Marcadores biológicos de necrosis miocárdica. Rev. Esp. Cardiol. 2003, 56, 703–720. [Google Scholar] [CrossRef]
- Hearse, D.J. The elusive coypu: The importance of collateral flow and the search for an alternative to the dog. Cardiovasc. Res. 2000, 45, 215–219. [Google Scholar] [CrossRef]
- Teunissen, P.F.; Horrevoets, A.J.; Van Royen, N. The coronary collateral circulation: Genetic and environmental determinants in experimental models and humans. J. Mol. Cell. Cardiol. 2012, 52, 897–904. [Google Scholar] [CrossRef]
- Cops, J.; Haesen, S.; De Moor, B.; Mullens, W.; Hansen, D. Current animal models for the study of congestion in heart failure: An overview. Heart Fail. Rev. 2019, 24, 387–397. [Google Scholar] [CrossRef]
- Costa, C.M.; Plank, G.; Rinaldi, C.A.; Niederer, S.A.; Bishop, M.J. Modeling the Electrophysiological Properties of the Infarct Border Zone. Front. Physiol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Humeres, C.; Frangogiannis, N.G. Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC: Basic Trans. Sci. 2019, 4, 449–467. [Google Scholar] [CrossRef]
- Richardson, W.J.; Clarke, S.A.; Quinn, T.A.; Holmes, J.W. Physiological Implications of Myocardial Scar Structure. Compr. Physiol. 2015, 5, 1877–1909. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Qu, Z.; Weiss, J.N. Cardiac fibrosis and arrhythmogenesis: The road to repair is paved with perils. J. Mol. Cell. Cardiol. 2014, 70, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kléber, A.G.; Rudy, Y. Basic Mechanisms of Cardiac Impulse Propagation and Associated Arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef] [PubMed]


| Week 1 | ||||
|---|---|---|---|---|
| Postoperative care | Day 0 | Day 1 | Day 2 | Day 3 |
| Surgery | Heat with infrared light | Heat with infrared light | Heat with infrared light | |
| Fluid Therapy (IV) | Fluid Therapy (IV) | Fluid Therapy (IV) | ||
| Analgesic treatment (SC) 0.03 mg/Kg/6 h | Analgesic treatment (SC) 0.03 mg/Kg/6 h | Analgesic treatment (SC) 0.03 mg/Kg/6 h | ||
| Anti-inflammatory treatment (SC) 0.2 mg/Kg/24 h | Anti-inflammatory treatment (SC) 0.2 mg/Kg/24 h | Anti-inflammatory treatment (SC) 0.2 mg/Kg/24 h | ||
| Antibiotic treatment (SC) 10 mg/Kg/24 h | Antibiotic treatment (SC) 10 mg/Kg/24 h | Antibiotic treatment (SC) 10 mg/Kg/24 h | ||
| Control of digestive functionality | Control of digestive functionality | Control of digestive functionality | ||
| General physical examination | General physical examination | General physical examination | ||
| Day 4 | Day 5 | Day 6 | Day 7 | |
| Heat with infrared light | - | - | - | |
| - | - | - | - | |
| Analgesic treatment: osmotic pump: 0.03 mg/Kg rate of 10 µL/h | Analgesic treatment: osmotic pump: 0.03 mg/Kg rate of 10 µL/h | Analgesic treatment: osmotic pump: 0.03 mg/Kg rate of 10 µL/h | Analgesic treatment: osmotic pump: 0.03 mg/Kg rate of 10 µL/h | |
| Anti-inflammatory treatment (SC) 0.2 mg/Kg/24 h | Anti-inflammatory treatment (SC) 0.2 mg/Kg/24 h | Anti-inflammatory treatment (SC) 0.2 mg/Kg/24 h | Anti-inflammatory treatment (SC) 0.2 mg/Kg/24 h | |
| Antibiotic treatment (SC) 10 mg/Kg/24 h | Antibiotic treatment (SC) 10 mg/Kg/24 h | Antibiotic treatment (SC) 10 mg/Kg/24 h | Antibiotic treatment (SC) 10 mg/Kg/24 h | |
| Control of digestive functionality | Control of digestive functionality | Control of digestive functionality | Control of digestive functionality | |
| General physical examination | General physical examination | General physical examination | General physical examination | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovés, P.; Arias-Mutis, Ó.J.; Parra, G.; Such-Miquel, L.; Zarzoso, M.; Del Canto, I.; Soler, C.; Díaz, A.; Blanch, E.; Alberola, A.; et al. Development and Long-Term Follow-Up of an Experimental Model of Myocardial Infarction in Rabbits. Animals 2020, 10, 1576. https://doi.org/10.3390/ani10091576
Genovés P, Arias-Mutis ÓJ, Parra G, Such-Miquel L, Zarzoso M, Del Canto I, Soler C, Díaz A, Blanch E, Alberola A, et al. Development and Long-Term Follow-Up of an Experimental Model of Myocardial Infarction in Rabbits. Animals. 2020; 10(9):1576. https://doi.org/10.3390/ani10091576
Chicago/Turabian StyleGenovés, Patricia, Óscar J. Arias-Mutis, Germán Parra, Luis Such-Miquel, Manuel Zarzoso, Irene Del Canto, Carlos Soler, Ana Díaz, Eva Blanch, Antonio Alberola, and et al. 2020. "Development and Long-Term Follow-Up of an Experimental Model of Myocardial Infarction in Rabbits" Animals 10, no. 9: 1576. https://doi.org/10.3390/ani10091576
APA StyleGenovés, P., Arias-Mutis, Ó. J., Parra, G., Such-Miquel, L., Zarzoso, M., Del Canto, I., Soler, C., Díaz, A., Blanch, E., Alberola, A., Such, L., & Chorro, F. J. (2020). Development and Long-Term Follow-Up of an Experimental Model of Myocardial Infarction in Rabbits. Animals, 10(9), 1576. https://doi.org/10.3390/ani10091576





