Expansion of Vertebrate Pest Exclusion Fencing and Its Potential Benefits for Threatened Fauna Recovery in Australia
Abstract
:Simple Summary
Abstract
1. Introduction
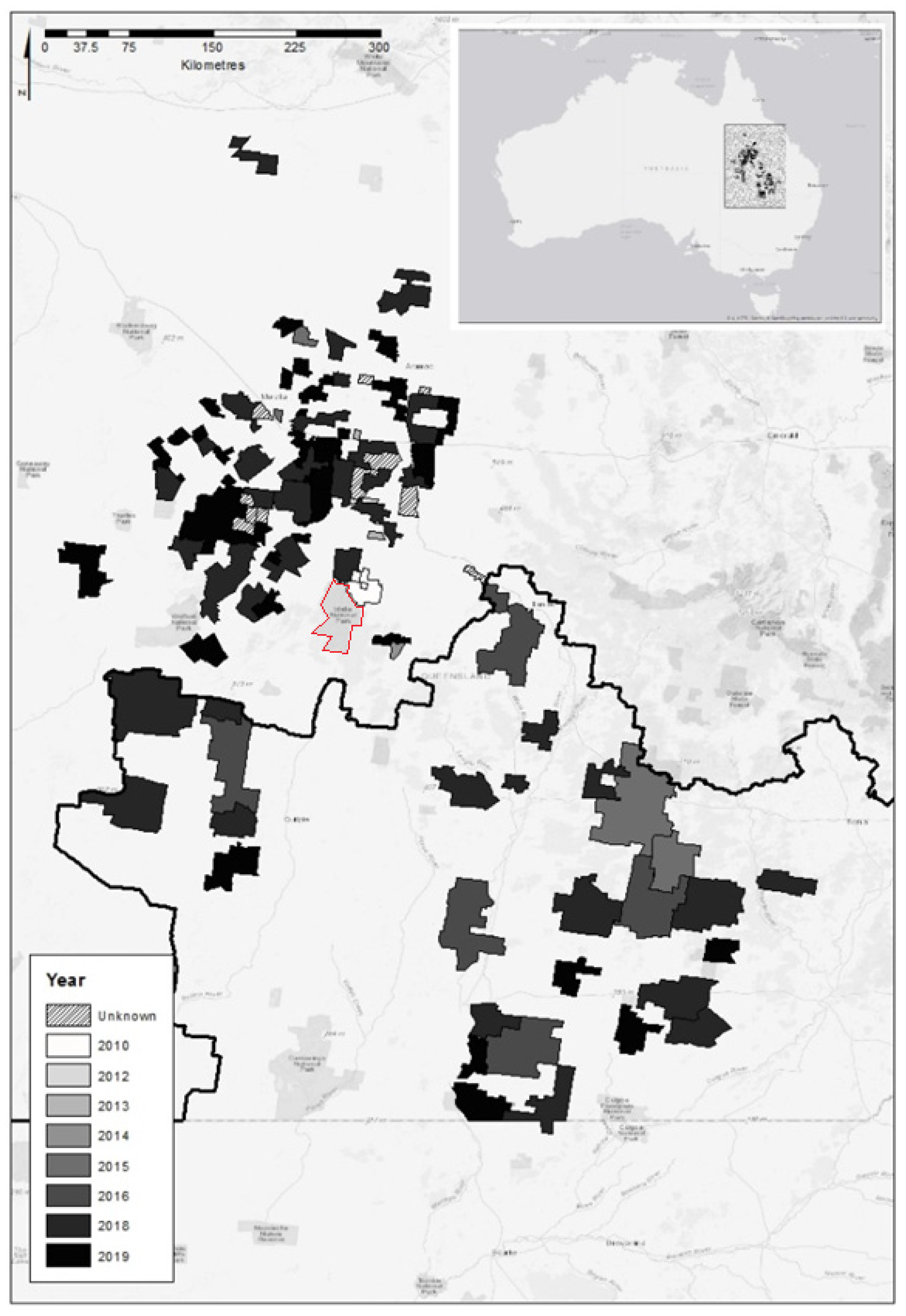
2. Materials and Methods
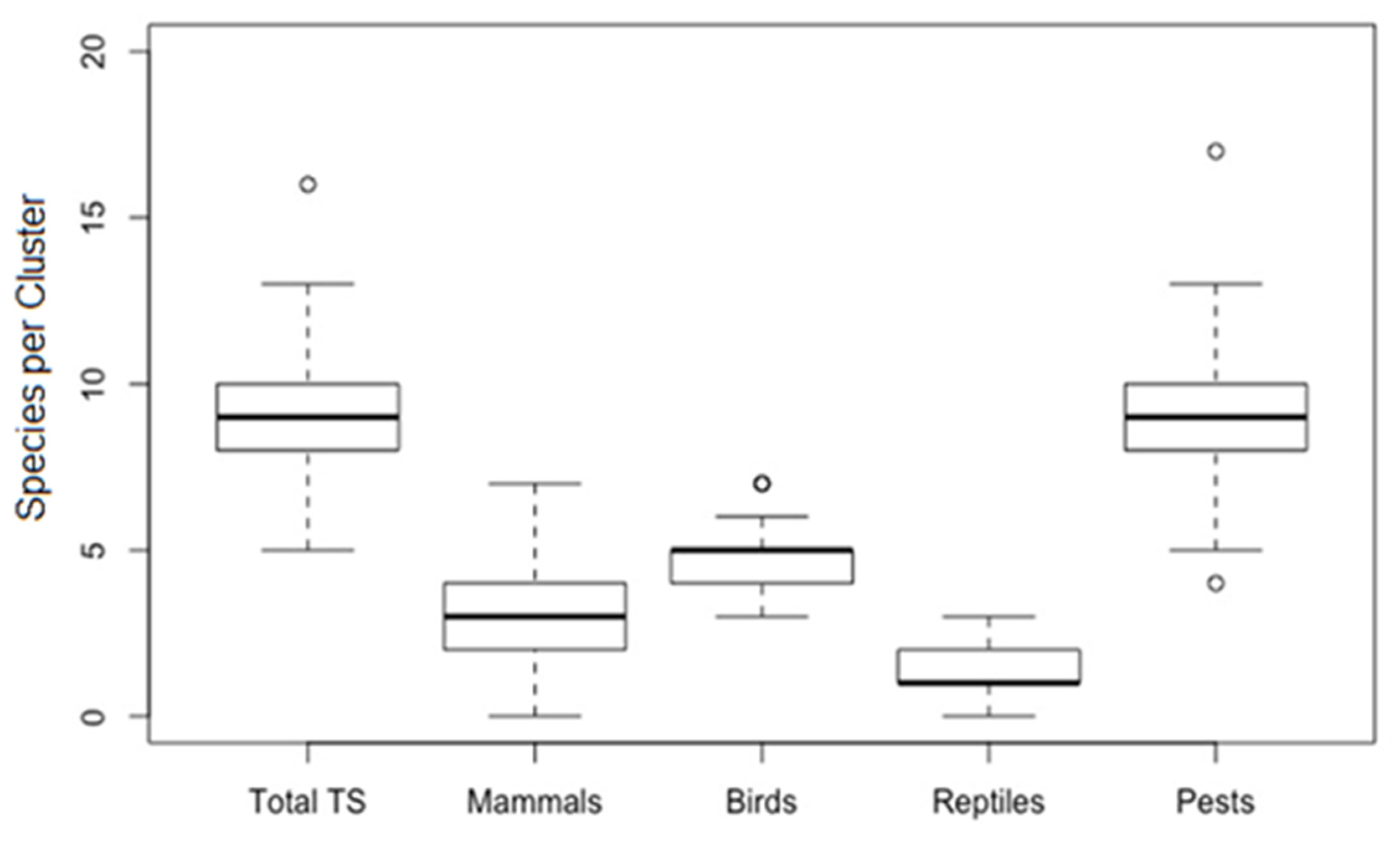
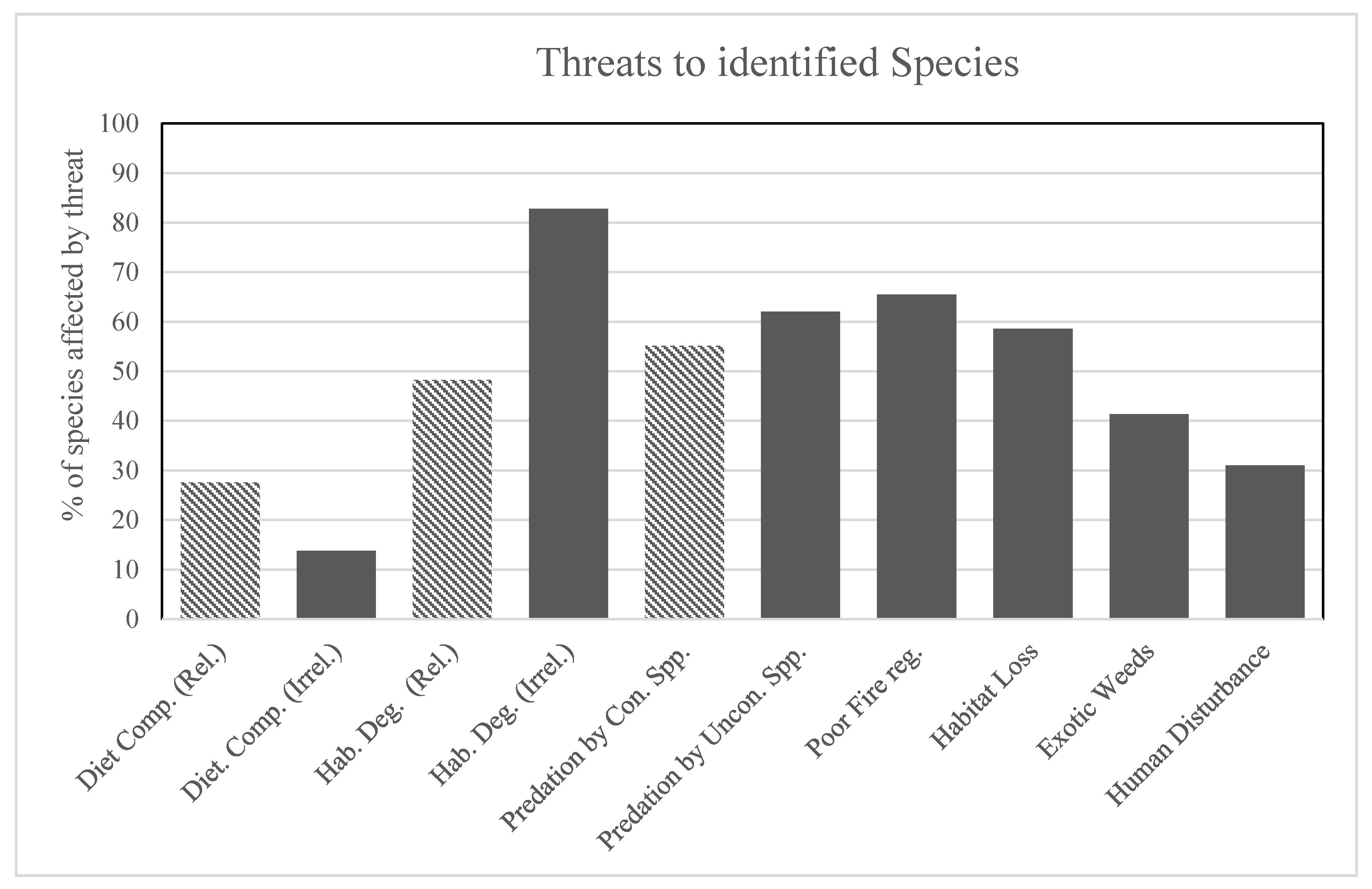
3. Results
4. Discussion
5. Conclusions
- Creation of new locations where it is possible to change the trajectory of extant threatened species populations;
- Creation of new locations where it is possible to reintroduce locally extinct species;
- Creation of new locations suitable for improving antipredator defences and overcoming prey naivety issues;
- Addition of new biomes not currently represented in the national fenced reserve system;
- Opportunities to develop private–public partnerships to share the costs of constructing high-security conservation fences (i.e., new, large conservation fences can be erected for a fraction of current costs);
- Reduction or shifting of ongoing fence maintenance costs to sources non-reliant on government, philanthropy or public donations;
- Alleviation of threatened species’ overpopulation in some of the current conservation reserves.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woinarski, J.; Burbidge, A.; Harrison, P. Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proc. Natl. Acad. Sci. USA 2015, 112, 4531–4540. [Google Scholar]
- Woinarski, J.; Burbidge, A.; Harrison, P. The Action Plan for Australian Mammals 2012; CSIRO Publishing: Clayton, Australia, 2014. [Google Scholar]
- McKenzie, N.; Burbidge, A.; Baynes, A.; Brereton, R.; Dickman, C.; Gordon, G.; Gibson, L.; Menkhorst, P.; Robinson, A.; Williams, M. Analysis of factors implicated in the recent decline of Australia’s mammal fauna. J. Biogeogr. 2007, 34, 597–611. [Google Scholar]
- Allen, B. A comment on the distribution of historical and contemporary livestock grazing across Australia: Implications for using dingoes for biodiversity conservation. Ecol. Manag. Restor. 2011, 12, 26–30. [Google Scholar]
- Ringma, J.; Legge, S.; Woinarski, J.; Radford, J.; Wintle, B.; Bode, M. Australia’s mammal fauna requires a strategic and enhanced network of predator-free havens. Nat. Ecol. Evol. 2018, 2, 410–411. [Google Scholar] [CrossRef]
- Hayward, M.; Kerley, G. Fencing for conservation: Restriction of evolutionary potential or a riposte to threatening processes? Biol. Conserv. 2009, 142, 1–13. [Google Scholar]
- Druce, H.; Pretorius, K.; Slotow, R. The response of an elephant population to conservation area expansion: Phinda Private Game Reserve, South Africa. Biol. Conserv. 2008, 141, 3127–3138. [Google Scholar]
- Moseby, K.; O’Donnell, E. Reintroduction of the greater bilby, Macrotis lagotis (Reid) (Marsupialia: Thylacomyidae), to northern South Australia: Survival, ecology and notes on reintroduction protocols. Wildl. Res. 2003, 30, 15–27. [Google Scholar] [CrossRef]
- McKnight, T. Barrier fencing for vermin control in Australia. Geogr. Rev. 1969, 59, 330–347. [Google Scholar]
- Smith, D.; King, R.; Allen, B. Impacts of exclusion fencing on target and non-target fauna: A global review. Biol. Rev. 2020. [Google Scholar] [CrossRef]
- Allen, B.; West, P. Influence of dingoes on sheep distribution in Australia. Aust. Vet. J. 2013, 91, 261–267. [Google Scholar]
- Cockfield, G.; Botterill, L.; Kelly, S. A prospective evaluation of contingent loans as a means of financing wild dog exclusion fences. Rangel. J. 2018, 40, 591–601. [Google Scholar]
- Darkoh, M.; Mbaiwa, J. Globalisation and the livestock industry in Botswana. Singap. J. Trop. Geogr. 2002, 23, 149–166. [Google Scholar] [CrossRef]
- Otto, T. Developing and Implementing Effective Black Bear Exclusion Fences to Protect Mobile Apiaries. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2013. [Google Scholar]
- Dickman, C. Fences or ferals? Benefits and costs of conservation fencing in Australia. In Fencing for Conservation; Somers, M., Hayward, M., Eds.; Springer: Berlin, Germany, 2012; pp. 43–63. [Google Scholar]
- Ringma, J.; Legge, S.; Woinarski, J.; Radford, J.; Wintle, B.; Bentley, J.; Burbidge, A.; Copley, P.; Dexter, N.; Dickman, C.; et al. Systematic planning can rapidly close the protection gap in Australian mammal havens. Conserv. Lett. 2019, 12, e12611. [Google Scholar]
- Legge, S.; Woinarski, J.; Burbidge, A.; Palmer, R.; Ringma, J.; Radford, J.; Mitchell, N.; Bode, M.; Wintle, B.; Baseler, M. Havens for threatened Australian mammals: The contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators. Wildl. Res. 2018, 45, 627–644. [Google Scholar]
- Abbott, I. Origin and spread of the cat, Felis catus, on mainland Australia, with a discussion of the magnitude of its early impact on native fauna. Wildl. Res. 2002, 29, 51–74. [Google Scholar]
- Rolls, E. They All Ran Wild: The Animals and Plants that Plague Australia; Angus & Robertson Publishers: Sydney, Australia, 1969. [Google Scholar]
- Doherty, T.; Davis, R.; van Etten, E.; Algar, D.; Collier, N.; Dickman, C.; Edwards, G.; Masters, P.; Palmer, R.; Robinson, S. A continental-scale analysis of feral cat diet in Australia. J. Biogeogr. 2015, 42, 964–975. [Google Scholar]
- Doherty, T.; Dickman, C.; Johnson, C.; Legge, S.; Ritchie, E.; Woinarski, J. Impacts and management of feral cats Felis catus in Australia. Mammal Rev. 2017, 47, 83–97. [Google Scholar]
- Burbidge, A.; McKenzie, N. Patterns in the modern decline of Western Australia’s vertebrate fauna: Causes and conservation implications. Biol. Conserv. 1989, 50, 143–198. [Google Scholar]
- Department of the Environment. SPRAT Database - List of Threatening Processes. Available online: http://www.environment.gov.au/cgi-bin/sprat/public/publicgetkeythreats.pl (accessed on 27 February 2019).
- Allen, B.; Fleming, P. Reintroducing the dingo: The risk of dingo predation to threatened vertebrates of western New South Wales. Wildl. Res. 2012, 39, 35–50. [Google Scholar]
- Allen, B.; Leung, L. Assessing predation risk to threatened fauna from their prevalence in predator scats: Dingoes and rodents in arid Australia. PLoS ONE 2012, 7, e36426. [Google Scholar]
- Major, R. Predation and Hybridisation by Feral Dogs (Canis Lupus Familiaris)—Key Threatening Process Listing; New South Wales Department of Environment, Climate Change, and Water: Sydney, Australia, 2009.
- Pedler, R.; Brandle, R.; Read, J.; Southgate, R.; Bird, P.; Moseby, K. Rabbit biocontrol and landscape-scale recovery of threatened desert mammals. Conserv. Biol. 2016, 30, 774–782. [Google Scholar]
- Australian Wildlife Conservancy. Feral Herbivore Control. Available online: https://www.australianwildlife.org/our-work/feral-herbivore-control/ (accessed on 9 September 2019).
- Australian Wildlife Conservancy. Feral Cat and Fox Control. Available online: https://www.australianwildlife.org/our-work/feral-cat-and-fox-control/ (accessed on 9 September 2019).
- Legge, S.; Murphy, B.; McGregor, H.; Woinarski, J.; Augusteyn, J.; Ballard, G.; Baseler, M.; Buckmaster, T.; Dickman, C.; Doherty, T.; et al. Enumerating a continental-scale threat: How many feral cats are in Australia? Biol. Conserv. 2017, 206, 293–303. [Google Scholar]
- Moseby, K.; Lollback, G.; Lynch, C. Too much of a good-thing, successful reintroduction leads to overpopulation in a threatened mammal. Biol. Conserv. 2018, 219, 78–88. [Google Scholar] [CrossRef]
- Short, J. The Characteristics and Success of Vertebrate Translocations within Australia; Australian Government Department of Agriculture, Fisheries and Forestry: Canberra, Australia, 2009.
- Department of Primary Industries and Fisheries. History of Barrier Fences in Queensland; Land Protection (Invasive Plants and Animals): Brisbane, Australia, 2007.
- RAPAD; QFPI. RAPAD Cluster Fencing Project Round 1: March—2016 April 2018 Final report: Section 1; Queensland Government: Queensland, Australia, 2018.
- Allen, B.; West, P. Re: Dingoes are a major causal factor for the decline and distribution of sheep in Australia. Aust. Vet. J. 2015, 93, 90. [Google Scholar]
- Pahl, L. Macropods, feral goats, sheep and cattle. 1. Equivalency in how much they eat. Rangel. J. 2019, 41, 497–518. [Google Scholar] [CrossRef]
- Waters, C.; McDonald, S.; Reseigh, J.; Grant, R.; Burnside, D. Insights on the relationship between total grazing pressure management and sustainable land management: Key indicators to verify impacts. Rangel. J. 2019, 41, 535–556. [Google Scholar] [CrossRef]
- Hacker, R.; Sinclair, K.; Pahl, L. Prospects for ecologically and socially sustainable management of total grazing pressure in the southern rangelands of Australia. Rangel. J. 2019, 41, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Allen, B. FOFI5M: Taking threatened species recovery to the next level. In Proceedings of the Restore, Regenerate, Revegetate: A Conference on Restoring Ecological Processes, Ecosystems and Landscapes in a Changing World, Armidale, New South Wales, Australia, 5–9 February 2017; pp. 1–2. [Google Scholar]
- Bode, M.; Brennan, K.; Morris, K.; Burrows, N.; Hague, N. Choosing cost-effective locations for conservation fences in the local landscape. Wildl. Res. 2012, 39, 192–201. [Google Scholar] [CrossRef]
- Department of Natural Resources‚ Mines and Energy. Rural Properites. 2014. Available online: http://www.informationweek.com/news/201202317 (accessed on 2 June 2012).
- Department of Natural Resources‚ Mines and Energy. Queensland Spatial Catalogue—QSpatial. Available online: https://www.dnrme.qld.gov.au/ (accessed on 2 June 2012).
- Clark, P.; Clark, E.; Allen, B. Sheep, dingoes and kangaroos: New challenges and a change of direction 20 years on. In Advances in Conservation through Sustainable Use of Wildlife; Baxter, G., Finch, N., Murray, P., Eds.; University of Queensland: Brisbane, Australia, 2018; pp. 173–178. [Google Scholar]
- Allen, B. Relationships between kangaroos, grass and livestock. In Proceedings of the Australian Rangelands Society Conference, Canberra, Australia, 2 September 2019. [Google Scholar]
- Helmstedt, K.; Possingham, H.; Brennan, K.; Rhodes, J.; Bode, M. Cost-efficient fenced reserves for conservation: Single large or two small? Ecol. Appl. 2014, 24, 1780–1792. [Google Scholar] [CrossRef]
- Long, K.; Robley, A. Cost Effective Feral Animal Exclusion Fencing for Areas of High Conservation Value in Australia; Department of the Environment and Heritage: Canberra, Australia, 2004. [Google Scholar]
- Moseby, K.; Read, J. The efficacy of feral cat, fox and rabbit exclusion fence designs for threatened species protection. Biol. Conserv. 2006, 127, 429–437. [Google Scholar]
- Threatened Species Scientific Committee. Conservation Advice: Petrogale xanthopus celeris, yellow-footed rock-wallaby (central-western Queensland). In Environment Protection and Biodiversity Conservation Act 1999; Threatened Species Scientific Committee: Canberra, Australia, 2016. [Google Scholar]
- Whitehouse, S. The diet of the dingo in Western Australia. Wildl. Res. 1977, 4, 145–150. [Google Scholar]
- Moseby, K.; Read, J.; Gee, P.; Gee, I. A Study of the Davenport Range Black- Footed Rock Wallaby Colony and Possible Threatening Processes; Wildlife Conservation Fund: Adelaide, Australia, 1998. [Google Scholar]
- Kinnear, J.; Krebs, C.; Pentland, C.; Orell, P.; Holme, C.; Karvinen, R. Predator-baiting experiments for the conservation of rock-wallabies in Western Australia: A 25-year review with recent advances. Wildl. Res. 2010, 37, 57–67. [Google Scholar]
- Lethbridge, M.; Alexander, P. Comparing population growth rates using weighted bootstrapping: Guiding the conservation management of Petrogale xanthopus xanthopus (yellow-footed rock-wallaby). Biol. Conserv. 2008, 141, 1185–1195. [Google Scholar]
- Lethbridge, M.; Harper, M.; Strauss, J. An Analysis of the Population Viability of the Yellow-Footed Rock-Wallaby at Selected Sites in South Australia; Flinders University & DEH Bounceback Program: Adelaide, Australia, 2010. [Google Scholar]
- Gordon, G.; McRae, P.; Lim, L.; Reimer, D.; Porter, G. The conservation status of the yellow-footed rock-wallaby in Queensland. Oryx 1993, 27, 159–168. [Google Scholar]
- Copley, P.; Ellis, M.; van Weenen, J. Petrogale xanthopus: The IUCN Red List of Threatened Species. Available online: http://dx.doi.org/10.2305/IUCN.UK.2016-2.RLTS.T16750A21955455.en (accessed on 12 November 2019).
- Pople, A.; Lowry, J.; Lundie-Jenkins, G.; Clancy, T.; McCallum, H.; Sigg, D.; Hoolihan, D.; Hamilton, S. Demography of bridled nailtail wallabies translocated to the edge of their former range from captive and wild stock. Biol. Conserv. 2001, 102, 285–299. [Google Scholar] [CrossRef]
- Hayward, M.; L’Hotellier, F.; O’Connor, T.; Ward-Fear, G.; Cathcart, J.; Cathcart, T.; Stephens, J.; Stephens, J.; Herman, K.; Legge, S. Reintroduction of Bridled Nailtail Wallabies Beyond Fences at Scotia Sanctuary - Phase 1. Proc. Linn. Soc. N. S. W. 2012, 134, A27–A37. [Google Scholar]
- Moseby, K.; Read, J.; Paton, D.; Copley, P.; Hill, B.; Crisp, H. Predation determines the outcome of 10 reintroduction attempts in arid South Australia. Biol. Conserv. 2011, 144, 2863–2872. [Google Scholar] [CrossRef]
- Short, J. Predation by feral cats key to the failure of a long-term reintroduction of the western barred bandicoot (Perameles bougainville). Wildl. Res. 2016, 43, 38–50. [Google Scholar] [CrossRef]
- West, R.; Read, J.; Ward, M.; Foster, W.; Taggart, D. Monitoring for adaptive management in a trial reintroduction of the black-footed rock-wallaby Petrogale lateralis. Oryx 2017, 51, 554–563. [Google Scholar]
- Fisher, D.; Hoyle, S.; Blomberg, S. Population dynamics and survival of an endangered wallaby: A comparison of four methods. Ecol. Appl. 2000, 10, 901–910. [Google Scholar]
- Environmental Resources Information Network. Interim Biogeographic Regionalisation for Australia, Version 7; Department of the Environment and Energy: Canberra, Australia, 2016. [Google Scholar]
- Bode, M.; Wintle, B. How to Build an Efficient Conservation Fence. Conserv. Biol. 2010, 24, 182–188. [Google Scholar] [CrossRef]
- Giumelli, J.; White, B. Exclusion Fences Keep Ferals at Bay; Kondinin Group: Perth, Australia, 2016. [Google Scholar]
- Norbury, G.; Hutcheon, A.; Reardon, J.; Daigneault, A. Pest fencing or pest trapping: A bio-economic analysis of cost-effectiveness. Austral Ecol. 2014, 39, 795–807. [Google Scholar] [CrossRef]
- Moseby, K.; Neilly, H.; Read, J.; Crisp, H. Interactions between a top order predator and exotic mesopredators in the Australian rangelands. Int. J. Ecol. 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Berger, J.; Swenson, J.; Persson, I. Recolonizing carnivores and naive prey: Conservation lessons from Pleistocene extinctions. Science 2001, 291, 1036–1039. [Google Scholar]
- Moseby, K.; Blumstein, D.; Letnic, M. Harnessing natural selection to tackle the problem of prey naivete. Evol. Appl. 2016, 9, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.; Letnic, M.; Blumstein, D.; Moseby, K. Reversing the effects of evolutionary prey naiveté through controlled predator exposure. J. Appl. Ecol. 2019, 56, 1761–1769. [Google Scholar] [CrossRef]


| Species | S | % | km2 | Threats | Potentially Alleviated | |
|---|---|---|---|---|---|---|
| 1 | Curlew Sandpiper, Calidris ferruginea | CE | 100.0 | - | 4, 8, 9, 10 | - |
| 2 | Plains-wanderer, Pedionomus torguatus | CE | 35.1 | 481.2 | 4, 5, 6, 7, 8, 9 | 5 |
| 3 | Eastern Curlew, Numenius madagascariensis | CE | 3.66 | - | 4, 8, 9, 10 | - |
| 4 | Star Finch, Neochmia ruficauda | E | 44.1 | - | 3, 4, 5, 6, 9 | 3, 5 |
| 5 | Black-throated finch, Poephila cinta | E | 10.1 | 4.2 | 3, 4, 5, 6, 7, 8, 9 | 3, 5 |
| 6 | Australian Painted Snipe, Rostratula australis | E | 99.5 | 208.3 | 3, 4, 5, 6, 9 | 3, 5 |
| 7 | Night Parrot, Pezoporus occidentalis | E | 26.0 | - | 1, 2, 3, 4, 5, 6, 7 | 1, 3, 5 |
| 8 | Bulloo Grey Grass-wren, Amytornis barbatus barbatus | E | 5.16 | 0 | 3, 4, 5, 6, 8, 9 | 3, 5 |
| 9 | Northern Quoll, Dasyurus hallucatus | E | 4.77 | 0 | 1, 4, 5, 6, 7, 8, 9 | 1, 5 |
| 10 | Bridled Nailtail Wallaby, Onychogalea fraenata | E | 1.84 | 0 | 1, 3, 4, 5, 6, 7, 9 | 1, 3, 5 |
| 11 | Squatter Pigeon, Geophaps scripta scripta | V | 22.9 | 124.5 | 3, 4, 5, 6, 8, 9, 10 | 3, 5 |
| 12 | Painted Honeyeater, Grantiella picta | V | 96.3 | 14,710.0 | 2, 3, 4, 6, 8, 10 | 3 |
| 13 | Red Goshawk, Erythrotriorchis radiatus | V | 37.8 | 0 | 1, 2, 4, 7, 8 | 1 |
| 14 | Masked Owl, Tyto novaehollandiae kimberli | V | 3.66 | - | 2, 3, 7, 8 | 3 |
| 15 | Greater Bilby, Macrotis lagotis | V | 40.9 | 0 | 1, 4, 5, 6, 7 | 1, 5 |
| 16 | Koala, Phascolarctos cinereus | V | 69.8 | 193.6 | 5, 7, 8, 10 | 5 |
| 17 | Julia Creek Dunnart, Sminthopsis douglasi | V | 34.0 | 686.7 | 1, 3, 5, 6, 8, 9 | 1, 3, 5 |
| 18 | Corben’s Long-eared Bat, Nyctophilus corbeni | V | 41.7 | 193.4 | 4, 5, 7, 8, 10 | 5 |
| 19 | Yellow-footed Rock-wallaby, Petrogale xanthopus | V | 46.3 | 4031.9 | 1, 3, 5, 7, 8 | 1, 3, 5 |
| 20 | Semon’s Leaf-nosed Bat, Hipposideros semoni | V | 3.66 | 0 | 4, 5, 6, 7, 8, 10 | 5 |
| 21 | Ghost Bat, Macroderma gigas | V | 4.47 | 0 | 1, 4, 6, 7, 8, 10 | 1 |
| 22 | Greater Glider, Petauroides volans | V | 3.66 | - | 4, 6, 7, 8 | - |
| 23 | Spectacled Flying Fox, Pteropus conspicillatus | V | 3.66 | 0 | 4, 7, 10 | - |
| 24 | Bare-rumped Sheathtail-bat, Saccolaimus nudicluniatus | V | 3.66 | 0 | 4, 7 | - |
| 25 | Plains Death Adder, Acanthophis hawkei | V | 45.5 | - | 3, 4, 6, 7 | 3 |
| 26 | Yakka Skink, Egernia rugosa | V | 49.1 | 2202.6 | 4, 5, 6, 7 | 5 |
| 27 | Ornamental Snake, Denisonia maculata | V | 12.9 | 0 | 3, 4, 6 | 3 |
| 28 | Adorned Delma, Delma torquata | V | 10.9 | 1.3 | 4, 7, 9 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, D.; Waddell, K.; Allen, B.L. Expansion of Vertebrate Pest Exclusion Fencing and Its Potential Benefits for Threatened Fauna Recovery in Australia. Animals 2020, 10, 1550. https://doi.org/10.3390/ani10091550
Smith D, Waddell K, Allen BL. Expansion of Vertebrate Pest Exclusion Fencing and Its Potential Benefits for Threatened Fauna Recovery in Australia. Animals. 2020; 10(9):1550. https://doi.org/10.3390/ani10091550
Chicago/Turabian StyleSmith, Deane, Kristy Waddell, and Benjamin L. Allen. 2020. "Expansion of Vertebrate Pest Exclusion Fencing and Its Potential Benefits for Threatened Fauna Recovery in Australia" Animals 10, no. 9: 1550. https://doi.org/10.3390/ani10091550






