Proline Protects Boar Sperm against Oxidative Stress through Proline Dehydrogenase-Mediated Metabolism and the Amine Structure of Pyrrolidine
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Media
2.2. Ethical Approval
2.3. Animals and Semen Collection
2.4. Experiment I
2.4.1. Semen Storage
2.4.2. Motility Analysis
2.4.3. Integrity of Membrane and Acrosome
2.4.4. Measurement of ATP Levels
2.4.5. Mitochondrial Membrane Potentials
2.5. Experiment II
2.5.1. Reactive Oxygen Species
2.5.2. Lipid Peroxidation
2.5.3. Measurement of Glutathione Levels
2.5.4. Analysis of Catalase and Superoxide Dismutase Activity
2.6. Experiment III
Rapid Cooling
2.7. Experiment IV
2.8. Experiment V
2.8.1. Western Blotting
2.8.2. Immunofluorescence Staining of PRODH in Boar Sperm
2.8.3. Analysis of Proline Dehydrogenase Activity
2.9. Statistical Analysis
3. Results
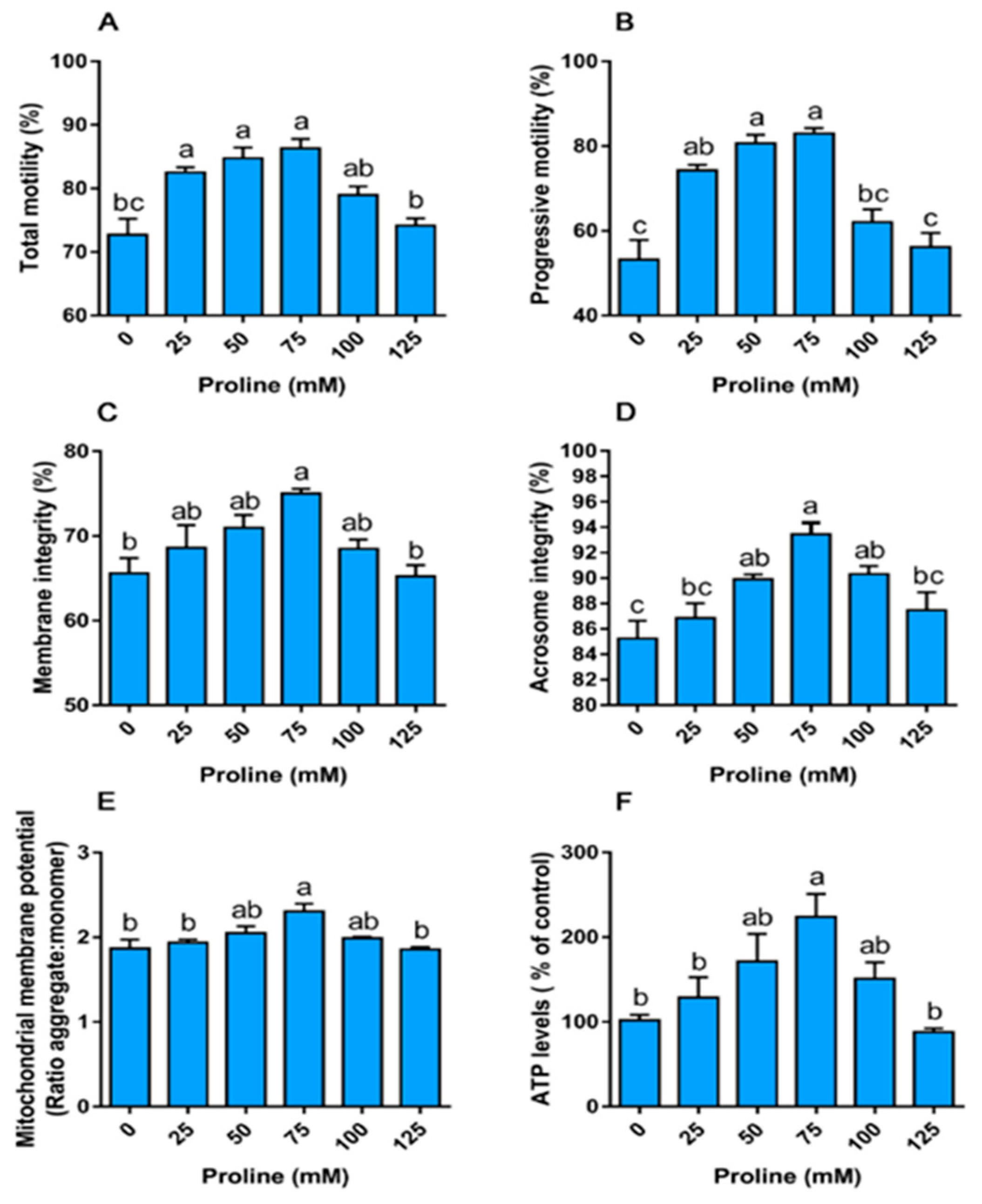
3.1. Experiment I
3.1.1. Proline Improves Sperm Motility
3.1.2. Proline Improves the Integrity of the Membrane and Acrosome
3.1.3. Proline Increases the Mitochondrial Membrane Potential and ATP Levels
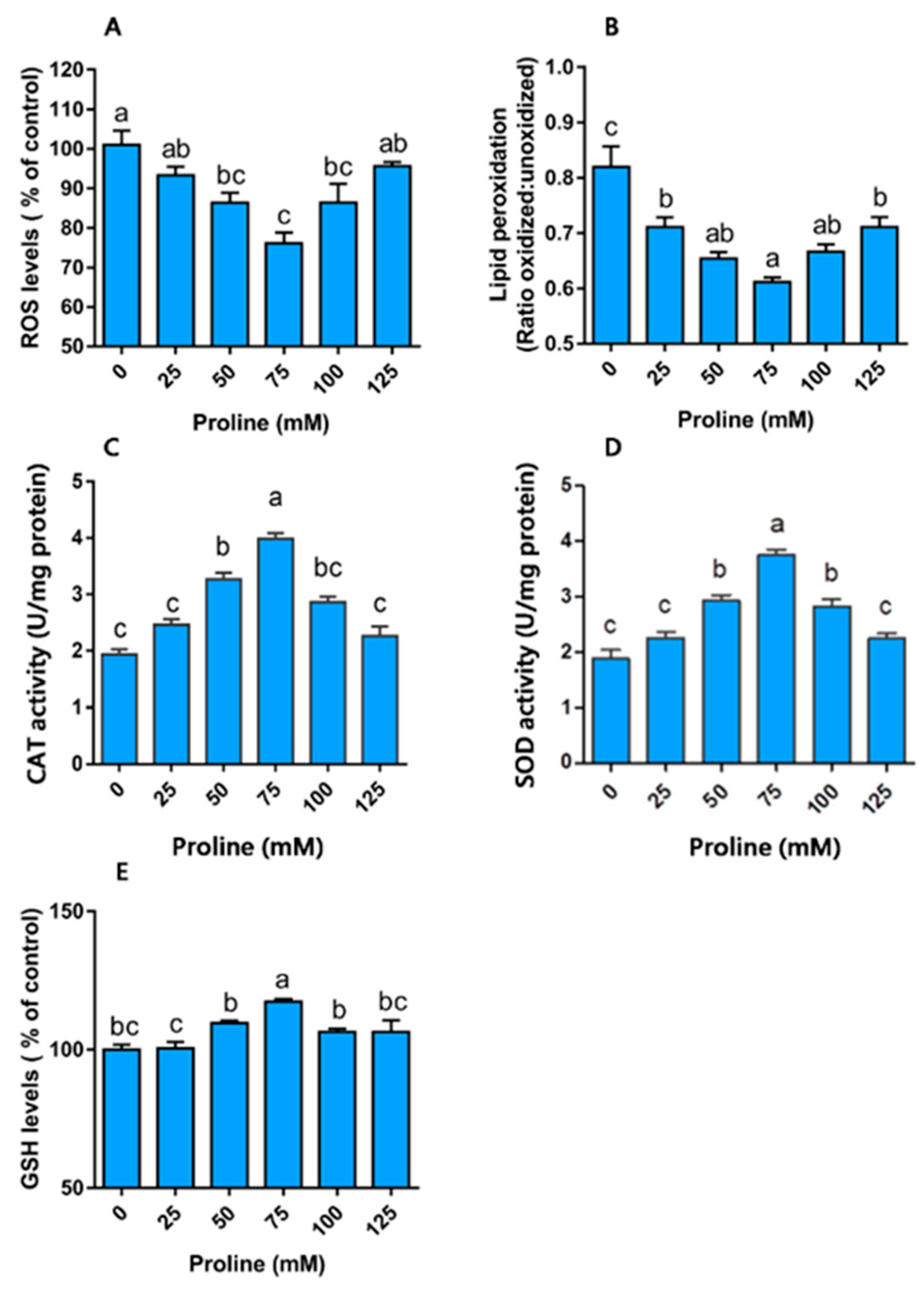
3.2. Experiment II
Proline Modulates Redox Homeostasis
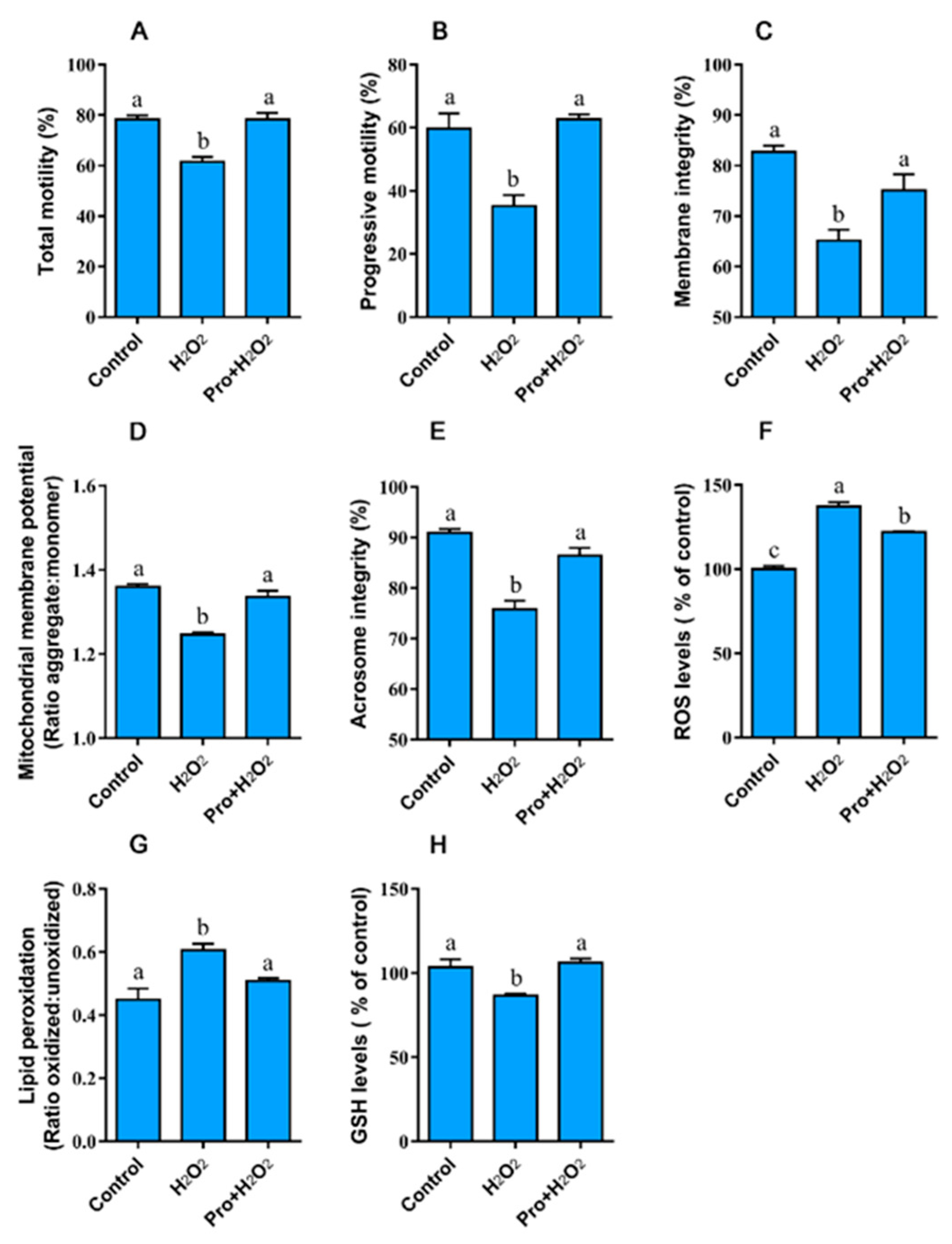
3.3. Experiment III
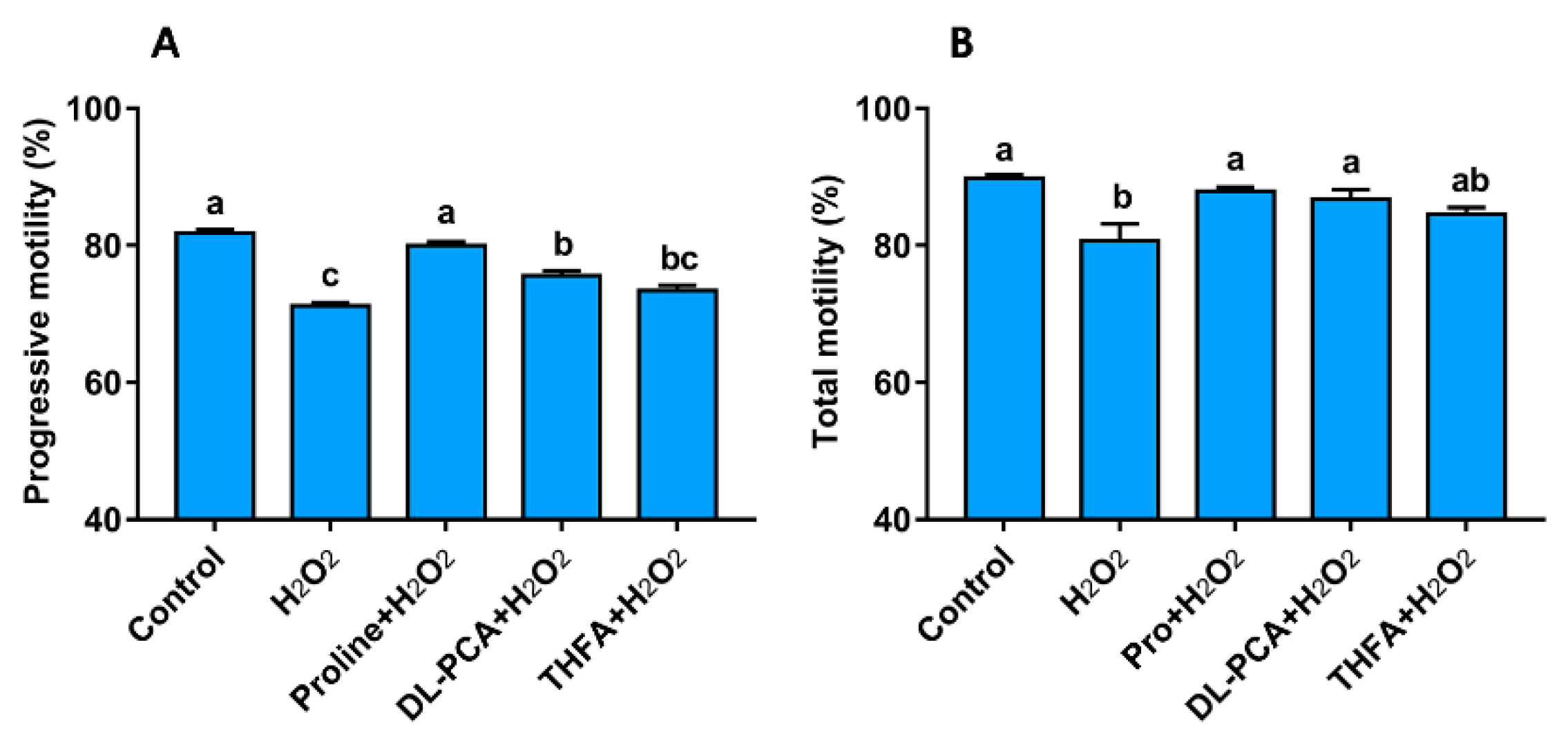
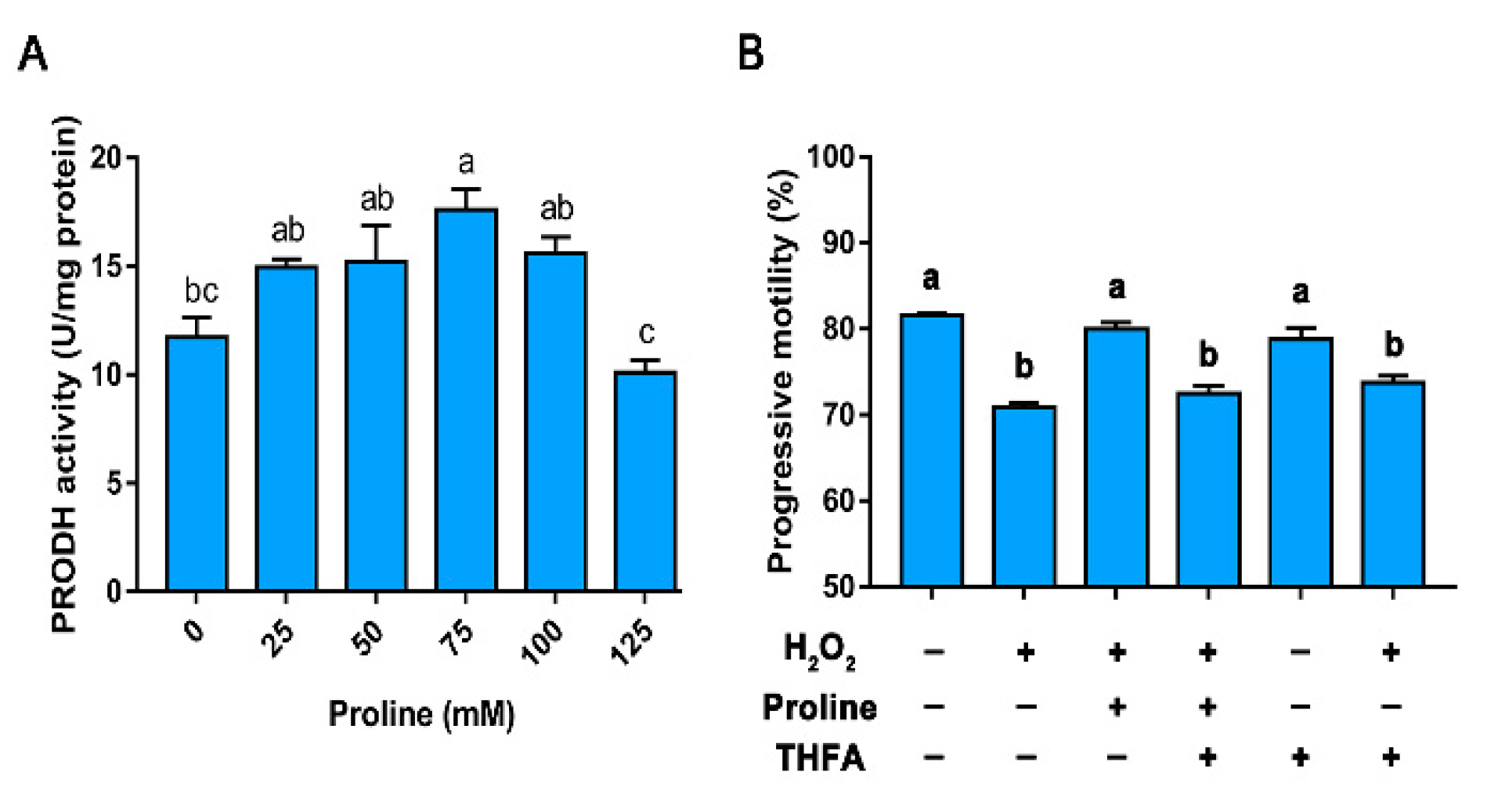
3.3.1. Proline Improves the Motility, Integrity, and Redox Environment when Sperm are Exposed to Hydrogen Peroxide
3.3.2. Proline Improves the Motility, Integrity, and Redox Environment During Rapid Cooling
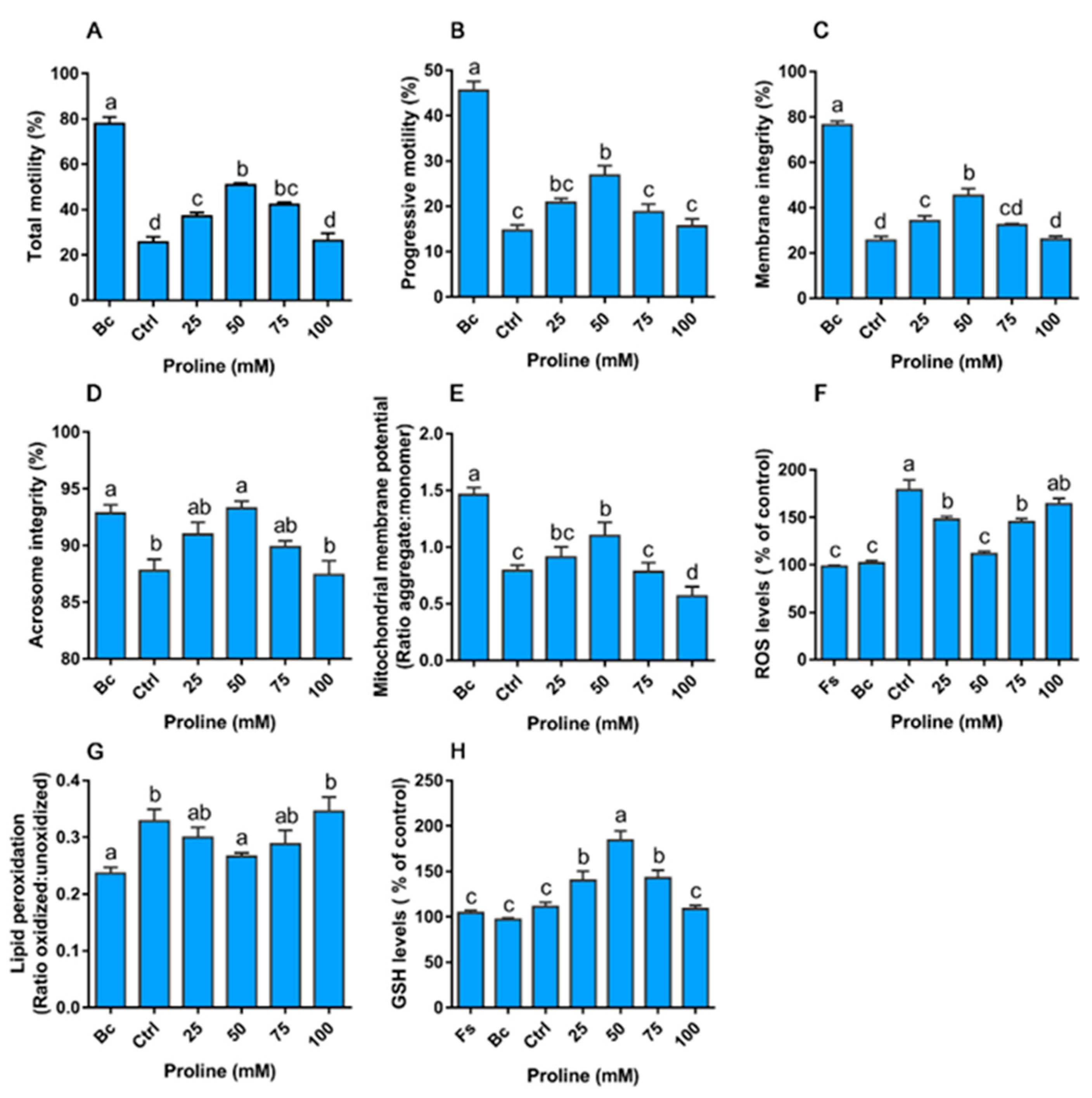
3.4. Experiment IV
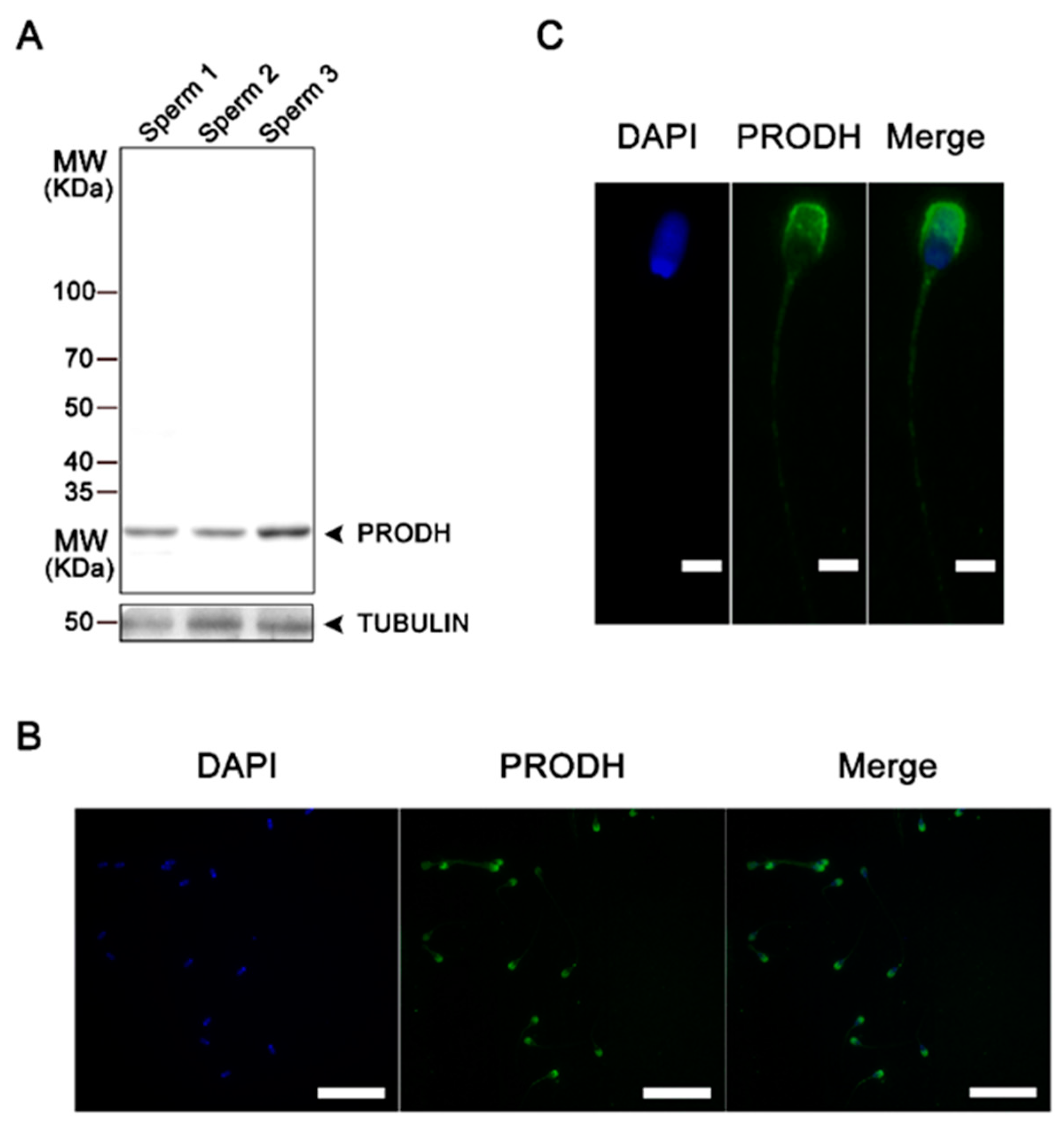
3.5. Experiment V
Proline Protects Sperm against ROS via Proline Dehydrogenase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roca, J.; Parrilla, I.; Bolarin, A.; Martinez, E.; Rodriguez-Martinez, H. Will AI in pigs become more efficient? Theriogenology 2016, 86, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.V. Artificial insemination in pigs today. Theriogenology 2016, 85, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Pezo, F.; Romero, F.; Zambrano, F.; Sánchez, R.S. Preservation of boar semen: An update. Reprod. Domest. Anim. 2019, 54, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Kuster, C.; Althouse, G. The impact of bacteriospermia on boar sperm storage and reproductive performance. Theriogenology 2016, 85, 21–26. [Google Scholar] [CrossRef]
- Kumaresan, A.; Kadirvel, G.; Bujarbaruah, K.; Bardoloi, R.; Das, A.; Kumar, S.; Naskar, S. Preservation of boar semen at 18 C induces lipid peroxidation and apoptosis like changes in spermatozoa. Anim. Reprod. Sci. 2009, 110, 162–171. [Google Scholar] [CrossRef]
- Waberski, D.; Henning, H.; Petrunkina, A. Assessment of storage effects in liquid preserved boar semen. Reprod. Domest. Anim. 2011, 46, 45–48. [Google Scholar] [CrossRef]
- Fu, J.; Li, Y.; Wang, L.; Zhen, L.; Yang, Q.; Li, P.; Li, X. Bovine serum albumin and skim-milk improve boar sperm motility by enhancing energy metabolism and protein modifications during liquid storage at 17 °C. Theriogenology 2017, 102, 87–97. [Google Scholar] [CrossRef]
- Wang, S.; Sun, M.; Wang, N.; Yang, K.; Guo, H.; Wang, J.; Zhang, Y.; Yue, S.; Zhou, J. Effects of L-glutamine on boar sperm quality during liquid storage at 17 °C. Anim. Reprod. Sci. 2018, 191, 76–84. [Google Scholar] [CrossRef]
- Zhang, X.G.; Yan, G.J.; Hong, J.Y.; Su, Z.Z.; Yang, G.S.; Li, Q.W.; Hu, J.H. Effects of bovine serum albumin on boar sperm quality during liquid storage at 17 °C. Reprod. Domest. Anim. 2015, 50, 263–269. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2011, 2011, 686137. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.F.; Silva, P.F.; Gadella, B.M. New assays for detection and localization of endogenous lipid peroxidation products in living boar sperm after BTS dilution or after freeze–thawing. Theriogenology 2005, 63, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Peña, S.T., Jr.; Gummow, B.; Parker, A.J.; Paris, D.B. Antioxidant supplementation mitigates DNA damage in boar (Sus scrofa domesticus) spermatozoa induced by tropical summer. PLoS ONE 2019, 14, e0216143. [Google Scholar] [CrossRef]
- Zakošek Pipan, M.; Mrkun, J.; Kosec, M.; Nemec Svete, A.; Zrimšek, P. Superoxide dismutase: A predicting factor for boar semen characteristics for short-term preservation. BioMed Res. Int. 2014, 2014, 105280. [Google Scholar] [CrossRef] [PubMed]
- Kowalowka, M.; Wysocki, P.; Fraser, L.; Strzezek, J. Extracellular superoxide dismutase of boar seminal plasma. Reprod. Domest. Anim. 2008, 43, 490–496. [Google Scholar] [CrossRef]
- Guthrie, H.; Welch, G. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef]
- Jofré, I.; Cuevas, M.; de Castro, L.S.; de Agostini Losano, J.D.; Torres, M.A.; Alvear, M.; Scheuermann, E.; Andrade, A.F.C.; Nichi, M.; Assumpção, M.E.O. Antioxidant Effect of a Polyphenol-Rich Murtilla (Ugni molinae Turcz.) Extract and Its Effect on the Regulation of Metabolism in Refrigerated Boar Sperm. Oxid. Med. Cell. Longev. 2019, 2019, 2917513. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—a clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, V.I. Selenium in Pig Nutrition and reproduction: Boars and semen quality—A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 730. [Google Scholar] [CrossRef]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.M.; Zeng, W.; Shimada, M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic. Biol. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef]
- Pintus, E.; Kadlec, M.; Jovičić, M.; Sedmíková, M.; Luis, J. Aminoguanidine Protects Boar Spermatozoa against the Deleterious Effects of Oxidative Stress. Pharmaceutics 2018, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.G.; Fang, Q.; Liu, Q.; Du, R.R.; Yang, G.S.; Wang, L.Q.; Hu, J.H. Supplemental effect of different levels of taurine in Modena on boar semen quality during liquid preservation at 17 °C. Anim. Sci. J. 2017, 88, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Coitiño, E.L.; Borsani, O.; Monza, J. Molecular mechanisms for the reaction between OH radicals and proline: Insights on the role as reactive oxygen species scavenger in plant stress. J. Phys. Chem. B 2013, 118, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Dickman, M.B.; Becker, D.F. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic. Biol. Med. 2008, 44, 671–681. [Google Scholar] [CrossRef]
- Chen, C.; Wanduragala, S.; Becker, D.F.; Dickman, M.B. Tomato QM-like protein protects Saccharomyces cerevisiae cells against oxidative stress by regulating intracellular proline levels. Appl. Environ. Microbiol. 2006, 72, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Saradhi, P.P.; AliaArora, S.; Prasad, K. Proline accumulates in plants exposed to UV radiation and protects them against UV-induced peroxidation. Biochem. Biophys. Res. Commun. 1995, 209, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Matysik, J.; Alia, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 525–532. [Google Scholar]
- Natarajan, S.K.; Zhu, W.; Liang, X.; Zhang, L.; Demers, A.J.; Zimmerman, M.C.; Simpson, M.A.; Becker, D.F. Proline dehydrogenase is essential for proline protection against hydrogen peroxide-induced cell death. Free Radic. Biol. Med. 2012, 53, 1181–1191. [Google Scholar] [CrossRef]
- Zhu, Z.; Ren, Z.; Fan, X.; Yang, P.; Shan, L.; Pan, C.; Lei, A.; Zeng, W.; Drevet, J.l.R. Cysteine protects rabbit spermatozoa against reactive oxygen species-induced damages. PLoS ONE 2017, 12, e0181110. [Google Scholar] [CrossRef]
- Dorado, J.; Acha, D.; Ortiz, I.; Gálvez, M.; Carrasco, J.; Gómez-Arrones, V.; Calero-Carretero, R.; Hidalgo, M. Effect of extender and amino acid supplementation on sperm quality of cooled-preserved Andalusian donkey (Equus asinus) spermatozoa. Anim. Reprod. Sci. 2014, 146, 79–88. [Google Scholar] [CrossRef]
- Sangeeta, S.; Arangasamy, A.; Kulkarni, S.; Selvaraju, S. Role of amino acids as additives on sperm motility, plasma membrane integrity and lipid peroxidation levels at pre-freeze and post-thawed ram semen. Anim. Reprod. Sci. 2015, 161, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Partida, L.; Maxwell, W.; Paleg, L.; Setchell, B. Proline and glycine betaine in cryoprotective diluents for ram spermatozoa. Reprod. Fertil. Dev. 1992, 4, 113–118. [Google Scholar] [CrossRef]
- Ollero, M.; Perez-Pe, R.; Muino-Blanco, T.; Cebrian-Perez, J. Improvement of ram sperm cryopreservation protocols assessed by sperm quality parameters and heterogeneity analysis. Cryobiology 1998, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kundu, C.; Das, K.; Majumder, G. Effect of amino acids on goat cauda epididymal sperm cryopreservation using a chemically defined model system. Cryobiology 2001, 42, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Partida, L.; Setchell, B.; Maxwell, W. Effect of compatible solutes and diluent composition on the post-thaw motility of ram sperm. Reprod. Fertil. Dev. 1998, 10, 347–358. [Google Scholar] [CrossRef]
- Sánchez-Partida, L.G.; Windsor, D.P.; Eppleston, J.; Setchell, B.P.; Maxwell, W.C. Fertility and its relationship to motility characteristics of spermatozoa in ewes after cervical, transcervical, and intrauterine insemination with frozen-thawed ram semen. J. Androl. 1999, 20, 280–288. [Google Scholar]
- Li, Y.; Si, W.; Zhang, X.; Dinnyes, A.; Ji, W. Effect of amino acids on cryopreservation of cynomolgus monkey (Macaca fascicularis) sperm. Am. J. Primatol. 2003, 59, 159–165. [Google Scholar] [CrossRef]
- Bottrel, M.; Acha, D.; Ortiz, I.; Hidalgo, M.; Gósalvez, J.; Camisão, J.; Dorado, J. Cryoprotective effect of glutamine, taurine, and proline on post-thaw semen quality and DNA integrity of donkey spermatozoa. Anim. Reprod. Sci. 2018, 189, 128–135. [Google Scholar] [CrossRef]
- Pena, A.; Barrio, F.; Quintela, L.; Herradon, P. Proline and glycine betaine in a diluent for freezing canine spermatozoa. Reprod. Fertil. Dev. 1998, 33, 5–9. [Google Scholar]
- Zhu, Z.; Fan, X.; Lv, Y.; Lin, Y.; Wu, D.; Zeng, W. Glutamine protects rabbit spermatozoa against oxidative stress via glutathione synthesis during cryopreservation. Reprod. Fertil. Dev. 2017, 29, 2183–2194. [Google Scholar] [CrossRef]
- Zhu, Z.; Fan, X.; Lv, Y.; Zhang, N.; Fan, C.; Zhang, P.; Zeng, W. Vitamin E analogue improves rabbit sperm quality during the process of cryopreservation through its antioxidative action. PLoS ONE 2015, 10, e0145383. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.X.; Terada, T. Effects of methyl-beta-cyclodextrin on cryosurvival of boar spermatozoa. J. Androl. 2001, 22, 111–118. [Google Scholar] [PubMed]
- Zarse, K.; Schmeisser, S.; Groth, M.; Priebe, S.; Beuster, G.; Kuhlow, D.; Guthke, R.; Platzer, M.; Kahn, C.R.; Ristow, M. Impaired Insulin/IGF1 Signaling Extends Life Span by Promoting Mitochondrial L-Proline Catabolism to Induce a Transient ROS Signal. Cell Metab. 2012, 15, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Donald, S.P.; Pandhare, J.; Liu, Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 2008, 35, 681–690. [Google Scholar] [CrossRef]
- Phang, J.M. Proline metabolism in cell regulation and cancer biology: Recent advances and hypotheses. Antioxid. Redox. Signal. 2019, 30, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Kononczuk, J.; Czyzewska, U.; Moczydlowska, J.; Palka, J.; Miltyk, W. Proline oxidase (POX) as a target for cancer therapy. Curr. Drug Targets 2015, 16, 1464–1469. [Google Scholar] [CrossRef]
- Liu, W.; Phang, J.M. Proline dehydrogenase (oxidase) in cancer. Biofactors 2012, 38, 398–406. [Google Scholar] [CrossRef]
- Hancock, C.N.; Liu, W.; Alvord, W.G.; Phang, J.M. Co-regulation of mitochondrial respiration by proline dehydrogenase/oxidase and succinate. Amino Acids 2016, 48, 859–872. [Google Scholar] [CrossRef]
- White, T.A.; Krishnan, N.; Becker, D.F.; Tanner, J.J. Structure and kinetics of monofunctional proline dehydrogenase from Thermus thermophilus. J. Biol. Chem. 2007, 282, 14316–14327. [Google Scholar] [CrossRef]
- Pandhare, J.; Donald, S.P.; Cooper, S.K.; Phang, J.M. Regulation and function of proline oxidase under nutrient stress. J. Cell. Biochem. 2009, 107, 759–768. [Google Scholar] [CrossRef]
- Ren, M.; Xu, Y.; Erdjument-Bromage, H.; Donelian, A.; Phoon, C.K.; Terada, N.; Strathdee, D.; Neubert, T.A.; Schlame, M. Extramitochondrial cardiolipin suggests a novel function of mitochondria in spermatogenesis. J. Cell. Biol. 2019, 218, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Zhu, Z.; Bai, W.; Li, R.; Zheng, Y.; Tian, X.; Wu, D.; Lu, H.; Wang, Y.; Zeng, W. Proline Protects Boar Sperm against Oxidative Stress through Proline Dehydrogenase-Mediated Metabolism and the Amine Structure of Pyrrolidine. Animals 2020, 10, 1549. https://doi.org/10.3390/ani10091549
Feng C, Zhu Z, Bai W, Li R, Zheng Y, Tian X, Wu D, Lu H, Wang Y, Zeng W. Proline Protects Boar Sperm against Oxidative Stress through Proline Dehydrogenase-Mediated Metabolism and the Amine Structure of Pyrrolidine. Animals. 2020; 10(9):1549. https://doi.org/10.3390/ani10091549
Chicago/Turabian StyleFeng, Chengwen, Zhendong Zhu, Wenjing Bai, Rongnan Li, Yi Zheng, Xiu’e Tian, De Wu, Hongzhao Lu, Yongjun Wang, and Wenxian Zeng. 2020. "Proline Protects Boar Sperm against Oxidative Stress through Proline Dehydrogenase-Mediated Metabolism and the Amine Structure of Pyrrolidine" Animals 10, no. 9: 1549. https://doi.org/10.3390/ani10091549
APA StyleFeng, C., Zhu, Z., Bai, W., Li, R., Zheng, Y., Tian, X., Wu, D., Lu, H., Wang, Y., & Zeng, W. (2020). Proline Protects Boar Sperm against Oxidative Stress through Proline Dehydrogenase-Mediated Metabolism and the Amine Structure of Pyrrolidine. Animals, 10(9), 1549. https://doi.org/10.3390/ani10091549





