1. Introduction
The lymphoepithelial cyst (LEC) is a rare benign lesion, uncommonly diagnosed in veterinary and human medicine. It has mainly been discovered in the human head-and-neck region, such as the oral cavity and salivary glands (more commonly, the parotid and, rarely, the submandibular gland), most often in the lateral cervical area just below the angle of the mandible, including the lymph nodes. It rarely affects the thyroid gland, lungs, and pancreas [
1].
The LEC of the salivary glands may be single or multiple, uni- or multilocular, or unilateral or bilateral, and its size varies from a few millimetres to centimetres (range 0.5–5 cm). It appears almost equally in both sexes as a soft, painless mass that grows slowly and may cause facial asymmetry. A complete surgical removal remains the treatment of choice and generally has a good prognosis. Human immunodeficiency virus (HIV)-related LEC is more prone to the transformation into B-cell lymphoma [
2,
3].
The LEC was first described in 1885 as a benign lymphocytic infiltration and an enlargement of the salivary and lacrimal glands, but the AIDS-related involvement of lymph nodes of the salivary glands was first reported in 1985 [
4,
5]. The LEC has been widely detected as a common cause of an enlarged parotid gland in patients infected with human immunodeficiency virus (HIV). The incidence of the salivary LEC in HIV-infected patients ranges between 3% and 6%. It is typically observed in middle-aged patients of both sexes and is usually large, bilateral, and multiloculated. Its presence should be considered as a hallmark of the early phases of HIV infection, whereas it is rarely observed in patients with an advanced stage of HIV infection [
6,
7]. It has been considered as nearly pathognomonic for HIV. In addition, its decreased incidence upon an antiretroviral treatment used as the first-line therapy has proved that this “linking” is not purely coincidental [
1].
Despite the growing interest in LEC due to its relation to HIV infection, its etiopathogenesis remains controversial, especially due to its occurrence in non-HIV patients [
8].
The diagnosis of HIV-associated LEC is usually established by the medical history, clinical appearance, blood tests for HIV, preoperative imaging, cytology (fine-needle aspiration), and histopathology (haematoxylin-eosin staining—HE) [
2,
3,
9]. There are only a few reports that confirmed HIV-associated LEC via HIV-1 p24 immunohistochemical stainings [
9,
10,
11,
12]. Sekikawa et al. [
9] suggested that, in HIV-patients with suspected LEC, this staining could help in the differential diagnosis. However, it is not offered in the standard practice of many laboratories.
Caprine arthritis-encephalitis (CAE) is a contagious disease of goats caused by the caprine arthritis-encephalitis virus (CAEV) classified as a small ruminant lentivirus (SRLV). SRLV belongs to the
Retroviridae family closely related to HIV. CAE is a chronic progressive disease manifesting as chronic non-purulent arthritis, wasting, less often indurative mastitis, and interstitial pneumonia [
13,
14]. The presence of LEC linked to SRLV-positive animals is unknown, and the pathological features of LEC have not been fully characterized so far. In this study, the histopathological and immunohistochemical characteristics of LEC in the salivary gland are described in a SRLV-positive dairy goat. The comparative aspect to its human counterpart is also discussed.
2. Materials and Methods
2.1. Case Presentation
A 5-year-old Polish White Improved dairy goat was seropositive for SRLV using ELISA (ID Screen MVV-CAEV Indirect Screening test, ID.vet Innovative Diagnostics, Grabels, France). The goat came from a herd with a long history of CAEV infection (with seroprevalence exceeding 90%) and the severity of pathological findings varying considerably among individual goats. The present goat showed clinical signs indicative of CAE—weight loss, marked dyspnoea, cyanosis of the oral mucosa, and the swelling of the carpal joints, suggesting arthritis. The animal was euthanized at the owner’s request and submitted for necropsy to the Department of Pathology and Veterinary Diagnostics, Warsaw University of Life Sciences (WULS). Additionally, other animals from the herd demonstrated clinical symptoms (lameness, emaciation, and interstitial mastitis) and macro- and microscopical lesions consistent with CAE.
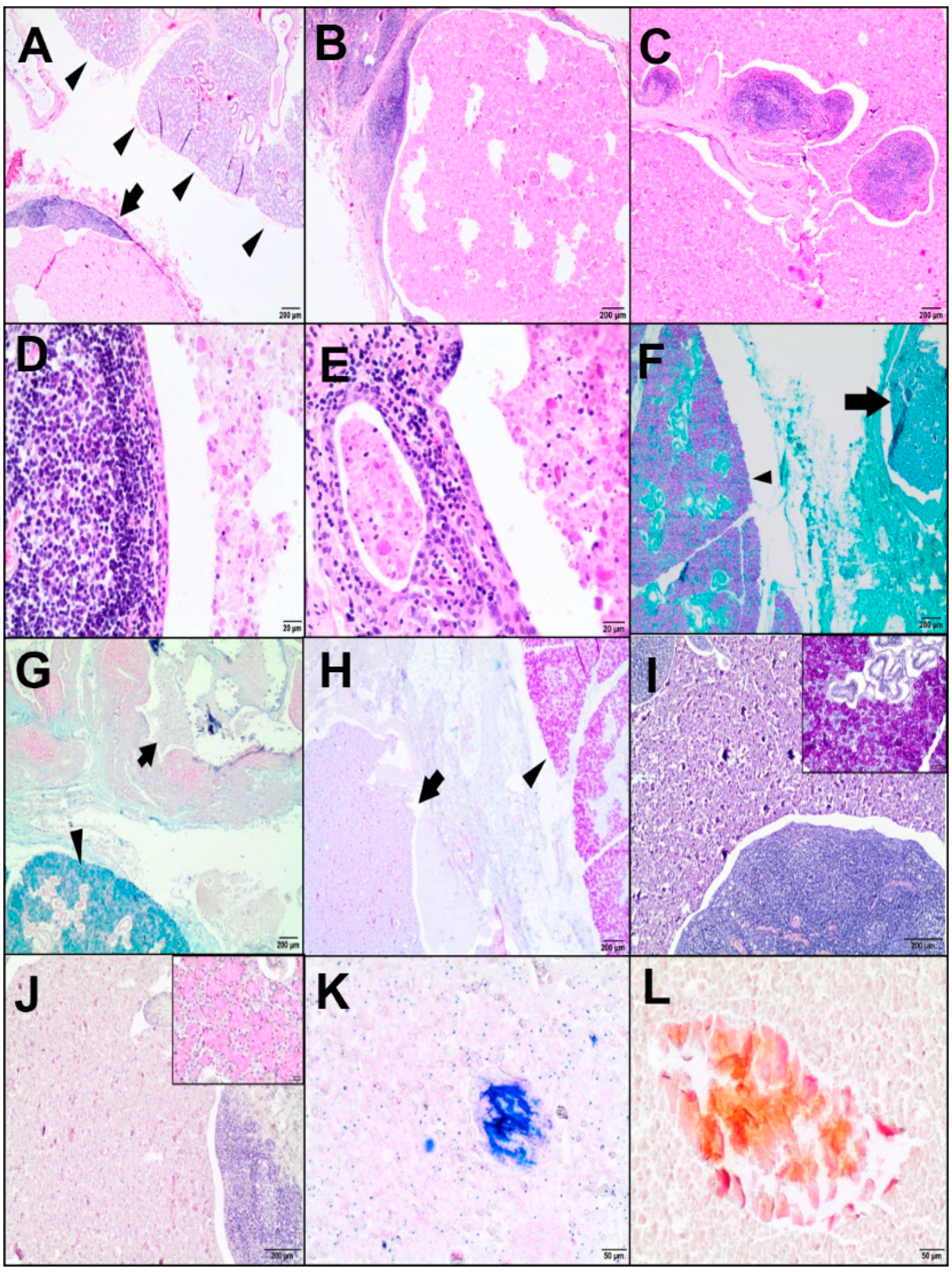
Grossly, the body condition of the goat was poor (2 on the 5-point scale) [
15]. Skin abrasions were also noted on the dorsal surfaces of the carpal joints and the sternum. The joint capsules were thickened and contained quite a small amount of synovial fluid. The surface of the articular cartilage was smooth. The mammary gland seemed to be empty. Mammary gland lymph nodes were congested. Pneumonia was the most evident lesion in the present case. The lungs were diffusely enlarged, firm, and congested, with consolidated cranioventral lobes and scattered grey-pink foci. The lungs did not collapse, and the hydrostatic test of the pulmonary samples showed flotation. Moreover, mainly the left cranial lobe showed fibrinous exudate on the cross-section, and its pleura was covered with fibrin deposits. Some fibrous adhesions extended onto the parietal pleura and pericardial sac. The tracheobronchial lymph nodes were enlarged and firm. The thoracic cavity contained blood-tinged and slightly cloudy fluid. Other pathological findings included pericarditis, splenic congestion, small duodenal haemorrhages, and renal pelvis calculi. Additionally, an oval, circumscribed mass of a cyst-like structure (about 2 cm in diameter) was found on the cross-section close to the right mandibular angle. The cyst was unilocular, surrounded by a thin wall (about 0.5 cm), and filled with a soft, pasty cream-coloured material (“muddy” appearance). The brain was not examined.
2.2. Histopathology
Tissue samples from the cyst-like lesion of interest, and from the lungs, spleen, heart, and duodenum, were fixed in 10% buffered formalin, routinely processed, and embedded in paraffin. The 4-μm-thick sections were stained with HE. Furthermore, the cystic mass with adjacent parenchyma was examined using histochemical stains, such as periodic acid Schiff (PAS), PAS with alcian blue (PAS-AB), alcian blue at pH 2.5, PAS-diastase (PAS/D), mucicarmine, Papanicolaou (Pap), and May-Grünwald-Giemsa (MGG). All samples were reviewed using an Olympus BX43 microscope (Olympus Corporation, Tokyo, Japan).
2.3. Immunohistochemistry
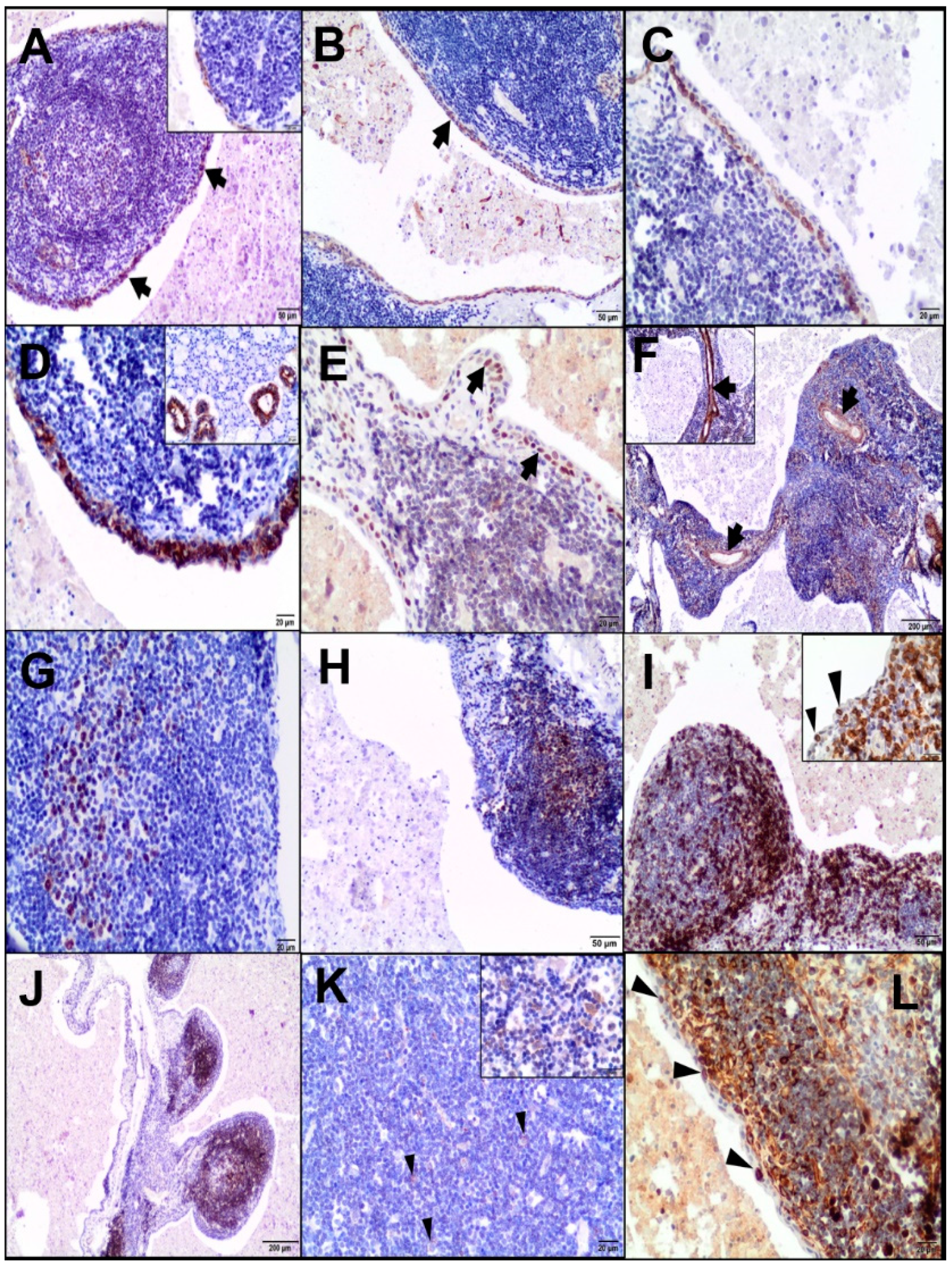
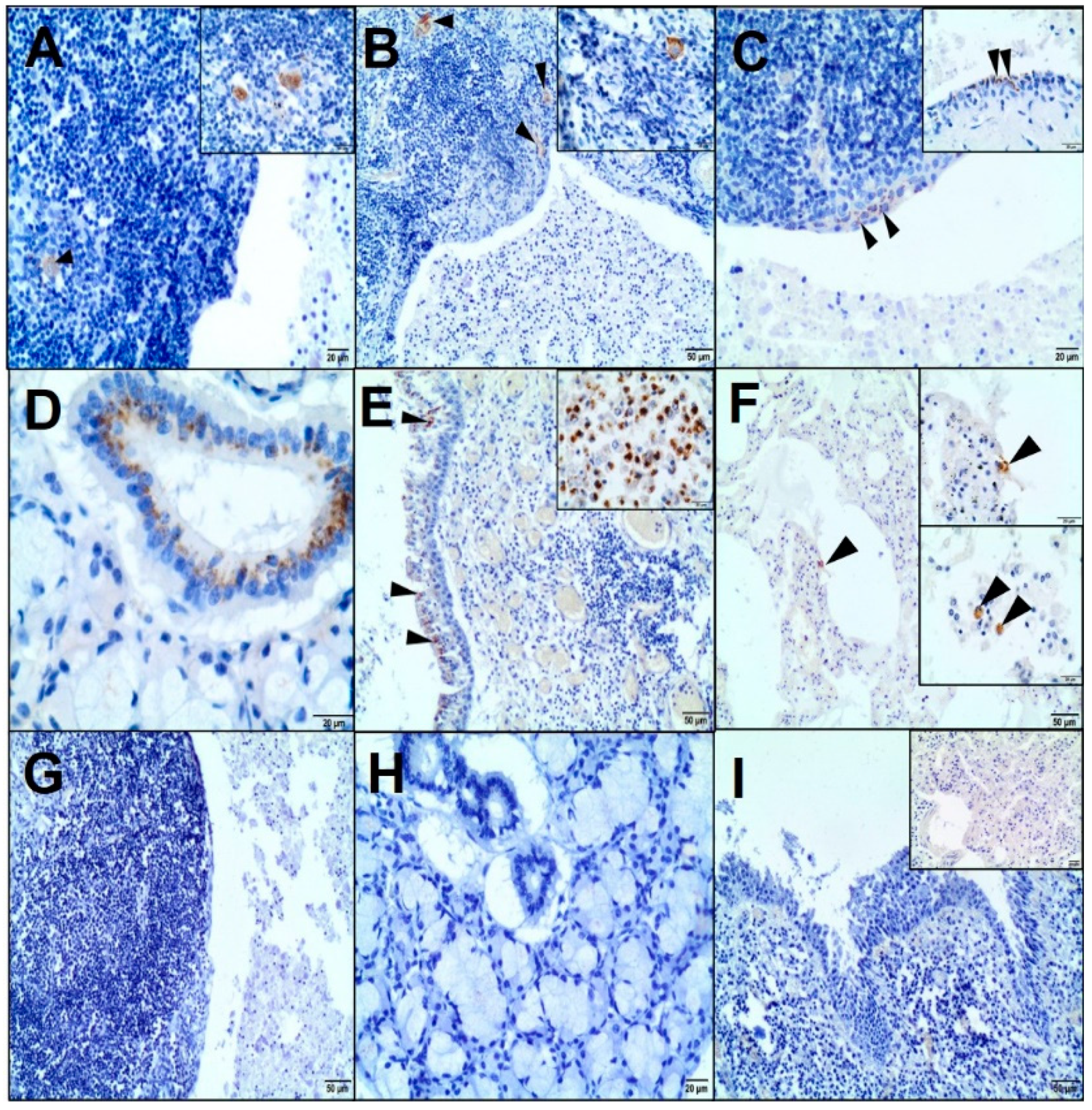
Immunohistochemistry (IHC) of the cystic lesion was performed using primary antibodies (
Table 1). Moreover, the lung specimens were analysed for the presence of a CAEV p28 antigen.
After dewaxing in xylene and rehydration in alcohols, the slides were microwaved in 0.02-M citrate buffer, pH 6.0, or in TRIS-EDTA, pH 9.0, at 600 W for 15 minutes and then treated with a 3% perhydrol solution for 15 minutes. The sections were incubated with the primary antibody for 1 hour at room temperature (RT) in a humid chamber; however, with anti-CAEV antibody overnight at 4 °C. After washing in Tris-buffered saline, they were incubated with a biotinylated secondary antibody (EnVision™ detection system, Peroxidase/DAB (3,3’-diaminobenzidine), Rabbit/Mouse, HRP, Dako, Agilent Tech., Santa Clara, CA, USA) and then visualized with 3,3’-diaminobenzidine (DAB) from Dako, Agilent Tech., Santa Clara, CA, USA. The sections were counterstained with Erlich’s haematoxylin and mounted using the DPX medium (Sigma-Aldrich Co, St. Louis, MO, USA). Negative controls were performed by omitting the primary antibody. Moreover, an isotypical monoclonal IgG was used as a negative control, replacing the anti-CAEV antibody. Tissues with known positive reactivity from a dog and goat (lungs, skin, mammary gland, salivary gland, spleen, tonsil, lymph nodes, and uterus) were used as positive controls.
4. Discussion
The morphological and immunohistochemical features of the cystic lesion that was incidentally found adjacent to the salivary gland in the present goat supported a LEC diagnosis. Our observations were in line with previous human studies that reported LEC as an epithelial-lined cyst in a lymph node adjacent to or embedded in the major salivary gland [
3]. Even though a cystic benign lymphoepithelial lesion has been more often found in the parotid glands in HIV-positive patients, there are some reports of a LEC of the submandibular gland also accompanying HIV infection [
2,
6].
Our goat had a medical history, clinical symptoms, laboratory confirmation, and necropsy diagnosis of CAE. In this animal, respiratory signs due to pneumonia were predominant. Despite the paucity of tissues from this case collected for histopathology, the CAEV infection was identified in the lungs by IHC. Therefore, it seems likely that, as in humans, SRLV-infected cells may migrate into the salivary glands. Additionally, both SRLV and HIV are closely related retroviruses that replicate in monocyte/macrophage cell lineages, affect mucosal membranes, have tropism to biological secretions, and cause latent and chronic multisystemic infections. Contrary to HIV, SRLV does not replicate in CD4
+ T lymphocytes and is unable to cause immunosuppression in infected goats [
14]. Although the main target cells for SRLV are macrophages, monocytes, and dendritic cells, many studies have reported that it could infect a broad range of cells, such as epithelial cells, oligodendrocytes, astrocytes, endothelial cells, fibroblasts, and adipocytes [
14].
In the present case, the immunohistochemical analysis confirmed the presence of CAEV (p28 Gag antigen) in the cells morphologically consistent with macrophages and dendritic cells of a LEC and epithelial cells of the salivary gland. Previous studies identified the CAE viral particles in multiple caprine tissues immunohistologically, e.g., lungs; brain; mammary gland; synovial fluid; bronchoalveolar lavage fluid; colostrum/milk (macrophages, macrophage-like cells, uterine, mammary epithelial cells, and bronchial epithelium); lymph nodes; and spleen (macrophages and dendritic cells) [
16].
The immunohistochemical studies on a LEC in HIV-infected patients have revealed HIV-1 p24 in residual dendritic cells of lymphoid follicles (follicular dendritic reticulum cells) and follicular and interfollicular macrophages [
9,
10,
12]. Some authors have shown that the lymphoepithelial cyst contained a large amount of HIV-1 p24 and RNA copies, suggesting this lesion to be a reservoir of HIV [
2,
12].
To the best of our knowledge, there are no published reports confirming the involvement of bacterial or fungal infections in LEC etiopathogenesis. In our study, these pathogens were not present in the lesion, and bacterial pneumonia occurred as a secondary infection.
The definition and etiopathogenesis of the LEC have not been established yet, and many theories exist in this respect. The term “benign lymphoepithelial cyst” was proposed by Bernier and Bhaskar in 1958 to distinguish this lesion from other cystic lesions derived from embryonic branchial pouch remnants [
17]. They emphasized an inclusion theory—according to which, the LEC is not an embryologic remnant. The cyst within lymph nodes associated with salivary glands results from the cystic degeneration of the salivary gland epithelium entrapped in the upper cervical lymph nodes during embryonic life. Recently, some authors have suggested sialadenitis-based pathogenesis (in non-HIV-cases), where the dilatation of a duct within the salivary gland is stimulated by focal sialadenitis. As a consequence, a lymphoepithelial cyst is formed by demarcation from the salivary gland because of the granulation tissue response, and it does not arise from intraparotid lymph nodes [
18]. For the present case, the inclusion hypothesis would seem a possible explanation for the LEC, whereas underlying sialadenitis was not observed. Additionally, we assume that the possible association with CAEV infection might be relevant to the development of a LEC. Another concept was that the LEC originated from the epithelial remnants of the cervical sinus or the branchial cleft retained during embryogenesis (classic theories: cervical sinus theory and branchial apparatus theory, respectively); therefore, the name “branchial cleft cyst” was coined [
17]. Although the final diagnosis of the lesion is based on the histopathological examination, the ability to establish the true nature of salivary LEC in relation to possible theories of origin remains difficult. To date, there have been no specific markers or features to distinguish them from each other. Despite different terms and theories, the branchial cleft cyst and the lymphoepithelial cyst shared similar microscopic features, thus remaining indistinguishable. On the other hand, due to their rarity and incompletely understood pathogenesis, they are still poorly characterized.
Given the different nature of HIV and CAEV infections, the comparisons regarding the clinical manifestation of the LEC is difficult. It is well-known that, in humans, the LEC diagnosis, especially in the parotid gland, is usually concomitant with the early clinical manifestation of HIV infection. However, in the present goat, the lesion did not cause a noticeable deformation, and it was discovered incidentally during necropsy in an animal manifesting the chronic disease. In turn, our observations might not reflect the real situation because of the lack of animals in the early stage of infection.
The pathophysiology of HIV-associated LEC is still debated. It has been postulated that reactive lymphoid proliferation in the parotid gland is responsible for the ductal obstruction and subsequent cyst formation. The other theory suggests that reactive lymphoid proliferation occurs in the lymph nodes of the parotid gland or submandibular gland. The metaplasia in the salivary ducts is caused by the migration of HIV-infected cells, which indirectly leads to cyst formation [
2,
3,
17].
Based on some studies, the lymphoid component of LECs are not as well-organized as the normal lymph nodes (e.g., cell arrangement, proliferative activity, and lack of sinuses and capsule). It may also be merged into the surrounding lymph nodes and completely replaced. On the other hand, LECs may also occur without the lymph nodes and arise outside the lymph nodes, originating from the intralobular ducts; hence, in such a location, the lymphoid component could occur as a secondary lymphocytic infiltration [
6,
11,
17,
18,
19]. Other authors concluded that it was impossible to determine if a LEC was derived from lymph nodes or from lymphocytic infiltration [
20].
Although HIV shows tropism to the lymphoid tissue and its high concentrations can be found within lymphoid nodes, most LEC cells do not contain viral antigens. The accumulation of the virus was reported in the cystic contents due to the shedding of virus-infected cells from the adjacent lymphoid tissues [
12]. Moreover, the regression of HIV-associated LEC after antiviral therapy was observed as a decline in the viral load, suggesting some degree of immune recovery [
7,
12]. Although the detection of HIV-1 p24 antigen by IHC should be performed on lymphoepithelial lesions in HIV-positive patients with salivary gland swelling, and its presence has been considered supportive evidence for the pathogenetic role in HIV-related LEC, there is no clear consensus on LEC etiopathogenesis. Furthermore, some studies have demonstrated the presence of other viruses within a LEC and showed that LECs share similar features in both HIV-positive and HIV-negative patients [
9,
10,
11,
18,
19].
Our observation may obviously be coincidental, given the scarcity of published data. Despite the high seroprevalence of CAEV in the herd and goats with clinical, pathological, and histopathological features of CAE, only one animal showed the presence of a LEC. Based on our observations, we can only speculate that this lesion may be very rare, as it has been reported in humans. The incidence of the salivary LEC corresponded to less than 6% of the HIV-infected patients, but the exact incidence of the LEC in non-HIV patients is unknown [
2,
8].
To date, only one study has demonstrated unilateral cysts in the upper neck in Anglo-Nubian goats [
21]. The authors suggested that the cysts were derived from the salivary gland ducts. However, a small number of lymphoid cells was noted only in one case. In contrast to our report, these cysts were filled with fluids or mucinous materials and were surrounded by a granulated tissue.
The histological findings in our case were in accordance with those reported in human medicine. The LEC was predominantly lined by a squamous epithelium surrounded by lymphoid tissue [
1,
3]. Although the tingible body macrophages, lymphocyte exocytosis, and multinucleated giant cells may be present [
18], the latter was not demonstrated in our case.
The LEC contained eosinophilic materials with foamy macrophages and lymphocytes; in addition, some crystalloids were dispersed. A variety of crystalloids have been reported in normal as well as neoplastic and non-neoplastic disorders of salivary glands; however, they are not common. The determination of crystalloid types may be helpful in differentiating the salivary gland tumours. Amylase crystalloids were reported as nonbirefringent geometrical crystalline structures of variable shapes and sizes (polygonal and plate-like) only in benign lesions, including the lymphoepithelial cyst [
22,
23]. They stained eosinophilic in HE, blue in MGG, bright orange in Pap, and did not react with mucous stains. It has been suggested that they can result from amylase-saturated saliva due to its stagnation in the cyst [
24].
Some studies have shown the immunohistochemical features of LEC to be similar in both HIV-associated and non-HIV-associated patients [
18,
19]. As far as we know, there are no data on the immunohistochemical analysis of LEC in the veterinary literature. In the present study, most T-positive lymphocytes were concentrated in the peripheral interfollicular region and at sites of the intraepithelial population, which was consistent with previously published reports [
7]. On the other hand, some authors have demonstrated that intraepithelial lymphocytes are derived predominantly from the B-cell lineage. The majority of lymphocytes of LEC in the lymphoepithelial islands were B cells in both HIV-associated and non-HIV-associated patients [
11,
25]. Given the previous reports, LEC showed a low proliferative index [
18,
19,
25]. The Bcl-2-positive lymphocytes were not observed in lymphoid germinal centres; however, they were interspersed throughout the lesion, including the intraepithelial population [
7,
25]. In human cases, one study demonstrated Bcl-2-positive intraepithelial lymphocytes in non-HIV-associated LEC [
25].